Introduction
Placebos and placebo effects have held an ambivalent place in health care for at least two centuries. On the one hand, placebos are traditionally used as controls in clinical trials to correct for biases. Among other factors, these include regression to the mean, the natural course of the disorder, and effective co-interventions. In this context, the placebo effect is viewed as an effect to be factored out in order to isolate and accurately measure the specific effects of the treatment. On the other hand, there is mounting scientific evidence that placebo responses represent complex psychoneurobiological events involving the contribution of distinct central nervous system as well as peripheral physiological mechanisms that influence pain perception, clinical symptoms, and substantially modulate the response to active analgesics.
In this review, we bring together three perspectives of placebo research including psychological mechanisms, neurobiological pathways and molecular substrates of placebo analgesia and their contribution to active pain medications. The emphasis is particularly on recent studies illuminating mechanisms underlying individual differences in placebo responsiveness.
Psychological aspects of placebo analgesia
From a psychological point of view, a series of recent studies supported the nature of the placebo effect as a learning phenomenon wherein a human being learns to produce a benefit via verbally-induced expectations, cued and contextual conditioning or social learning [9; 11]. Placebo analgesic effects can be elicited by verbal instructions that anticipate a benefit, thus creating expectations of analgesia and recalling previously acquired experiences of pain relief. These verbally-induced expectations can be reinforced through manipulations in which a placebo treatment is paired with reduced pain intensities so that subjects come to experience analgesia and thereby enhance their expectations of future pain relief [28; 8; 11]. This procedure typically evokes much stronger and more stable placebo analgesic effects compared to verbally-induced effects [8; 16]. Interestingly, recent evidence suggests that these effects can be triggered by contextual cues that are not consciously perceived suggesting that placebo analgesic responses can operate outside of conscious awareness [15]. It is also noteworthy that conditioning can be induced by repetitive exposure to pharmacological treatments and produces drug-like effects when the active drug is replaced by a placebo. These effects, termed pharmacological conditioning, are quite robust in the field of pain and other conditions. Intriguingly, placebos given after preexposure to pharmacological treatments mirror the action of the pharmacological agent such as analgesics, for example, morphine and ketorolac [1], the immunosuppressant cyclosporin A [21], the dopamine-agonist apomorphine [5], the benzodiazepine receptor agonist midazolan and antagonist flumazenil [23], supporting the fact that placebos induce physiologically specific effects via learning processes.
Placebo analgesic effects can also occur without formal conditioning and direct prior experience because crucial information necessary to build up expectations of analgesia can be acquired through social learning. Colloca and Benedetti showed that substantial placebo analgesic responses were present after observing a benefit in another person undergoing an analgesic treatment [9]. Remarkably, the placebo analgesic effects following the observation of a benefit in another person were similar in magnitude to those induced by directly experiencing the benefit through a conditioning procedure and were positively correlated with the individual empathy traits of the observer. These observations emphasize that contextual cues and the entire atmosphere surrounding the participant or patient contribute to induce expectations of clinical benefit and recall memories of pain relief and thereby substantially modulate the individual placebo analgesic response.
Neurochemistry of placebo analgesia
The above-described mechanisms are associated with specific central nervous system and peripheral physiological responses. Beginning with experiments in the 1960s, evidence from indirect pharmacological approaches and molecular imaging studies with positron emission tomography (PET) indicated that placebo analgesia is mediated by the release of endogenous neuromodulators, including opioids, cholecystokinin, cannabinoids and dopamine. Levine, Gordon and Fields [18] first demonstrated that placebo analgesia can be antagonized by naloxone, suggesting the involvement of endogenous release of opioids. Since then, the contribution of opioidergic neurotransmission in placebo analgesia has been corroborated by behavioral and functional magnetic resonance imaging (fMRI) studies using the opioid antagonist naloxone and PET studies using in vivo receptor binding approaches with opioidergic ligands [1; 33; 32; 11]. The changes in opioidergic neurotransmission are associated with the modulation of the dopaminergic system suggesting that both endogenous opioids and dopamine contribute to the individual placebo analgesic response [26]. However, the distinct role of the dopaminergic system in placebo analgesia needs to be further investigated.
