Abstract
Pannexin1 (Panx1), a protein related to the gap junction proteins of invertebrates, forms nonjunctional channels that open upon depolarization and in response to mechanical stretch and purinergic receptor stimulation. Importantly, ATP can be released through Panx1 channels, providing a possible role for these channels in non-vesicular signal transmission. In this study we expressed exogenous human and mouse Panx1 in the gap junction deficient Neuro2A neuroblastoma cell line and explored the contribution of Panx1 channels to cell–cell communication as sites of ATP release. Electrophysiological (patch clamp) recordings from Panx1 transfected Neuro2A cells revealed membrane conductance that increased beyond 0 mV when applying voltage ramps from −60 to +100 mV; threshold was correlated with extracellular K+, so that at 10 mM K+, channels began to open at −30 mV. Evaluation of cell–cell communication using dual whole cell recordings from cell pairs revealed that activation of Panx1 current in one cell of the pair induced an inward current in the second cell after a latency of 10–20 s. This paracrine response was amplified by an ATPase inhibitor (ARL67156, 100 µM) and was blocked by the ATP-degrading enzyme apyrase (6.7 U/ml), by the P2 receptor antagonist suramin (50 µM) and by the Panx1 channel blocker carbenoxolone. These results provide additional evidence that ATP release through Panx1 channels can mediate nonsynaptic bidirectional intercellular communication. Furthermore, current potentiation by elevated K+ provides a mechanism for enhancement of ATP release under pathological conditions.
Keywords: Purinergic receptor, Panx1, Hemichannel, Neuroblastoma, Intercellular signaling, Paracrine
Introduction
Studies on a wide range of non-excitable cell types and under a wide range of conditions has made it clear that two distinct processes contribute to long range cell signaling: direct intercellular diffusion of second messenger molecules through gap junction channels (most likely IP3, although Ca2+ and other molecules may also contribute) and a nonjunctional pathway in which ATP and other molecules are released from a stimulated cell to act on membrane receptors of adjacent cells [1]. With respect to ATP, its release can elevate Ca2+ in adjacent cells through activation of both metabotropic and ionotropic purinergic P2 receptors.
Mechanisms that have been postulated for ATP release from non-excitable cells include exocytosis, transporters and channels that are permeable to such large anions. Candidate channels include volume sensitive outwardly rectifying anion channels (VSOR), the plasmalemmal form of the mitochondrial voltage dependent anion channel (plVDAC), gap junction hemichannels (or connexons) in particular those formed of Cx43, and most recently discovered, channels (pannexons) formed of Pannexin1 (Panx1) [2, 3]. Pannexons are mechano- and voltage-sensitive channels that can also be activated following P2 receptor stimulation and by elevated extracellular K+; their activation by these stimuli and their large pore diameter ideally suit them for ATP release [4–7], for which recent evidence has been provided [7–11].
Several studies have begun to characterize the electrophysiological and pharmacological properties of pannexons both in endogenous systems such as the J774 macrophage cell line [5, 12] and primary cultures of astrocytes [13], and when expressed exogenously in Xenopus oocytes [6, 7, 14] and in HEK193 cells [15]. We now report results from studies using the Neuro2A neuroblastoma cell line, whose virtual lack of endogenous expression of connexin gap junction proteins has made it the cell line of choice for characterization of biophysical properties of cloned connexins [16]. Neuro2A cells exhibit very low expression of Panx1 [9], allowing evaluation of physiological consequences of exogenous expression [17]. Dual whole cell recordings from Panx1 transfected cell pairs never revealed the presence of high conductance gap junction channels as would be expected if they were formed by this protein. However, these recordings evidenced the contribution of Panx1 channels to non-synaptic paracrine signaling; activation of Panx1 channel opening led to exonucleotidase-sensitive inward currents in an adjacent cell, implicating release of ATP through Panx1 channels from the stimulated cell. Thus, depolarization induced ATP release through Panx1 channels can mediate signaling through activation of ionotropic and metabotropic P2 receptors in this neuroblastoma cell line.
Materials and Methods
Cell Culture
The mouse neuroblastoma cell line Neuro2A originally obtained from American Tissue Type Collection (Rockville, MD, USA) as CCL 131 was used in this study. Cells were cultured in DMEM, supplemented with 10% fetal calf serum (Gibco) and 1% Penicillin/Streptomycin and maintained in a humidified chamber with 5% CO2 at 37°C. Cells were generally passaged twice weekly and not used beyond twentieth passage.
