Abstract
Sex differences are observed in both epidemiological and clinical aspects of major depressive disorder (MDD). The cortico-limbic-striatal neural system, including the prefrontal cortex, amygdala, hippocampus, and striatum, have shown sexually dimorphic morphological features and have been implicated in the dysfunctional regulation of mood and emotion in MDD. In this study, we utilized a whole-brain, voxel-based approach to examine sex differences in the regional distribution of gray matter (GM) morphological abnormalities in medication-naïve participants with MDD. Participants included 29 medication-naïve individuals with MDD (16 females and 13 males) and 33 healthy controls (HC) (17 females and 16 males). Gray matter morphology of the cortico-limbic-striatal neural system was examined using voxel-based morphometry analyses of high-resolution structural magnetic resonance imaging scans. The main effect of diagnosis and interaction effect of diagnosis by sex on GM morphology were statistically significant (p<0.05, corrected) in the left ventral prefrontal cortex, right amygdala, right hippocampus and bilateral caudate when comparing the MDD and HC groups. Posthoc analyses showed that females with MDD had significant GM decreases in limbic regions (p<0.05, corrected), compared to female HC; while males with MDD demonstrated significant GM reduction in striatal regions, (p<0.05, corrected), compared to HC males. The observed sex-related patterns of abnormalities within the cortico-limbic-strial neural system, such as predominant prefrontal-limbic abnormalities in MDD females vs. predominant prefrontal-striatal abnormalities in MDD males, suggest differences in neural circuitry that may mediate sex differences in the clinical presentation of MDD and potential targets for sex-differentiated treatment of the disorder.
Keywords: Major depressive disorder, Magnetic resonance imaging, Voxel-based morphometry, Caudate, Amygdala, Hippocampus
Introduction
Sex differences are observed in both epidemiological and clinical aspects of major depressive disorder (MDD). Studies have consistently shown that MDD is more prevalent in females than males (Kessler et al., 2005; Kessler et al., 1994; Kuehner, 2003). Females with MDD are more likely to show increased anxiety including higher rates of comorbid anxiety disorders (Kornstein et al., 2000; Marcus et al., 2005), while males with MDD are more likely to show more psychomotor agitation and to have comorbid substance abuse disorders (Kessler et al., 1997; Marcus et al., 2005; Roeloffs et al., 2001). Although females with MDD are more likely to attempt suicide than males (Marcus et al., 2005), males with MDD are more likely to be successful when they attempt suicide, and thus are at higher risk for completed suicide (Blair-West et al., 1999; Oquendo et al., 2001). While sex differences in the clinical presentation of MDD are apparent, the neural mechanisms that underlie these differences remain unclear.
The cortico-limbic-striatal neural system including the prefrontal cortex (PFC), amygdala, hippocampus and striatum, is implicated in the dysfunctional regulation of emotion in MDD (Drevets, 1998; Marchand, 2010). The sexually dimorphic development of cortico-limbic-striatal neural system has been implicated to contribute to sex differences in psychiatric disorders (Giedd et al., 1997; Giedd et al., 1996; Lenroot et al., 2007; Neufang et al., 2009; Teicher et al., 2004). For example, regions (PFC, amygdala and hippocampus) subserving emotions might be related with increased depression or anxiety risk in females (Davis et al., 2012; Kessler et al., 1993; Lieberwirth et al., 2012), and regions (PFC and striatum) subserving impulse control might be associated with increased risk for substance abuse (Nagoshi et al., 1991; Nolen-Hoeksema et al., 2006). Additionally, preclinical studies indicate particular vulnerability to stress in the PFC-amygdala/hippocampus system in females, with estrogen-dependent effects observed, while this system appears relatively resilient in males. However, males may be more vulnerable to stress effects on the PFC-striatum system than females;for example, estrogen has been shown to be protective in caudate (Arnsten et al., 2004; Dluzen et al., 2000; McEwen, 2010; Shansky et al., 2010). The sexually dimorphic features in cortico-limbic-striatal neural system may reflect the sex differences in clinical observations of MDD, such as MDD females with more comorbid anxiety disorders, and MDD males with more comorbid substance abuse and higher risk for completed suicide.