Recently, placebo analgesia has also been linked to the cannabinoid system [3]. This system seems to underlie placebo analgesia after pharmacological conditioning with the non-steroidal anti-inflammatory drug (NSAID) ketorolac In this case, placebo analgesic responses were reversed by the CB1 receptor antagonist rimonabant, indicating that the effects elicited by NSAID conditioning are partially mediated by the endogenous release of cannabinoids [3]. Importantly, placebo analgesia can be negatively modulated by the release of cholecystokinin as indicated by the antagonist action of proglumide [4].
Overall, these findings support the notion that the neurobiological effects of placebo analgesia are related to neuromodulators that are released in our brain under different contexts. Further research needs to clarify the interactions between the different systems involved in placebo analgesia under physiological and pathological conditions (e.g., acute and chronic pain). It is important to understand where in the brain these changes take place and what the mechanisms initiating and mediating these changes in neurochemistry are.
Neurophysiology of placebo analgesia
Functional neuroimaging studies indicate that placebo analgesia involves a top-down activation of endogenous analgesic activity via the descending pain modulatory system. Specifically, placebo analgesia has been shown to be associated with activity changes and enhanced functional coupling of the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate cortex (ACC) and distinct subcortical structures such as the hypothalamus, amygdalae and the periaqueductal grey (PAG) [31; 6; 11; 19]. Within this network of brain regions the DLPFC seems to be crucially involved in the initiation of the placebo analgesic response, whereas rACC to PAG connectivity has been shown to correlate with the reduction of pain-related responses in somatosensory pain areas and the behavioral changes in pain reports [31; 6; 11; 19].
Moreover, neuroimaging studies indicate that the reduced pain ratings during placebo analgesia are paralleled by decreased activity in the classical pain processing areas including the thalamus, insula and the somatosensory cortex [24; 31; 6; 25; 11; 20; 30]. Evidence from spinal cord fMRI further revealed that pain-related activity in the ipsilateral dorsal horn, corresponding to painful stimulation, is substantially reduced under placebo [12] and thereby provides evidence for spinal inhibition during placebo analgesia. Together these studies support the notion that altered pain experience during placebo analgesia, at least in part, results from active inhibition of nociceptive activity as depicted in Figure 1.
Figure 1.
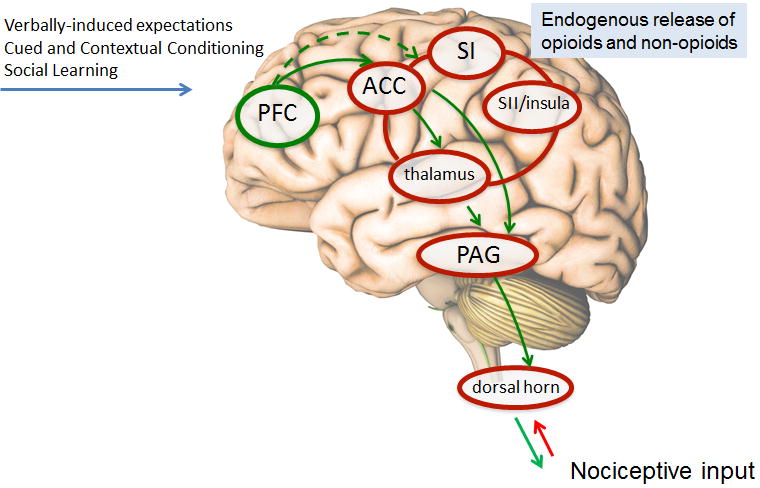
Psychological mechanisms such as verbally-induced expectations, cued and contextual conditioning and social learning trigger the cascade of endogenous opioids and non-opioids. The result is an alteration of the pain experience that at least in part, induces an active inhibition of nociceptive activity and modulation of brain areas predicting placebo analgesic responses.