Electrophysiology
Cells were plated on coverslips for 12–24 h at low confluence prior to recordings. The single or dual whole cell patch clamp configurations [16] were performed at room temperature on cells bathed in external solution containing (mM): NaCl 147, Hepes 10, glucose 13, CaCl2 2, MgCl2 1 and KCl 2, pH 7.4. Patch pipettes (resistance 4–6 MOhms) were filled with solution containing (mM): CsCl 130, EGTA 10, Hepes 10, CaCl2 0.5, and connected to an Axopatch 1D amplifier (Molecular Devices). In some experiments, external K+ was varied from 0.5 to 10 mM; osmolarity was adjusted by reducing NaCl. Membrane potential was generally held at −60 mV. Voltage activation of Panx1 channels was achieved using voltage ramps from −60 to +100 mV or −60 to +60 mV (16 mV/sec) at 20 s intervals. Data were acquired with Clampex 6.0 or 8.2 software, digitized using an Axon Instruments Digitizer and analyzed with Clampfit 9.0 software (Molecular Devices).
Pharmacological Agents
Drugs used (from Sigma) included tetanus toxin (3 nM, #T3194) to inhibit vesicular release, the gap junction inhibitor carbenoxolone (50 µM, Sigma), the ectoATPase inhibitor ARL67156 (100 µM), the ectoATPase apyrase (stock of 200U/ml dH2O diluted 1:60 in external solution for final concentration of 6.7 U/ml), ATP and BzATP (50 µM prepared in dH2O), the non-specific P2R antagonist suramin (50 µM,) and KN62 (1 µM), antagonist of the murine P2X7R.
Transfection with Panx1 cDNA
Neuro2A cells were transfected using 6 µl oligofectamine reagent (Invitrogen) in 1.5 ml Optimen (Gibco) with 2 µg of untagged human (h) Panx1 in pCDNA3.1 vector or fluorescent protein (FP) tagged mouse (m) Panx1 in pEYFP or GFP-N1 vectors. The hPanx1 construct was originally obtained from Dr. Gerhard Dahl (U Miami School of Medicine) in a cloning vector; the mPanx1 construct was originally obtained from Dr. Georg Zoidl (Ruhr University, Bochum, Germany) in the pEYFP vector and the mPanx1 sequence was PCR amplified and inserted into the monomeric GFP vector. After overnight exposure, transfection reagents were removed and cells replated on coverslips for an additional 24–36 h incubation in DMEM-FBS medium. Brightly fluorescent cells were chosen for recordings.
Results
Non-synaptic, Panx1-Mediated ATP Release from Neuro2A Cells
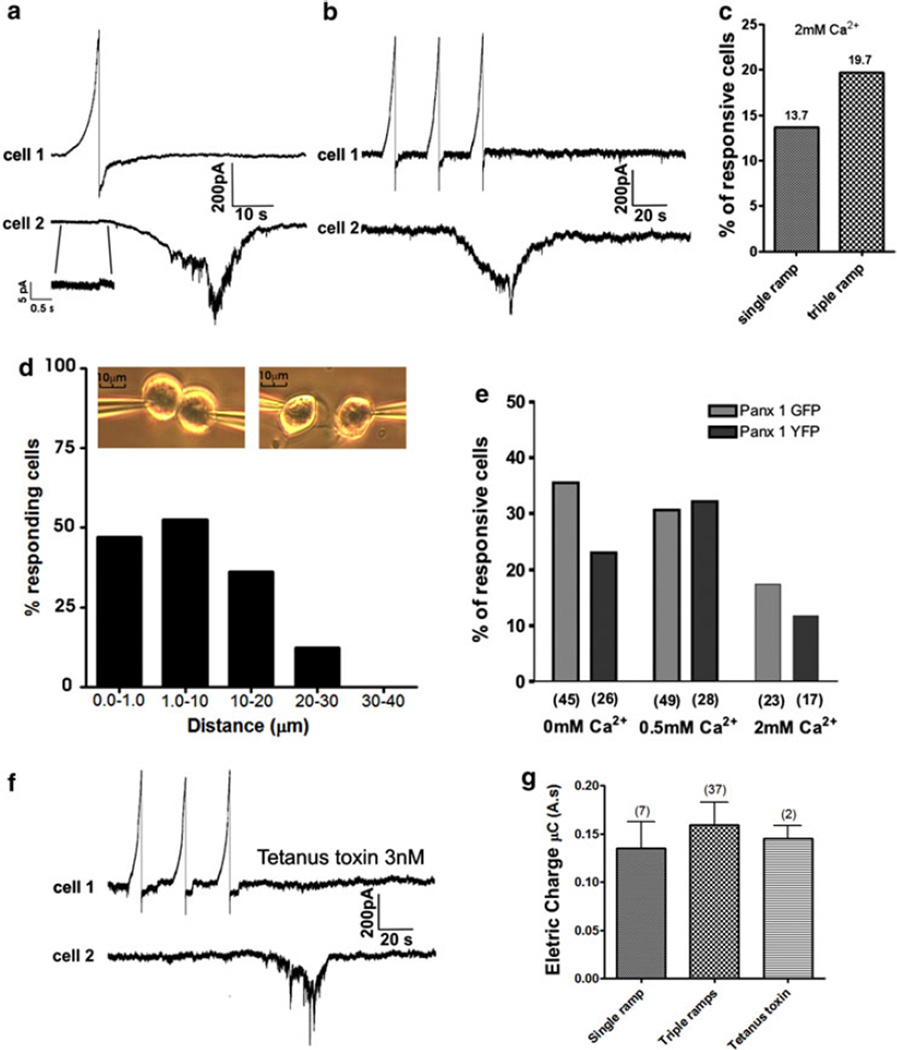
Recordings from pairs of Panx1-transfected Neuro2A cells that were either in physical contact or separated from each other by distances up to 50 µm were used to evaluate whether Panx1 could mediate paracrine signaling to nearby cells. For these experiments, both cells were held at −60 mV and a voltage ramp (−60 to +100 mV) was applied to one cell of the pair while continuously recording from both cells (see photographs in Fig. 1d, inset). In 5 of 202 cell pairs that were in physical contact (2.5%), we recorded in the non-pulsed cells small currents (mean conductance: 32 ± 12 pS) that were attributable to the presence of gap junction channels providing a low incidence of a very low degree of electrical coupling between the cell pairs (Fig. 1a, inset). Regardless of whether cells displayed such electrotonic interaction, activation of Panx1 currents (Ipanx) in one cell by voltage ramps resulted in an inward current in the other cell that began shortly after the end of the voltage ramp and peaked at variable time (~20–30 s) later (Fig. 1a, b). Using a paradigm in which single voltage ramps were applied to one cell of the pair, we found