Sex differences in cortico-limbic-striatal morphology have been reported in human brain studies (Biswal et al., 2010; Cosgrove et al., 2007; Goldstein et al., 2001). Recently, our group found sexually dimorphic effects of child maltreatment (CM) on cortico-limbic-striatal morphology in adolescents. In adolescent females, CM was associated with more robust effects in brain regions that subserve emotional regulation, including the PFC, amygdala and hippocampus, whereas in adolescent males, effects were more prominent in brain regions that subserve impulse control, including PFC and striatum (Edmiston et al., 2011). Several morphological studies using region of interest (ROI) tracing technique examined sex differences in the cortico-limbic-striatal neural system in MDD; however there are some inconsistencies in findings (Frodl et al., 2002; Hastings et al., 2004; Vakili et al., 2000). In this study, we utilized a whole-brain, voxel-based approach to examine sex differences in gray matter (GM) density and volume within the cortico-limbi-striatal neural system in medication-naïve participants with MDD. Given the sex differences in clinical features of MDD, we anticipated that GM morphological alterations in cortico-limbic-striatal brain regions subserving emotional regulation might be more prominent in MDD females, while GM morphological changes in MDD males might be more apparent in region subserving impulse control.
Methods
The MDD group was comprised of 29 participants [mean age 29.5±SD 6.84 years, 16 females (55%), 13 males (45%)] who met DSM-IV criteria for MDD, were currently depressed as determined by the consensus of two psychiatrists using the Structured Clinical Interview for DSM-IV (First et al., 1995), had a score of at least 24 on the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960), and had never taken any psychotropic medications. No MDD participants had current comorbid Axis I diagnosis. The healthy control (HC) group included 33 participants with neither personal Axis I disorder or first-degree relatives with psychiatric disorder [mean age 29.9±SD 8.27 years, 17 females (51%), 16 males (49%)]. No participant had a history of neurological illness, head trauma with loss of consciousness of 5 minutes or more, or major medical disorder. After a complete description of the study, written informed consent was obtained from all participants in accordance with the human investigation committees of China Medical University.
High-resolution structural magnetic resonance imaging (MRI) scans were obtained on a 3T MR scanner (General Electric, Milwaukee, USA) using a three-dimensional Fast Spoiled Gradient-Echo (FSPGR) T1-weighted sequence (TR=7.1ms, TE=3.2ms, FOV=240×240mm2, matrix=240×240, slice thickness=1.0 mm without gap). Images were processed according our previous protocol (Blumberg et al., 2008; Kalmar et al., 2009; Tang et al., 2007; Wang et al., 2011). In brief, segmentation function in the Statistical and Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm) was used for bias correction, segmentation and spatial normalization. Segmented unmodulated GM images (representing gray matter density, GMD) and modulated GM images (representing gray matter volume, GMV) were normalized to Montreal Neurological Institute (MNI) space using the SPM5 GM tissue probability map (voxel size 2×2×2 mm3) as a template and spatially smoothed using an 8-mm full width at half maximum Gaussian kernel.
Two-way analysis of variance (ANOVA) with diagnosis (MDD/HC) and sex (M/F) as between subject factors was used to compare demographic data (age and education) and HDRS with SPSS 13.0 software (SPSS Inc, Chicago, Illinois). Two-sample t-test was used to compare illness duration between males and females with MDD. Full factorial ANOVA (two-way ANOVA) was performed in SPM5 with group (MDD/HC) and sex (male/female) as between-subject factors to investigate the morphological differences between HC and MDD groups. Significant diagnostic group by sex interactions were interpreted using graphical displays and by performing post-hoc two-sample t-tests separately for males and females or HC and MDD groups. To test region-based hypotheses regarding group differences, we performed region of interest (ROI) analyses. ROIs included the bilateral amygdala, hippocampus, striatum, and PFC, including Brodmann areas (BA) 9–12, 24, 25, 32, and 44–47, defined by WFU PickAtlas Utility (http://www.fmri.wfubmc.edu/cms/software#WFU_PickAtlas). Consistent with our previous study (Blumberg et al., 2008; Tang et al., 2007; Wang et al., 2009), findings were considered significant at p<0.005 (uncorrected) for the hypothesized regions. To minimize false discovery, cluster-level correction was applied to the hypothesized regions using AlphaSim (http://afni.nimh.nih.gov/) correction. The program determined a minimum cluster size in each ROI with Monte Carlo simulation to achieve a corrected significance of p < 0.05 with a voxelwise threshold of p < .0005 (see program AlphaSim by B.D. Ward in AFNI software). Additionally, potential associations between the morphological measurements (GMD or GMV) and HDRS, as well as duration of illness were performed separately in MDD females and MDD males and were corrected in AlphaSim.
