Abstract
Lower serum concentrations of sex-hormone binding globulin (SHBG) are associated with increased risk for several obesity-related diseases in women including hormone-sensitive cancers, type 2 diabetes, metabolic syndrome, and cardiovascular disease. Previous investigations have reported that body composition, specifically central obesity, and/or higher insulin concentrations are key factors associated with lower SHBG in overweight and obese women; however, these studies were limited by their cross-sectional design. We hypothesized that intra-abdominal adipose tissue (IAAT), a fat depot linked with an abnormal metabolic profile, is inversely and independently associated with SHBG. Therefore, we determined the longitudinal associations among SHBG, insulin, and IAAT in 107 premenopausal women enrolled in a weight loss study. Overweight (BMI 27–30 kg/m2) women were weight reduced until BMI of ≤24 was achieved. Body composition and IAAT were measured at baseline and after weight loss with dual-energy X-ray absorptiometry and computed tomography, respectively. Serum concentrations of insulin and SHBG were determined. Paired t-test showed that insulin and IAAT decreased significantly and SHBG increased significantly following weight loss (P < 0.0001 for all). Simple correlations from baseline showed no association with insulin and SHBG (r = −0.142, P = 0.143) and a significant inverse association between IAAT and SHBG (r = −0.43, P < 0.0001). Repeated measures mixed-model showed that after adjusting for age and time (weight loss), IAAT was significantly inversely associated with SHBG (P = 0.0002) and there was no association with insulin and SHBG (P = 0.180). We conclude that SHBG concentrations are influenced by IAAT and not insulin in premenopausal women.
INTRODUCTION
Sex-hormone binding globulin (SHBG) is a protein produced by the liver that transports the sex-steroid hormones, testosterone, and estradiol in the blood. The binding of SHBG to testosterone and estradiol regulates the bioavailibity of these hormones. In premenopausal women, lower blood concentrations of SHBG are indicative of higher blood concentrations of free androgens (1). Some data suggest that higher concentrations of free androgens are associated with increased risk for cancers of the breast (2) and endometrium (3) and cardiovascular disease (4). Several recent studies have reported that lower concentrations of SHBG are associated with metabolic syndrome (5) and type 2 diabetes (6).
Obesity results in lower serum SHBG concentrations (7). However, the physiological factors that link obesity with decreased SHBG remain unclear. One potential physiological mechanism linking obesity and lower SHBG is higher blood concentrations of insulin. Insulin is elevated in obesity and has been shown to suppress hepatocyte production of SHBG in vitro 8). More recent in vitro investigations have implicated other metabolic processes associated with obesity such as hepatic lipogenesis, peroxisome-proliferator activated receptor-γ and thyroid hormone in mediating SHBG production (9–11). Epidemiological studies in female populations have provided some evidence that insulin is negatively correlated with SHBG (12–14). However, these studies were limited by their cross-sectional design and did not directly measure body fat or specific fat depots rather they depended on waist circumference (12), waist-to-hip ratio (14), or BMI as indicators of adiposity (13).
The accumulation of intra-abdominal adipose tissue (IAAT) is related to insulin resistance and type 2 diabetes but may also be a fat depot independently associated with lower SHBG. One recent study found that the ratio of trunk fat to limb fat is significantly inversely correlated with SHBG in premenopausal African-American and European-American women after controlling for homeostasis model assessment–insulin resistance, estradiol, physical activity, and caloric intake (15). However, trunk fat is a general indicator of central adiposity and does not reflect a specific fat compartment. As reviewed by Jenson (16), IAAT is an important fat depot that is associated with an abnormal metabolic profile and therefore may influence hepatic production of SHBG. Tchernof et al. reported that IAAT was significantly associated with SHBG independent of insulin in a female population (17); however, this cross-sectional study was limited by its relatively small sample size. Currently, the associations among IAAT, insulin, and SHBG in healthy premenopausal women are unclear.
Therefore, the purpose of this study was to longitudinally determine the independent associations among SHBG, IAAT, and insulin in premenopausal women enrolled in a weight loss study.
METHODS AND PROCEDURES
Study participants
Participants were overweight (BMI 27–30), premenopausal women reporting to have normal menstrual cycles (approximately every 28 days) and not taking oral contraceptives. Further eligibility criteria included sedentary (defined as exercising <1 time per week for the past year); negative for a history of eating disorders; normoglycemic (determined by 2-h blood glucose following 75-g oral glucose load); nonsmoker; not taking medications known to alter metabolism; and self-identified as either European-American or African-American.