that 13.7% (7 of 51) of the neighboring cells responded with an inward current. When three identical ramps were successively applied (at 20 s intervals between ramps) to one cell, we found that 19.7% (11 of 56 cells) of the adjacent cells displayed such inward currents (Fig. 1b, c). Such current responses were not seen when the triple ramp protocol was applied to untransfected Neuro2A cells (0/20 pairs, see Suppl. Fig. 1 for example). Neither the peak amplitudes nor total charge transfer differed significantly following single or triple ramp stimulation of Panx1 transfected cells (peak currents 283.2 ± 127.8 vs. 283.2 ± 52.2 pA, total charge 0.15 ± 0.07 vs. 0.16 ± 0.02 µC; Fig. 1g). Because the percentage of responding cells was >40% higher using triple than single ramp stimulation, we used this triple ramp paradigm for subsequent experiments.
Fig. 1.
Paired recordings from Pannexin1 transfected Neuro2A cells demonstrate that currents evoked by depolarizing voltage ramps (−60 to +100 mV) lead to responses in adjacent cells that are dependent on distance and do not require extracellular Ca2+. a Response of a cell to which a voltage ramp was applied (top trace) is shown to be followed by a larger, long lasting inward current in an adjacent cell (lower trace). Note that in some cell pairs a low degree of junctional coupling was present (gj = 32 ± 12 pS, recorded from 5 cell pairs in which junctional conductance was nonzero), b representative traces for a triple ramp protocol. Inward currents were similar to those in the cell pair illustrated in part A following the ramp applied to the first cell. c Quantification of responsive cell for the single and triple ramps protocols with 2 mM Ca2+. d Quantification of the percentage of responding cells as a function of distances separating them from the depolarized cell. First bar represents cells likely in direct contact. Note that as distance increased beyond 20 µm, the percentage of responding cells dramatically decreased. Insets show representative photographs acquired for the quantification of the percentage of depolarized Neuro2A cells as a function of distance separating them. e Percentages of responsive cells obtained in Neuro2A cell transfected with mPanx1 pEYFP or GFP-N1 at high extracellular Ca2+ (2.0 mM), at low extracellular Ca2+ (0.5 mM) and in the absence of extracellular Ca2+. Note that cell responsiveness does not depend on extracellular Ca2+ concentration and is actually enhanced at low extracellular Ca2+ levels. Numbers of voltage clamped cells are in parentheses. f Representative traces obtained following tetanus toxin application. g Inward currents (quantified as electrical charge) were not significantly different when induced by single or triple ramps applied to the first cell or when recorded in the presence of tetanus toxin. Numbers in parentheses represent number of cells for which currents were observed in second cell and analyzed
The presence of a moderate latency (3–20 s) in the response evoked in one cell following voltage ramp stimulation of a neighbor appeared likely to be due to the release of a signaling molecule from the electrically stimulated cell. Consistent with this hypothesis, quantification of the frequency of cells responding with an inward current revealed that such responses were more frequent in cases where cells were close to one another than when distance between cells exceeded 30 µm, in which case no response was detected (Fig. 1d).