Results
There was no significant effect of diagnosis, sex or interaction of diagnosis and sex in age and education. The effect of diagnosis in HDRS was significant, with significant higher HDRS scores in the MDD group, compared to the HC group. There was no significant effect of sex in HDRS. Two-sample t-tests showed no difference in the illness duration between MDD female and MDD male subgroups (Table 1).
Table 1.
Demographic and Clinical Data of Subjects
| Healthy Controls | MDD Participants | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Number | 16 | 17 | 13 | 16 |
| Age (years, mean±S.D.) [range] | 31.81±7.05 [18–45] | 28.00±9.09 [18–44] | 31.23±8.39 [21–45] | 28.88±9.71 [18–45] |
| Education (years, mean±S.D.) [range] | 14.50±3.06 [9–19] | 13.53±2.83 [9–17] | 13.08±2.63 [9–17] | 12.01±3.16 [6–16] |
| HDRS (mean±S.D.) [range] | 0.5±1.03 [0–3] | 1.12±1.87 [0–4] | 27.69±5.12 [24–37] | 29.56±5.11 [24–38] |
| Duration of illness (Month, mean±S.D.) [range] | N/A | N/A | 12.1±13.07 [2–48] | 13.91±17.65 [0.5–48] |
S.D.: standard deviation
MDD: major depressive disorder
HDRS: Hamilton Depression Rating Scale
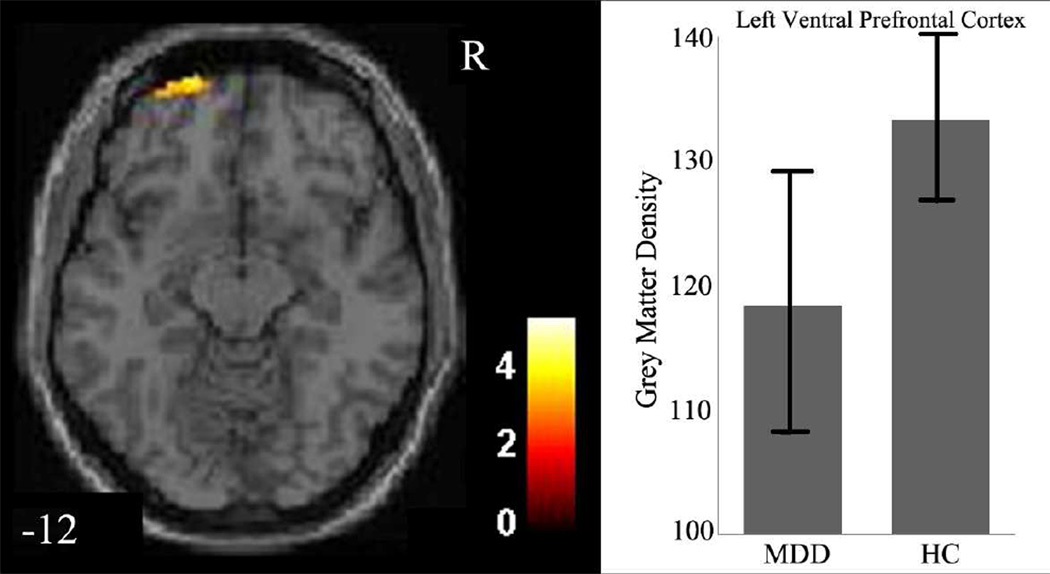
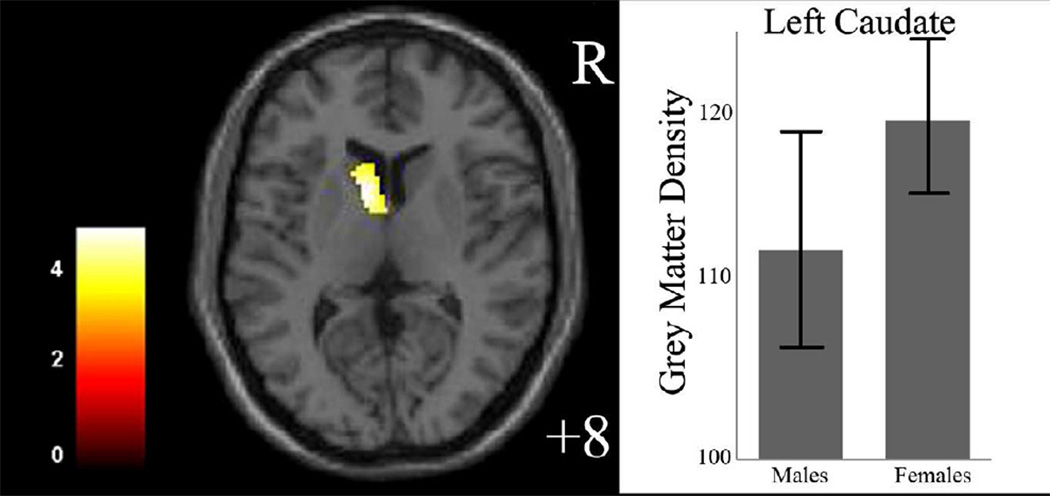
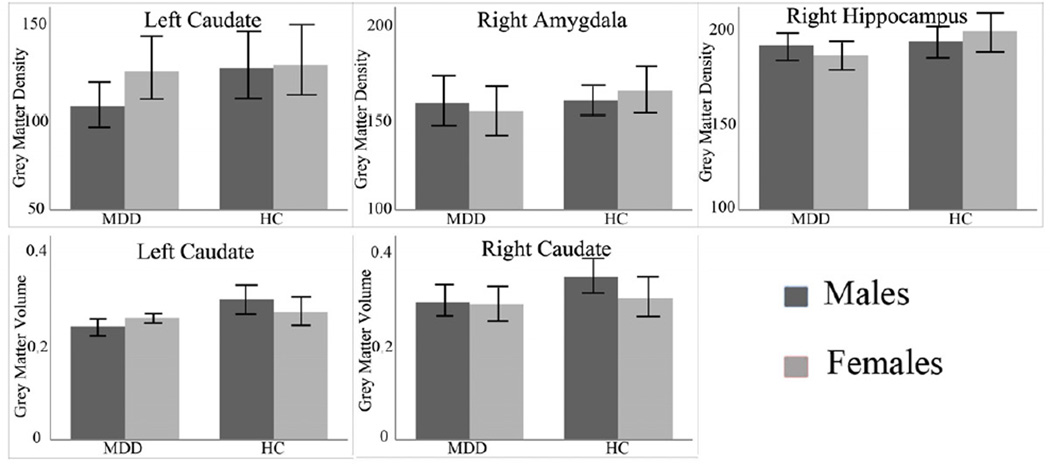
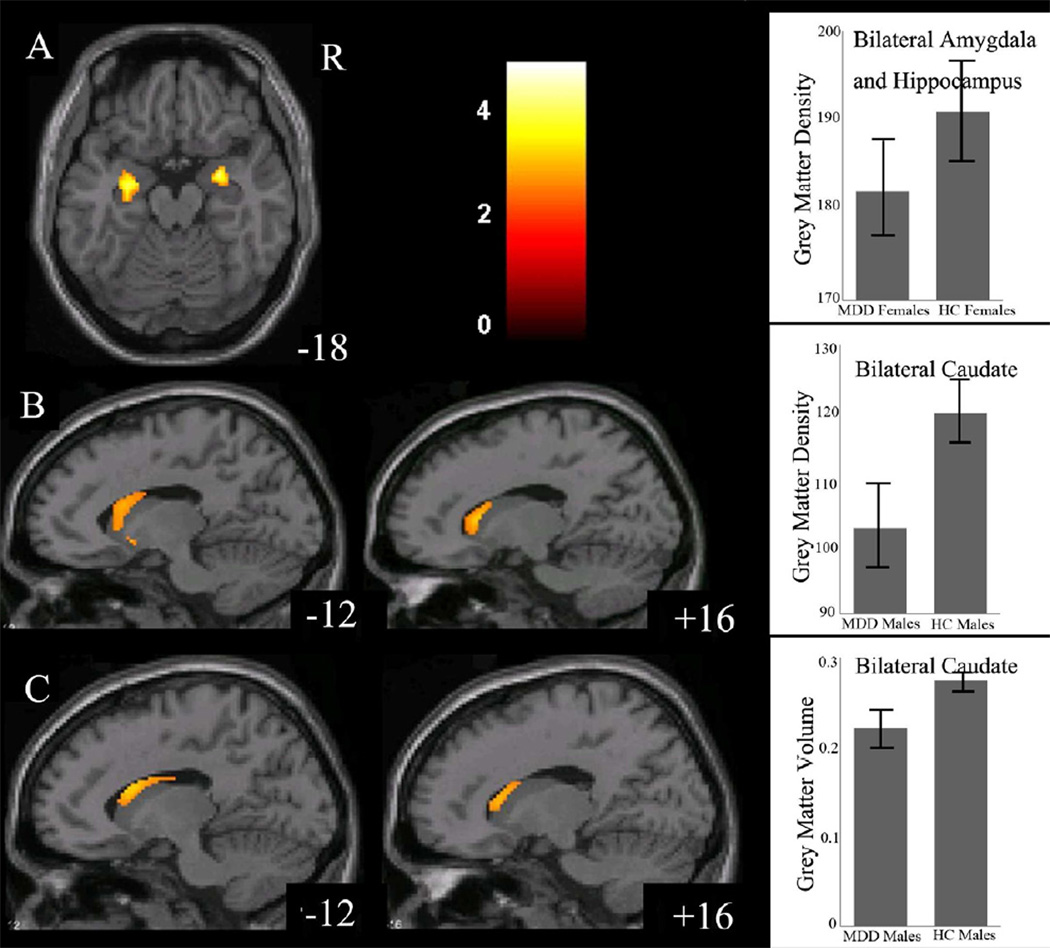