PFC, prefrontal cortex; ACC, anterior cingulate cortex; SI, primary somatosensory cortex; SII secondary somatosensory cortex; PAG, periacqueductal gray.
However, other neuroimaging studies on placebo analgesia involving novel methodological approaches including multivariate pattern analyses and meta-analyses of brain imaging studies, support the relevance of changes in intracortical, emotion-related circuitry to induce and predict placebo analgesia [29]. Future studies have to unravel the distinct contribution and potential interaction of these mechanisms.
Relation between the pharmacodynamics of a drug and the placebo component
Placebo effects are inherent to every treatment and significantly contribute to clinical outcome even in the presence of strong analgesic treatments such as the opioid agonist morphine in post-operative patients or remifentanil in healthy subjects [10; 7]. This is best illustrated in the so-called open/hidden drug paradigm [2]. In this paradigm, identical concentrations of the same analgesic are administrated under two conditions: an open condition, in which the patient is aware of the time-point at which the medication is administrated by a health practitioner and a hidden condition in which the patient is unaware of the medication being delivered by a preprogrammed infusion machine. The comparison of both conditions allows for the dissociation of the genuine pharmacodynamic effect of the treatment (hidden treatment) and the additional analgesic benefit of the psychosocial context in which the treatment is provided. By using this paradigm, post-surgery patients who received their analgesic treatments in presence of a physician required a much lower dose of morphine to reduce their pain by 50% than those who received the medication from a preprogrammed infusion machine [10]. These studies provide compelling evidence that the simple awareness of a being treated considerably enhances the overall analgesic effect in both experimental and clinical settings. These findings have recently been corroborated by brain imaging approaches. Remifentanil administrated overtly as compared to an administration given covertly, enhanced substantially the analgesic changes in the neural activity of brain regions involved with the coding of pain intensity [7]. One of the crucial yet unanswered questions is whether placebo responses and pharmacologically-induced analgesia combine in an additive or interactive manner. Clinically speaking, pharmacologically-induced analgesia and the distinct endogenous cascades triggered by placebo mechanisms may combine in additive or interactive manner depending on the given analgesic treatment (i.e. opioidergic or non-opioidergic analgesics). Future studies involving different methodologies and designs should aim at unraveling how placebo responses and pharmacological analgesia combine or interact at a receptor/molecular level.
Interindividual differences and predictability of placebo analgesic responses
In both experimental and clinical conditions, the placebo response varies tremendously among individuals. The individual placebo analgesic response can range from no effect (‘non-responders’) to complete pain relief. Much effort is currently being dedicated to the identification of psychosocial and biological markers that moderate individual proneness to form placebo responses. Available evidence supports the putative relevance of several psychosocial variables for placebo responsiveness. Many studies have explored different psychological traits as potential predictors of placebo responses. These include, among others, trait and state anxiety, dispositional optimism, hypnotic suggestibility, coping abilities and the locus of control. Many of these studies however, included small sample sizes what might contribute to the often conflicting results.
Only recently the ability to trigger the cascade of endogenous opioids has been directly linked to psychological traits. Higher levels of endogenous opioids in a placebo paradigm have been observed in those subjects who scored high on personality traits such as agreeableness and resilience, qualities that allow people to have an optimistic view of human nature and cope with stress and adversity [22].
Another factor that may partially account for an individual’s placebo analgesic responsiveness is the variation in genetic variables. This has recently been documented in patients with irritable bowel syndrome (IBS) [13]. These chronic pain patients were assigned to one of three treatment arms: no-treatment (‘waitlist’), placebo treatment with a ‘limited’ patient-health practitioner interaction and placebo treatment with an ‘augmented’ supportive patient-health practitioner interaction. The primary outcome, change from baseline in IBS-Symptom Severity Scale after three weeks of treatment, was correlated with the number of methionine alleles in the COMT val158met polymorphism (rs4633). Patients who were met/met homozygotes showed the strongest placebo analgesic effect under the augmented placebo arm whilst patients who were val/val homozygotes were less responsive to warm and caring physicians and thus minimally benefiting from placebo responses [13].