To evaluate whether Ca2+-dependent regulated exocytosis contributes to this paracrine signaling, experiments were performed on cells bathed either in low Ca2+ solution or treated with the SNARE degrading tetanus toxin (3 nM). In contrast to what would be expected for a Ca2+-dependent exocytic process, we found that the frequency of responsive cell partners was higher at moderate and low Ca2+ concentrations than in the presence of 2 mM extracellular Ca2+ (Fig. 1e). Moreover, 20–60 min incubation in zero extracellular Ca2+ with 3 nM tetanus toxin did not significantly change the number of cells displaying inward current (25% cells; 8 cell pairs tested). Furthermore, total charge transfer following tetanus toxin treatment was 0.14 ± 0.09 µC, similar to that seen with single and triple ramp stimuli (Fig. 1g).
To test whether Panx1 channels were involved in this type of cell–cell communication and to disclose the nature of the molecule(s) released from the electrically stimulated cell, we applied pharmacological agents known to affect Panx1 channels and ATP signaling. As illustrated in Fig. 2a–c, inward current amplitudes (total charge 0.16 ± 0.02 µC, peak current 283.2 ± 52.2 pA; N = 23 cells) in response to triple ramps were inhibited by CBX (0.02 ± 0.00 µC or 32.8 ± 21.0 pA; N = 7 cells), at a concentration that is shown in Fig. 2 to block voltage activated Ipanx1 (161.2 ± 19.1 pA, see also Fig. 2f). Moreover, CBX blockade was partially reversible after rinsing (recovering to 0.07 ± 0.012 µC or 234.8 ± 21.6 pA) (Fig. 2c). Summary of this quantification is shown in Fig. 2g, h.
Fig. 2.
Evidence that ATP released through pannexon channel acts on purinergic receptors in adjacent cells. a Response obtained in cell 2 in response to a triplet of ramps applied to cell 1 (control). b Inhibition of the depolarization induced current in cell one and inward current evoked is cell 2 by 10 min incubation with 50 µM CBX. c Restoration of currents in cell 1 and cell 2 thorough rinsing. d Amplification of the currents in cell 2 to ramps applied in cell 1 following 10 min incubation with 100 µM ARL67156. e Virtual disappearance of response in the second cell when cells were incubated for 10 min in the presence of apyrase (6.7 U/mL). f Quantification of amplitude of outward currents in cell 1 at the peak of the ramp after exposed to ARL67156 (P > 0.05), CBX (P < 0.05) and apyrase (P > 0.05) and after recovery. Bars represent SEM. Quantification of charge transfer (g) and current amplitude (h) in cell2 in the presence of CBX, ARL67156 and apyrase. Numbers in parentheses represent the number of cells tested
These results thus indicate that Panx1 is involved in this paracrine signaling. One likely candidate molecule released from the cell through Panx1 channels is ATP, although ATP degradation products such as ADP and adenosine are also active in certain systems and release of other transmitter substances is also possible. In order to evaluate the contribution of ATP, we examined the effects of inhibition or enhancement of ATP degradation in the bathing solution using the ATPase inhibitor ARL67156 (100 µM) or the ATP-degrading enzyme apyrase (6.7 U/ml). For each reagent, we compared the amplitudes of Ipanx1 evoked at +100 mV of the voltage ramp in the stimulated cell before, during and after drug application, as well as the amplitudes and total charges of the inward currents recorded in the nonelectrically stimulated cells. With regard to outward Ipanx1 in the stimulated cell, neither apyrase (337.8 ± 32 pA; N = 7 cells) nor ARL67156 (383.0 ± 28.75 pA; N = 11 cells) significantly altered these currents when compared to those recorded from control, untreated cells (430.6 ± 49.7 pA; N = 37 cells; Fig 2f). With regard to charge transfer and peak amplitudes of inward currents elicited in the cell adjacent to the electrically stimulated one, apyrase caused a large decrease in these parameters (from 0.14 ± 0.03 to 0.017 ± 0.01 µC, and from 263.2 ± 22 to 13.6 ± 9 pA; N = 5 cells; Fig. 2g, h), and ARL67156 significantly enhanced these responses (from 0.15 ± 0.02 to 0.29 ± 0.03 µC, and from 293.1 ± 27 to 487.5 ± 106.2 pA; N = 11 cell pairs; Fig. 2g, h).