The effect of diagnosis on GMD was significant in the left ventral prefrontal cortex (VPFC; BA 11/10, cluster size=107 voxels, maximal point MNI coordinate: x=−20mm, y=66mm, z=−12mm, T = 3.75, p<0.05, corrected) (Figure 1), with significantly reduced GMD in the VPFC in the MDD group, compared to the HC group. The effect of sex on GMD was significant in the left caudate (cluster size=131 voxels, maximal point MNI coordinate: x=−8mm, y=8mm, z=8mm, T = 4.94, p<0.05, corrected), with significantly reduced GMD in the caudate in the male participants, compared to the female participants (Figure 2). Diagnosis by sex effects on GMD was significant in the right amygdala, right hippocampus, and left caudate (Table 2, Figure 3). Post-hoc two-sample t-tests indicated MDD females had significantly reduced GMD in the bilateral amygdala and hippocampus (left side: cluster size=291 voxels, maximal point MNI coordinate: x=−26mm, y=−14mm, z=−22mm, T = 4.31; right side: cluster size=204 voxels, maximal point MNI coordinate: x=28mm, y=0mm, z=−16mm, T = 4.46, p<0.05, corrected) compared to HC females (Figure 4 A). Moreover, the affected voxel clusters in the post-hoc analyses contained regions that showed significant diagnosis by sex effects in the primary analyses, including right amygdala and right hippocampus. Post-hoc analyses also showed that MDD males had significantly reduced GMD in the bilateral caudate extending to the left ventral striatum (left side: cluster size=288 voxels, maximal point MNI coordinate: x=−8mm, y=10mm, z=−6mm, T = 3.56; right side: cluster size=93 voxels, maximal point MNI coordinate: x=16mm, y=20mm, z=6mm, T = 3.29, p<0.05, corrected) compared to the HC males (Figure 4 B). The affected voxel clusters in post-hoc analyses included regions with significant diagnosis by sex effects in primary analyses, including left caudate. Based on the above analyses, it appeared that the contribution to diagnosis by sex effects on GMD were driven by reduced GMD in the amygdala and hippocampus in female MDD and reduced GMD in the caudate in male MDD. Additional correlation analyses did not detect any relationship between GMD and clinical variables in MDD female or male groups.
Figure 1.
Regions of decreased in gray matter density in medication-naïve participants with major depressive disorder compared to healthy controls
The axial images (z= −12mm Montreal Neurological Institute coordinate plane) show the regions of significantly decreased gray matter density in left ventral prefrontal cortex in all medication-naïve participants with major depressive disorder (MDD), compared to healthy controls (HC) (p<0.005, uncorrected). The color bar represents the range of F values. R=right
The graph shows left ventral prefrontal cortex gray matter density and standard deviation for the MDD group and the HC group.
Figure 2.
Regions of differences in gray matter density between male and female participants The axial image (z= 8mm Montreal Neurological Institute coordinate plane) shows the regions of significantly decreased gray matter density in left caudate in male participants, compared to female participants (p<0.005, uncorrected).