Protocol
Data and biological samples for this study were from a previous research study conducted between March 2001 and May 2005 at the University of Alabama at Birmingham described in detail elsewhere (18). At baseline, following 4 weeks of energy balance and weight maintenance, blood was collected following an overnight fast during the follicular phase of the menstrual cycle (within 10 days of menses). Participants underwent body composition analyses and were then randomized to one of three weight loss groups: (i) diet alone; (ii) diet with aerobic exercise; or (iii) diet with resistance training, based on matching for age, race, and BMI. All participants consumed 800 kcal/day with 20–22% of energy as fat, 18–22% as protein, and 58–62% from carbohydrate. Participants were weighed regularly until their weight loss reached BMI ≤24. Once the weight loss goal was achieved participants again underwent 4 weeks of weight maintenance and energy balance. Blood was collected following an overnight fast and body composition measurements were again assessed during the follicular phase of the menstrual cycle. Written informed consent was obtained from all participants before testing. All procedures and protocols pertaining to the study were approved by the University of Alabama at Birmingham Institutional Review Board.
Body composition measurements
BMI was calculated using weight in kilograms divided by height in meters squared. Dual-energy X-ray absorptiometry (GE-Lunar Prodigy running software version 1.33) was used to assess total and regional body fat composition. Computed tomography scanning with HiLight/Advantage Scanner was used to determine IAAT. A 5-mm scan at the L4–L5 region was taken while subjects were in the supine position with arms above their head. Cross sectional area of adipose tissue was analyzed using density contour as we have previously described (18).
Serum assays
SHBG was assayed in duplicate using a commercially available solid-phase immunoradiometric assay kit from Siemens (Los Angeles, CA). The mean intra- and interassay coefficient of variation were 6.72% and 11.52%. Insulin was assayed in duplicate using double-antibody radioimmunoassay kits from Linco Research (St Charles, MO). The mean intra- and inter-assay coefficient of variation were 5.94% and 6.50%.
Statistical analyses
Insulin and SHBG were not normally distributed and were log (ln) transformed. The geometric mean and 95% confidence interval are shown for these variables. The mean and s.e. are shown for all other normally distributed variables. In initial exploratory analyses, we used multivariate repeated measures ANOVA to test whether weight loss group or race were associated with SHBG and found no effect for either of these variables and therefore combined the groups in all subsequent analyses. The difference in baseline and post weight loss body composition measurements and serum analytes was determined using paired t-tests. Simple correlation coefficients were determined for baseline SHBG and weight, total fat mass, lean mass, BMI, IAAT, and insulin. We used two methods to investigate the association between SHBG and insulin and measures of adiposity. Change scores (baseline—follow-up values) were generated for these variables and simple correlation coefficients were determined for the change score for SHBG with the change score for insulin and the measures of adiposity. In addition, repeated-measures mixed-models, a more robust method for longitudinally assessing the associations among changes in variables, were used to determine the associations among the independent variables insulin and IAAT with the dependent variable SHBG after adjusting for age and time (weight loss). JMP version 8.0 (SAS Institute, Cary NC) was used for statistical analyses.
RESULTS
Fifty African-American and fifty seven European-American premenopausal women mean age 34.3 ± 0.62 years were included in this study. Following weight loss, ~22 weeks, there were significant reductions in all body composition parameters (P < 0.0001 for weight, BMI, fat mass, percent fat, lean mass, and IAAT) (Table 1). There was a significant reduction in serum insulin (P < 0.0001) whereas serum concentrations of SHBG significantly increased (P < 0.0001) following weight loss.
Table 1.
Baseline and weight-reduced measurements of body composition and serum analytes
| Baseline | Weight-reduced | P for time | |
|---|---|---|---|
| Body compositiona | |||
| Weight, kg | 78.0 ± 7.24 | 65.9 ± 6.48 | <0.0001 |
| BMI | 28.3 ± 0.13 | 23.9 ± 0.11 | <0.0001 |
| Fat mass, kg | 32.4 ± 0.49 | 21.2 ± 0.43 | <0.0001 |
| Lean mass, kg | 45.6 ± 0.39 | 44.8 ± 0.39 | <0.0001 |
| IAAT cm2 | 81.0 ± 3.06 | 50.9 ± 2.25 | <0.0001 |
| Serum analytesb | |||
| Insulin, μIU/ml | 11.20 (10.5, 11.9) | 8.60 (7.96, 9.25) | <0.0001 |
| SHBG, nmol/l | 55.5 (50.5, 60.9) | 70.4 (64.5, 76.9) | <0.0001 |
Mean ± s.e.e.