Together, these results are consistent with the hypothesis that opening of Panx1 channels in the depolarized cells leads to the release of ATP that acts through purinergic P2 receptors to activate an inward current in the adjacent cell.
Pharmacolgoical Characterization of Purinergic Receptors in Neuro2A Cells
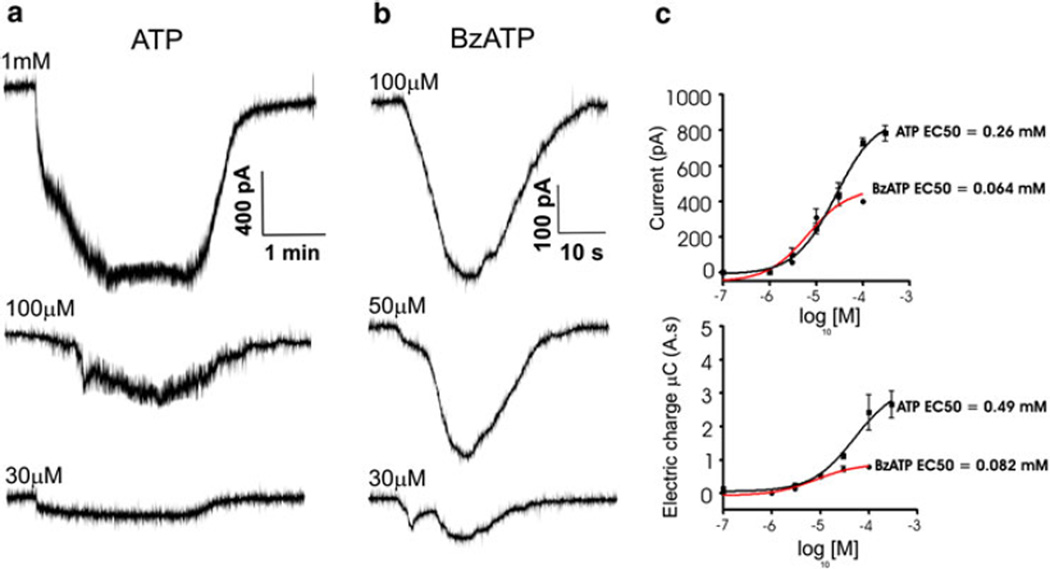
In order to evaluate which purinergic receptor subtypes were likely to be responsible for the inward current activated by the ATP in Neuro2A cells, we applied P2 receptor agonists and antagonists in voltage clamped cells. Both ATP and the P2X receptor agonist BzATP induced inward currents in Neuro2A cells transfected with mPanx1-GFP (Fig. 3a, b); such biphasic currents are typical of the P2X7 receptor [18], where the first phase is attributable to the P2X7 receptor itself and the second to the Panx1 channel opening (see ref. 12). Dose–response curves obtained for ATP and BzATP, where responses were quantified in terms of inward current amplitudes, yielded an EC50 value of 255 µM for ATP and an EC50 value of 64 µM for BzATP; in terms of electrical charge, EC50 values were 497 µM for ATP and 82 µM for BzATP (Fig 3c).
Fig. 3.