The color bar represents the range of T values. R=right
The graph shows left caudate gray matter density and standard deviation for male and female participants.
Table 2.
Areas of diagnosis by sex effects on gray matter density/volume in medicated naïve participants with major depressive disorder compared to health control participants
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Areas | Cluster Size | x | y | z | F values |
| Gray Matter Density | |||||
| Left Caudate | 128 | −18 | 10 | 22 | 17.46 |
| Right Amygdala | 36 | 28 | 0 | −16 | 16.21 |
| Right Hippocampus | 25 | 30 | −22 | −14 | 15.99 |
| Gray Matter Volume | |||||
| Left Caudate | 81 | −16 | 14 | 20 | 14.73 |
| Right Caudate | 36 | 16 | 14 | 18 | 9.57 |
Figure 3.
Gray matter density/volume and standard deviation of male or female medication naïve participants with major depressive disorder and health control participants in the regions showing significant interaction of diagnosis by sex effects from two-way analysis of variance.
Figure 4.
Regions of differences from posthoc two-sample t-tests for gray matter density and volume between medication naïve males and females with major depressive disorder and healthy control participants
A: The axial images (z= −18mm Montreal Neurological Institute coordinate plane) show the regions of significantly decreased gray matter density in bilateral amygdala and hippocampus in medication-naïve females with major depressive disorder (MDD), compared to healthy control (HC) females (p<0.005, uncorrected).
The graph shows bilateral amygdala and hippocampus gray matter density and standard deviation for MDD and HC females.
B: The sagittal images (z= −12mm and 16mm Montreal Neurological Institute coordinate planes) show the regions of significantly decreased gray matter density in bilateral caudate extending to left ventral striatum in medication-naïve males with major depressive disorder (MDD), compared to healthy control (HC) males (p<0.005, uncorrected).
The graph shows bilateral caudate gray matter density and standard deviation for MDD and HC males.
C. The sagittal images (z= −12mm and 16mm Montreal Neurological Institute coordinate planes) show the regions of significantly decreased gray matter volume in bilateral orbitofrontal cortex, bilateral caudate in medication-naïve males with major depressive disorder (MDD), compared to healthy control (HC) males (p<0.005, uncorrected).
The color bar represents the range of T values. R=right
The graph shows bilateral caudate gray matter volume and standard deviation for MDD and HC males.
There were no significant effect of diagnosis or sex on GMV; however the effect of diagnosis by sex effect was significant in the bilateral caudate (Table 2, Figure 3). Post-hoc two-sample t-tests showed that MDD males had significant volume reduction in the bilateral caudate when compared to HC males (left side: cluster size=149 voxels, maximal point MNI coordinate: x=−14mm, y=12mm, z=20mm, T = 3.40; right side: cluster size=89 voxels, maximal point MNI coordinate: x=16mm, y=22mm, z=8mm, T = 3.08, p<0.05, corrected) (Figure 4 C). The affected clusters in post-hoc analyses included regions that had significant diagnosis by sex effects in the primary analyses, including bilateral caudate. Given this, it appeared that that reduced caudate GMV in MDD males contributed to the diagnosis by sex effect on GMV in this region. Additional correlation analyses did not detect any relationship between GMV and clinical variables in MDD female or male groups.
Discussion
In this study, we found that VPFC morphological abnormalities in MDD females and males with significant sex differences in abnormalities of the limbic and striatal regions. We observed sex-specific patterns of morphological abnormalities within the cortico-limbic-striatal neural system. Prefrontal-limbic abnormalities were predominantly observed in MDD females; while prefrontal-striatal abnormalities were primarily found in MDD males. Morphology in a cortico-limbic-striatal neural system has shown sexually dimorphic patterns (Filipek et al., 1997; Giedd et al., 1997; Lenroot et al., 2007; McCormick et al., 2007; Neufang et al., 2009). Evidence further suggests that sex is a key mediator of the effects of genes and/or environment on regional vulnerabilities to MDD (Qureshi et al., 2010; Shansky et al., 2004; Walf et al., 2006). The interaction among hormonal, genetic, and environmental factors might result in sex-specific patterns of morphological abnormalities within the cortico-limbic-striatal neural system in MDD observed in this study (McCarthy et al., 2009; Qureshi et al., 2010).