Geometric mean (95% CI).
CI, confidence interval; IAAT, intra-abdominal adipose tissue.
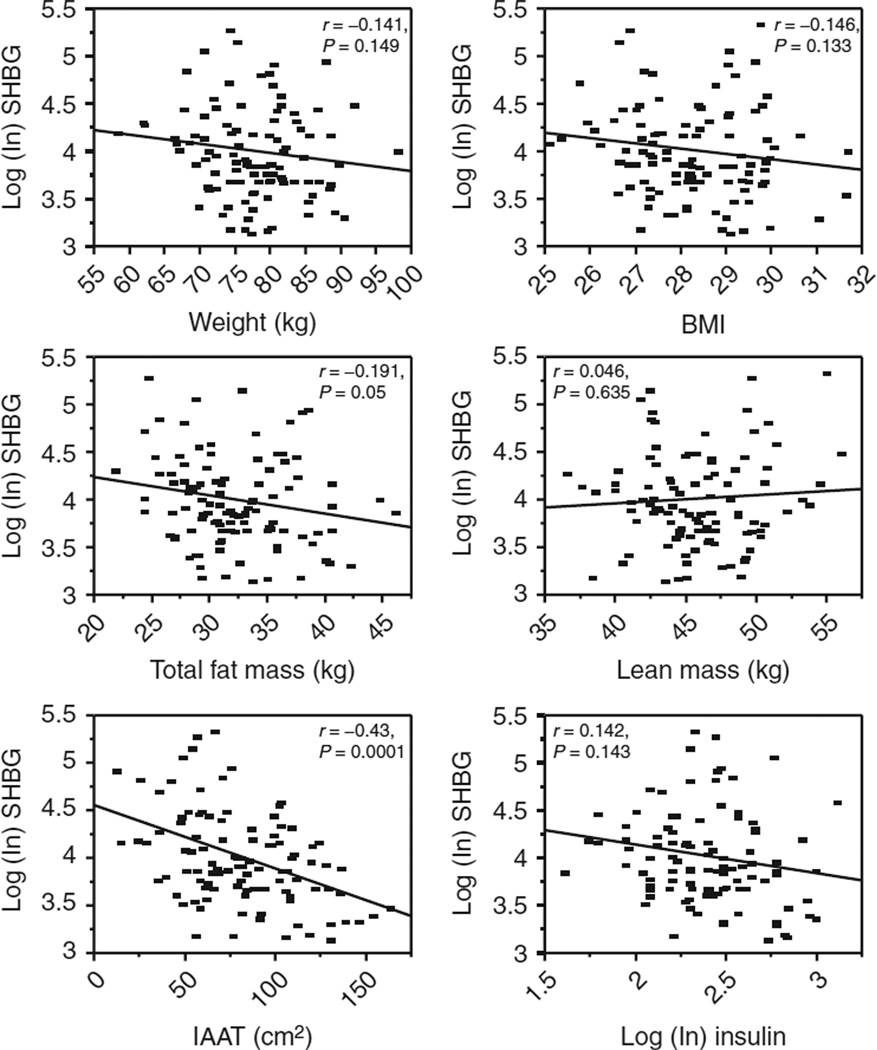
The simple correlations between baseline SHBG and baseline body weight (kg), BMI, total fat mass (kg), lean mass (kg), IAAT (cm2), and insulin (μIU/ml) are shown in Figure 1. There were significant correlations between SHBG and total fat mass (r = −0.191, P = 0.05) and IAAT (r = −0.43, P < 0.0001). The results from simple correlations between the change score (baseline-follow-up values) for SHBG and change scores for insulin and body composition measurements were not statistically significant.
Figure 1.
Simple correlation coefficients for baseline sex-hormone binding globulin (SHBG), body composition measurements, and insulin. SHBG was significantly inversely correlated with fat mass (r = −191, P = 0.05) and intra-abdominal adipose tissue (IAAT) (r = −0.43, P < 0.0001).