Purinergic receptor agonists induce inward currents in Neuro2A cells. a Representative traces of inward currents induced by application of 30, 100 and 1000 µM ATP. b Representative traces for currents induced by 30, 50 and 100 µM BzATP. Cells were held at −60 mV during agonist application. c Dose response current of peak current or total charge recorded in response to application of a range of ATP and BzATP concentrations. EC50 values for ATP and BzATP presented were obtained as best fits to the logistic equation
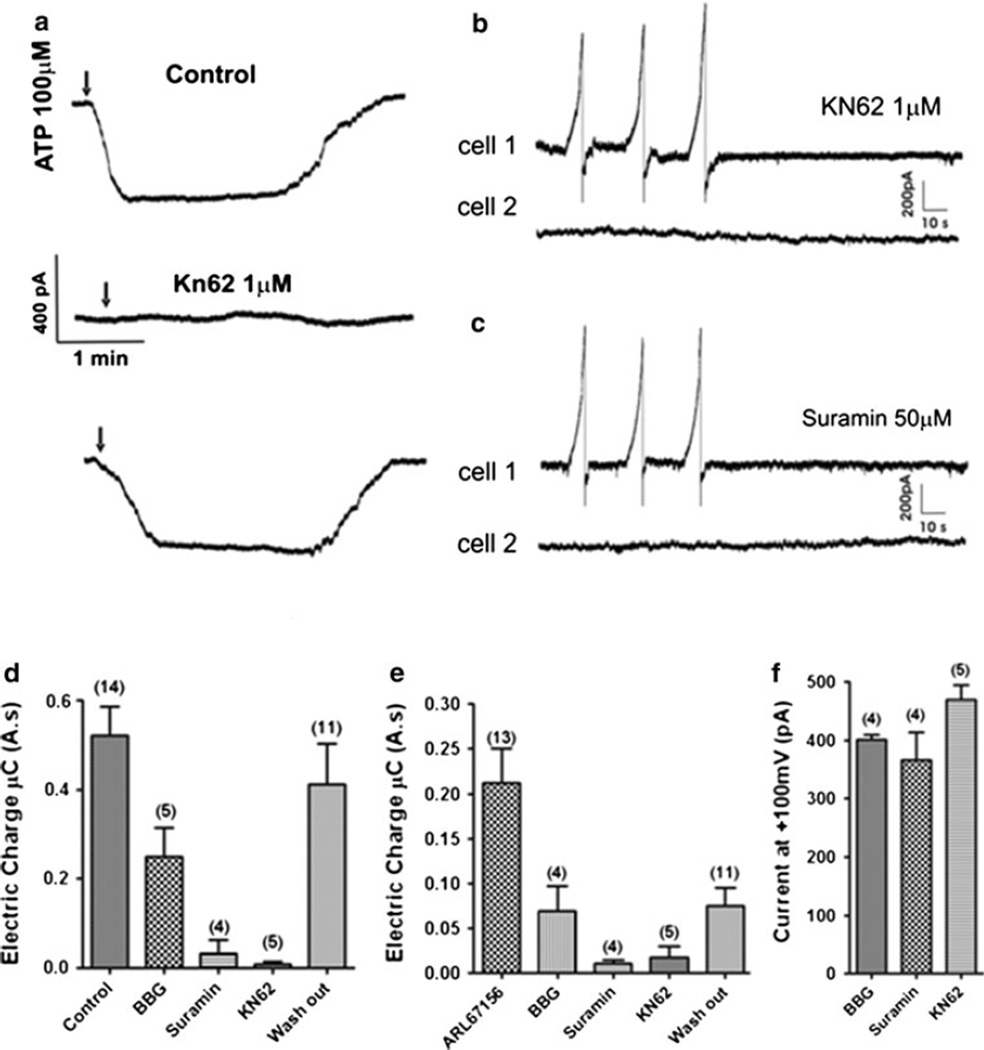
Application of suramin (50 µM, a non-specific P2R antagonist) and KN-62 (1 µM, an antagonist of the murine P2X7R) both reduced ATP-induced inward currents substantially in Neuro2A cells transfected with mPanx1-GFP (after treatment with suramin, electrical charge was reduced from 0.52 ± 0.1 to 0.010 ± 0.02 µC, peak amplitude from 320 ± 39 to 35 ± 9 pA; N = 4 cells; KN62 treatment reduced electrical charge from 0.52 ± 0.2 to 0.01 ± 0.012 µC, peak amplitude from 347 ± 13 to 12 ± 5; N = 5 cells (Fig 4a, d and e). Brilliant Blue G (BBG, a rat P2X7R antagonist) at 100 nM did not totally block ATP induced currents (decreasing electrical charge from 0.42 ± 0.8 to 0.25 ± 0.11 µC and peak current from 335 ± 19 to 135 ± 69 pA; N = 5 cells), even when incubated or perfused for more than 10 min. Inward currents recorded from cells bathed in the presence of ARL67156 induced by triple ramps were decreased by 100nM BBG (from 0.21 ± 0.08 to 0.07 ± 0.05 µC for electrical charge, 238.8 ± 32.2 to 102 ± 52 pA peak current; N = 4 cell pairs), by 50 µM suramin (from 0.21 ± 0.09 to 0.01 ± 0.00 µC for electrical charge, 262.8 ± 11.2 to 22 ± 9 pA for peak current; N = 4 cell pairs; Fig. 4c) and by 1 µM KN62 (from 0.21 ± 0.08 to 0.018 ± 0.02 µC for electrical charge, 302.8 ± 41.2 to 37 ± 12 pA; N = 5 cells pairs; Fig. 4b). ATP induced inward currents during the drug treatment are presented as total electrical charge in Fig. 4d and with respect to electric charge are shown in Fig. 4e. Amplitudes of outward currents evoked in the stimulated cell at 100 mV are quantified before and after each of these treatments in Fig. 4f.