Our findings may reflect the sex differences in clinical features of MDD; females with MDD are prone to anxiety while males with MDD tend toward impulsiveness. Dysfunctional prefrontal-limbic neural circuitry is involved in development of anxiety (Davidson, 2002; Etkin, 2010). Specifically, the prefrontal cortex may participate in controlling excessive anxiety by moderating the activation of limbic structures such as the amygdala and hippocampus. Both the amygdala and hippocampus are critical structures in anxiety and may play important roles in mediating the effects of estrogen on behaviors related to anxiety (Walf et al., 2006). The prefrontal-striatal neural circuitry underlies behavioral disinhibition (Ferry et al., 2000; Petrides et al., 2007; Sinha, 2008). Behavioral disinhibition has been associated with increased suicidal behavior, psychomotor agitation, and impulse dyscontrol, as well as increased comorbid substance abuse disorders in males with MDD (Blair-West et al., 1999; Kessler et al., 1997; Marcus et al., 2005; Oquendo et al., 2001; Roeloffs et al., 2001). Interestingly, estrogen appears to have a neuroprotective effect on the striatum, implicating decreased vulnerability in this region in females and increased vulnerability in males (Dluzen, 2000). Based on these previous findings, the prominent prefrontal-limbic abnormalities in female MDD participants found herein may account for increased anxiety and comorbid anxiety disorders, as well as more atypical features, observed in females with MDD; while the predominant prefrontal-striatal abnormalities in male MDD participants may account for increased suicidal behaviors and comorbid substance abuse disorders seen in males with MDD. These conclusions are tentative, and further investigation of sex-specific association between abnormalities in the cortico-limbic-striatal system and aforementioned clinical features is needed.
There have been several ROI tracing studies examining sex differences in the cortico-limbic-striatal neural system in MDD. Hastings et al found that depressed males who were without medication at the time of scan had smaller anterior cingulate cortex volumes, compared to healthy control males; while depressed females had smaller amygdala compared with healthy control females. No significant volumetric differences were noted in the hippocampus or orbitofrontal cortex (Hastings et al., 2004). Frodl et al. found first episode MDD males had smaller hippocampal volume than healthy control males; while no significant differences in hippocampal volume were found between first episode MDD and healthy control females (Frodl et al., 2002). Vakili et al did not find significant differences in hippocampal volume between medicated patients with MDD and healthy controls; however, they observed a significant correlation between left hippocampal volume and HDRS baseline measures in males, as well as significant increases in mean right hippocampal volume in female fluoxetine responders compared to nonresponders (Vakili et al., 2000). Their findings among MDD females highlight the potential confounding effects of psychotropic medications.
The sex-specific patterns of neural abnormalities found in this study were not likely the result of differences in medication exposure between males and females with MDD, as the MDD participants were naïve to psychotropic medications. This raises interesting questions regarding the influence of pharmacotherapy on these abnormalities and the associated neural circuitries, as well as whether psychotropic medications differentially affect brain regions in males and females. Particularly, antidepressant medications have been shown to have neurotrophic and neuroprotective effects that may significantly influence brain morphology (Duman et al., 2006; Moore et al., 2000; Savitz et al., 2010). Medication-related differences in regional brain volumes have been observed in patients with mood disorders (Blumberg et al., 2006; Brambilla et al., 2002; Chang et al., 2005). Studies of medication-naïve patients could better elucidate brain abnormalities that are more directly related to MDD. Moreover, as males and females may have differential responses to psychotropic medications and these medications likely influence the neural circuitry of MDD, the study of a medication-naïve sample to discern sex-specific neural circuitry features of MDD is important. Our group has previously examined morphological abnormalities in medication-naïve females and found smaller amygdala and ventral anterior cingulate cortex; males were not included in that study (Tang et al., 2007). Future studies of the influence of psychotropic medications on the cortico-limbic-striatal system and possible sex differences in response to these medications may elucidate the mechanisms underlying the amelioration of MDD with certain psychotropic medications and aid in tailoring treatment strategies to individual characteristics.
There were some limitations to this study. First, our sample sizes were relatively small, especially for sex difference comparisons. This may limit the generalizability of our results as well as our ability to detect relationships between clinical variables and neuroimaging findings in this study. Additionally, the cross-sectional design of this study did not allow us to observe neural changes in MDD with respect to illness progression and treatment response. Future studies using larger sample sizes and longitudinal design are needed. Furthermore, as our study examined participants during acute phases of MDD, future studies examining individuals at high risk for MDD, such as first degree relatives of MDD, are also needed for further understanding of sex differences in the development of MDD.