Table 2 shows the results from repeated measures mixed-models for the dependent variable SHBG after adjusting for age. As shown in model 1, there was a significant effect of time (weight loss) on SHBG (P < 0.0001). In model 2, after entering insulin into the model, time (weight loss) remained statistically significant (P < 0.0001) and insulin was not associated with SHBG. This indicated that the change in insulin had no effect on the change in SHBG. In model 3, the significant effect of time (weight loss) disappeared with the addition of IAAT into the model. This indicates that the change in IAAT (P = 0.0002) was significantly associated with the change in SHBG.
Table 2.
Repeated measures mixed models for dependent variable SHBG
| Term | Estimate | s.e. | P | |
|---|---|---|---|---|
| Model 1 | ||||
| Intercept | 4.533 | 0.227 | <0.0001 | |
| Time | 0.122 | 0.014 | <0.0001 | |
| Model 2 | ||||
| Intercept | 4.608 | 0.242 | <0.0001 | |
| Time | 0.114 | 0.017 | <0.0001 | |
| Insulin | −0.007 | 0.007 | 0.357 | |
| Model 3 | ||||
| Intercept | 4.689 | 0.233 | <0.0001 | |
| Time | 0.035 | 0.024 | 0.151 | |
| Insulin | −0.010 | 0.007 | 0.180 | |
| IAAT | −0.005 | 0.001 | 0.0002 |
Bold values indicate significant effects.
SHBG, sex-hormone binding globulin; IAAT, intra-abdominal adipose tissue.
DISCUSSION
In this study, we determined that SHBG significantly increased following weight loss. Our findings are consistent with previous reports that significant weight loss as a result of decreased energy intake increases serum SHBG (7), a protein associated with risk for type 2 diabetes (6), cardiovascular disease (4), metabolic syndrome (5), and hormone-sensitive cancers in women (2, 3).
A previous cross-sectional study reported that there was no significant independent association between insulin and SHBG in healthy premenopausal women rather IAAT was significantly inversely associated with SHBG (17). Based on this evidence, we longitudinally explored the associations among SHBG and measures of body composition and insulin in a group of premenopausal women before and after weight loss. In univariate analyses, we found that SHBG was marginally but statistically significantly correlated with total fat mass at baseline. Conversely, there was a strong significant inverse correlation between baseline SHBG and IAAT whereas we found no correlation with insulin. In subsequent analysis, we used repeated measures mixed models, a robust method for assessing change over time to determine the effects of insulin and IAAT on SHBG (19). After adjusting for age, we found that there was a significant effect of time (weight loss) associated with the change in SHBG. The addition of insulin into the model did not change the association between time (weight loss) and SHBG; however, when IAAT was entered into the model the effect of time (weight loss) disappeared. These analyses indicate that the change in IAAT over the course of the weight loss had a significant effect on SHBG independent of insulin. Our results are in contrast to several previous observational studies that have shown insulin is negatively associated with SHBG (12–14). However, these studies were cross-sectional and did not directly account for IAAT and this may explain the discrepancy in our findings and these previous investigations. Of note, we performed additional analyses to test whether more robust measures of glucose and insulin metabolism were associated with SHBG. In repeated-measure mixed-models that included insulin sensitivity and acute insulin response to glucose as determined by intravenous glucose tolerance test, we did not observe an association between these independent variables with SHBG although IAAT remained statistically significant (data not shown).
Indeed, IAAT appears to play an important role in metabolic disease risk (16). Our data suggest that IAAT is associated with hepatic metabolism resulting in decreased production of SHBG. The gene expression of SHBG is influenced by several nutritional and metabolic factors (5). IAAT is a metabolically active fat depot and source of free-fatty acids (FFAs) that drain into the hepatic portal vein (20). At the transcriptional level, the SHBG promoter region is centrally controlled by the transcription factor hepatic nuclear factor-4 (21). This transcription factor has a ligand-binding domain for endogenous FFAs that can modulate the transcriptional activity of the SHBG gene (22, 23). Selva et al. showed that the promoter region has a secondary binding site to which peroxisome-proliferator activated receptor-γ can bind and decrease SHBG expression (10). This is believed to be through peroxisome-proliferator activated receptor-γ binding of endogenous fatty acids and repression of SHBG transcription (10). Although speculative, we hypothesize that endogenous FFAs may potentially be an important link between IAAT and SHBG.
A recent study that included middle-aged European men and women reported that hepatic fat is independently related to SHBG after adjusting for IAAT and total fat (24). Hepatic fat and IAAT tend to be positively correlated with one another (25). We did not measure hepatic fat and cannot rule out the possibility that hepatic fat is associated with SHBG in our study. However, IAAT is likely a source of FFAs and hepatic fat accumulation (20) and our data suggest it is a pivotal fat depot associated with serum SHBG concentrations. Future studies examining the association between obesity and SHBG should consider IAAT, FFAs, and hepatic fat as potential factors inversely associated with SHBG.