Fig. 4.
Inward currents recorded in cell 2 are due to activation of purinergic P2 receptors, as identified through use of receptor antagonists a Representative traces showing the inhibition of ATP induced inward currents under control condition, after incubation with 1 µM Kn62 for 10 min and after rinsing and recovery. Inhibition of the response obtained in cell 2 in response to a triplet of ramps applied to the first cell 10 min after incubation in 1 µM Kn62 (b) and after treatment with 50 µM suramin (c). d Quantification of single cell ATP induced currents and of responses in cell 2 to triplet ramp protocol following treatment with 100nM BBG (*P < 0.05), 50 µM suramin (***P < 0.05) and 1 µM Kn62 (***P < 0.05) and wash out (P > 0.05). e Quantification of amplitude of outward currents in cell 1 to BBG (P > 0.05), suramin (P > 0.05), and Kn62 (P > 0.05). Numbers of recordings are given in parentheses
Threshold for Pannexin1 Activation is Reduced when Extracellular K+ is Elevated
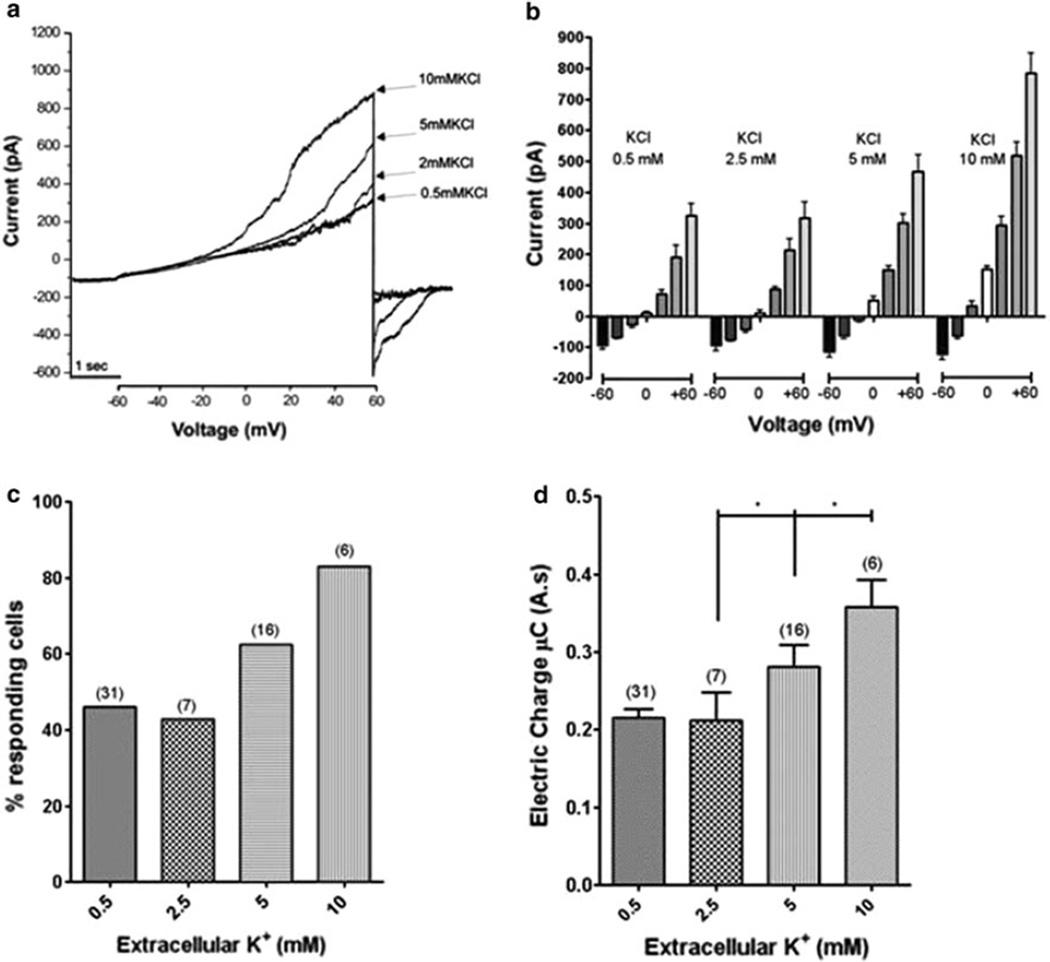
The strong depolarization used to evoke Panx1 channel opening may raise the issue of whether this mechanism would be expected to operate under physiological situations. We thus evaluated whether elevating extracellular K+, a treatment previously shown by Silverman et al. [11] to facilitate Panx1 activation, would favor signal transmission and channel opening in our transfectants. As illustrated in Fig. 5a, activation of Panx1 current occurred at lower voltages as K+ level was increased from 0.5 to 10 mM. In Fig. 5b the voltage dependent Panx1 current in response to 5 s voltage ramps from −60 to +60 mV is illustrated. Note that the voltage at which the Panx1 current is activated is directly related to concentration of potassium in the external solution (threshold −4.6 ± 2.1 mV for 0.5 mM, 3.7 ± 1.1 mV for 2.5 mM, −15.7 ± 0.7 mV for 5 mM and −26.3 ± 0.9 mV for 10 mM K+). Figure 5c shows that the fraction of responding cells also increases as concentration of extracellular potassium, is increased. Furthermore, quantification of responses in neighboring cells (Fig. 5d) indicates that the concentration of the amplitude of electric charge transfer in the non stimulated cells also increased at high K+.
Fig. 5.