In summary, this study demonstrated sex differences in morphological abnormalities of the cortico-limbic-striatal system in MDD, in which MDD females had abnormalities predominant in the prefrontal-limbic circuitry while MDD males had abnormalities primarily in the prefrontal-striatal circuitry. These distinct sex-related patterns of neural deficits strongly suggest different mechanisms in the development and pathophysiology of MDD for males and females. They may contribute to sex differences in the clinical presentation of MDD. Furthermore, they may have important implications for developing sex-specific and more effectively-targeted treatment of MDD.
Acknowledgments
We thank Gang Zhu, Zhe Wang, Xuesheng Fan, Huan Ma, Yongjin Huang and Feng Wu for helping to recruit participants with major depressive disorder, and Ling Ren, Ting Liu and Jian Li for the assistance in MRI scanning.
Role of Funding source:
This work was supported by the National Institute of Health (NIH) [K01MH086621 to F.W.]; the National Alliance for Research on Schizophrenia and Depression (Great Neck, NY) [F.W.]; the Klingenstein Foundation [F.W.]; the National Natural Science Foundation of China (81071099 and 81271499 to Y.T.) and the Liaoning Science and Technology Foundation (2008225010-14 to Y.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Drs. Tang, Xu and Wang designed the study. Drs. Kong, Chen and Jiang acquired the data. Drs. Chen, Luo, Driesen, and Liu analyzed the data. Drs. Womer, Blumberg and Wang wrote the article. All authors contributed to and have approved the final manuscript.
References
- Arnsten AF, Shansky RM. Adolescence: vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Annals of the New York Academy of Sciences. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML. Lifetime suicide risk in major depression: sex and age determinants. Journal of Affective Disorders. 1999;55:171–178. doi: 10.1016/s0165-0327(99)00004-x. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, Martin L, Gerard E, Charney DS, Peterson BS. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biological Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Wang F, Chepenik LG, Kalmar JH, Edmiston E, Duman RS, Gelernter J. Influence of vascular endothelial growth factor variation on human hippocampus morphology. Biological Psychiatry. 2008;64:901–903. doi: 10.1016/j.biopsych.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Haley T, Duvoisin RM, Raber J. Measures of anxiety, sensorimotor function, and memory in male and female mGluR4(−)/(−) mice. Behavioural Brain Research. 2012;229:21–28. doi: 10.1016/j.bbr.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. Journal of Neurocytology. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Neuroprotective role of estrogen upon methamphetamine and related neurotoxins within the nigrostriatal dopaminergic system. Annals of the New York Academy of Sciences. 2000;914:112–126. doi: 10.1111/j.1749-6632.2000.tb05189.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics and Adolescent Medicine. 2011;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. Journal of Comparative Neurology. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. American Journal of Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Ryan CE, Hess AL, Harrison W, Davis SM, Keller MB. Gender differences in chronic major and double depression. Journal of Affective Disorders. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatrica Scandinavica. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Hormones and Behavior. 2012 doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct. 2010;215:73–96. doi: 10.1007/s00429-010-0280-y. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. Journal of Neuroscience. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry and Behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, neural adaptation to a changing environment: mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR, Rodriguez LA. Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcoholism, Clinical and Experimental Research. 1991;15:661–667. doi: 10.1111/j.1530-0277.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. Journal of General Psychology. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Ellis SP, Greenwald S, Malone KM, Weissman MM, Mann JJ. Ethnic and sex differences in suicide rates relative to major depression in the United States. American Journal of Psychiatry. 2001;158:1652–1658. doi: 10.1176/appi.ajp.158.10.1652. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Progress in Brain Research. 2010;186:77–95. doi: 10.1016/B978-0-444-53630-3.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeloffs CA, Fink A, Unutzer J, Tang L, Wells KB. Problematic substance use, depressive symptoms, and gender in primary care. Psychiatric Services. 2001;52:1251–1253. doi: 10.1176/appi.ps.52.9.1251. [DOI] [PubMed] [Google Scholar]
- Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, Bain EE, Price JL, Zarate C, Manji HK, Cannon DM, Marrett S, Charney DS, Drevets WC. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: the impact of medication. Neuroimage. 2010;49:2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Molecular Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebral Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Research. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biological Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, Spencer L, Blumberg HP. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134:2005–2012. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]