Latest evidence supports the notion that also an individual’s brain anatomy including structural and functional measures predicts the capacity for placebo analgesic responses in healthy subjects. Stein et al. [27] indicated that white matter integrity in dorsolateral prefrontal cortex and rostral anterior cingulate cortex and their pathways to the periaqueductal grey are positively associated with individual placebo analgesic responses in healthy subjects. This finding supports the importance of structural brain connectivity in determining the individual ability to form placebo analgesic responses. Along these lines, resting state functional connectivity between prefrontal and insular/parietal cortices has also been shown to predict individual placebo analgesic responses in patients suffering from chronic low back pain [14], and expectancy-related modulation of pain in healthy volunteers [17].
Given that placebo analgesic responses fundamentally contribute to the overall analgesic outcome, a more detailed knowledge about the individual markers of placebo responsiveness may help to personalize therapeutic protocols and optimize patient-clinician interactions as well as patient stratification in clinical trials.
Concluding remarks
In summary, the available scientific evidence indicates that placebo analgesic responses are mediated by psychoneurobiological mechanisms and molecular targets and that these effects substantially contribute to the overall effectiveness of analgesic treatments. Recent advances in the field have paved the way for how neuropsychological, genetic and brain-related variables may predict individual differences in placebo responsiveness. Further insights into the mechanisms of placebo analgesia, its modulation of analgesic drug pharmacodynamics, and importantly, its predictability is urgently needed to guide future translational research and improve the methodology of clinical trials and clinical practice (see Part 2 of this review on clinical applications).
Acknowledgments
This research was funded by intramural NCCAM and NIMH (L.C.); grants by the Deutsche Forschungsgemeinschaft (FOR 1328/1), (Kl 1350/3-1) (R.K.), (Fl 156/33-1) (H.F.) and (BI 789/2-1) (U.B.); and grants by the Bundesministerium für Bildung und Forschung (01GQ0808) (U.B.).
Footnotes
Conflict of interests: The authors have not conflicts of interest to declare.
Disclosure: The opinions expressed by LC are those of the author and do not necessarily reflect the position or policy of the National Institutes of Health, the Public Health Service, or the Department of Health and Human Services.
References
- 1.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90(3):205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26(46):12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7(6):587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 6.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1–2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 8.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124(1–2):126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1–2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3(11):679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 11.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326(5951):404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 13.Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, Conboy LA, Kelley JM, Kokkotou E, Kaptchuk TJ. Catechol-O-Methyltransferase val158met Polymorphism Predicts Placebo Effect in Irritable Bowel Syndrome. PLoS One. 2012;7(10):e48135. doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. Brain networks predicting placebo analgesia in a clinical trialfor chronic back pain. Pain. 2012;153(12):2393–2402. doi: 10.1016/j.pain.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A. 2012;109(39):15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128(1–2):31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. Functional connectivity of frontoparietal network predicts cognitive modulation of pain. Pain. 2012 doi: 10.1016/j.pain.2012.12.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 19.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. Pain. 2010;151(3):816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Lyby PS, Aslaksen PM, Flaten MA. Variability in placebo analgesia and the role of fear of pain - an ERP study. Pain. 2011;152(10):2405–2412. doi: 10.1016/j.pain.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Ober K, Benson S, Vogelsang M, Bylica A, Gunther D, Witzke O, Kribben A, Engler H, Schedlowski M. Plasma noradrenaline and state anxiety levels predict placebo response in learned immunosuppression. Clin Pharmacol Ther. 2012;91(2):220–226. doi: 10.1038/clpt.2011.214. [DOI] [PubMed] [Google Scholar]
- 22.Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta JK. Personality Trait Predictors of Placebo Analgesia and Neurobiological Correlates. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127(1–2):63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 27.Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153(11):2210–2217. doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48(1):47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wager TD, Fields H. Placebo analgesia. Textbook of Pain. in press. [Google Scholar]
- 31.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 32.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