The strengths of this study are the longitudinal design and the inclusion of premenopausal women with similar BMI in the overweight and weight-reduced states. In addition, we used computed tomography for the direct measurement of IAAT allowing us to investigate the associations of this fat depot with SHBG.
In conclusion, decreased concentrations of SHBG have been linked with increased risk for several chronic diseases. Weight loss increased SHBG in healthy premenopausal women and intra-abdominal adiposity was significantly inversely associated with SHBG. Although, the mechanisms underlying this association are unclear, it does not appear to be mediated by insulin.
ACKNOWLEDGMENTS
RO1DK49779, NCI Cancer Prevention & Control Training Program (R25 CA047888), UAB Nutrition and Obesity Research Center (P30 DK56336), UAB Diabetes Research and Training Center (P60 DK079626) and UL 1RR025777 for core lab support. The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab. 1983;57:304–310. doi: 10.1210/jcem-57-2-304. [DOI] [PubMed] [Google Scholar]
- 2.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 3.Potischman N, Hoover RN, Brinton LA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996;88:1127–1135. doi: 10.1093/jnci/88.16.1127. [DOI] [PubMed] [Google Scholar]
- 4.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. SWAN Investigators. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 5.Pugeat M, Nader N, Hogeveen K, et al. Sex hormone-binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol Cell Endocrinol. 2010;316:53–59. doi: 10.1016/j.mce.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab. 1993;77:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- 7.Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2008;66:506–516. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormonebinding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 9.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharideinduced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117:3979–3987. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selva DM, Hammond GL. Peroxisome-proliferator receptor gamma represses hepatic sex hormone-binding globulin expression. Endocrinology. 2009;150:2183–2189. doi: 10.1210/en.2008-1289. [DOI] [PubMed] [Google Scholar]
- 11.Selva DM, Hammond GL. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor- 4alpha. J Mol Endocrinol. 2009;43:19–27. doi: 10.1677/JME-09-0025. [DOI] [PubMed] [Google Scholar]
- 12.Nayeem F, Nagamani M, Anderson KE, et al. Dietary beta-tocopherol and linoleic acid, serum insulin, and waist circumference predict circulating sex hormone-binding globulin in premenopausal women. J Nutr. 2009;139:1135–1142. doi: 10.3945/jn.108.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio M, Lauretani F, Basaria S, et al. Sex hormone binding globulin levels across the adult lifespan in women–the role of body mass index and fasting insulin. J Endocrinol Invest. 2008;31:597–601. doi: 10.1007/BF03345608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquali R, Vicennati V, Bertazzo D, et al. Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-Menopause- Health Group. Metab Clin Exp. 1997;46:5–9. doi: 10.1016/s0026-0495(97)90159-1. [DOI] [PubMed] [Google Scholar]
- 15.Yeung EH, Zhang C, Hediger ML, Wactawski-Wende J, Schisterman EF. Racial differences in the association between sex hormone-binding globulin and adiposity in premenopausal women: the BioCycle study. Diabetes Care. 2010;33:2274–2276. doi: 10.2337/dc10-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenson MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchernof A, Toth MJ, Poehlman ET. Sex hormone-binding globulin levels in middle-aged premenopausal women. Associations with visceral obesity and metabolic profile. Diabetes Care. 1999;22:1875–1881. doi: 10.2337/diacare.22.11.1875. [DOI] [PubMed] [Google Scholar]
- 18.Hunter GR, Brock DW, Byrne NM, et al. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity (Silver Spring) 2010;18:690–695. doi: 10.1038/oby.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York: Oxford University Press; 2003. pp. 3–15. [Google Scholar]
- 20.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity (Silver Spring) 2006;14(Suppl 1):20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 21.Jänne M, Hammond GL. Hepatocyte nuclear factor-4 controls transcription from a TATA-less human sex hormone-binding globulin gene promoter. J Biol Chem. 1998;273:34105–34114. doi: 10.1074/jbc.273.51.34105. [DOI] [PubMed] [Google Scholar]
- 22.Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 23.Wisely GB, Miller AB, Davis RG, et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 2002;10:1225–1234. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 24.Peter A, Kantartzis K, Machann J, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]