Evidence that elevated extracellular K+ facilitates Pannexin1 channel opening. a Representative traces showing facilitation of Panx1 currents as concentration of extracelluar K+ is increased. Note that the voltage at which Panx1 current is activated in these ramps from −60 to +60 mV is lower at higher K+ concentration and that maximal current at the end of the ramp is increased at higher K+. b Quantification of currents in 10 mV increments from −60 to 60 mV obtained from voltage ramps in the presence of K+ concentrations 0.5 (n = 31), 25 (n = 7), 5 (n = 16), and 10 mM KCl (n = 6). Asterisk indicates P < 0.05. c, d Increase in number of responding cells and amplitude of responses as K+ concentrations were increased from 2.5 to 10 mM
In order to determine the extent to which amplitude of response in the second cell was dependent on current evoked in the first cell, we correlated values obtained in the experiments where K+ was varied. When amplitudes of responses in the second cell were compared to maximal amplitures of currents in stimulated cells, the relationship was striking, with r2 = 0.73 (Suppl. Fig. 2). This result provides further evidence that a signal carried through Panx1 channels (likely ATP) mediates the response of the second cell.
Discussion
Whole cell recordings from pairs of Panx1-transfected Neuro2A cells did not detect formation of inter-cellular channels in Panx1-transfectants, in contrast to reports in Xenopus oocytes and C6 glioma cells [14, 19]. However, currents induced by depolarizing voltages in one cell led to inward currents in the other cell. These currents in the second cell were blocked by treatments that decreased the Panx1 current in the first cell and were not affected by blockade of vesicular release (treatment with tetanus toxin or low extracellular Ca2+), indicating that a substance released from the first cell in response to Panx1 channel opening caused a conductance change in the second cell. BBG and suramin had significant blockade on the voltage stimulated current, consistent with recent demonstrations by others that ATP and certain P2 receptor antagonists can block pannexon channels [15, 20].
One likely candidate for the mediating signal is ATP, permeating the Panx1 channels and acting upon purinergic receptors in the adjacent cells. Although metabolites such as ADP, AMP and adenosine might also be active, their major contribution is inconsistent with our findings that blockade of ATPase with ARL67156 potentiated the response and that apyrase reduced it, both of which imply that ATP itself is the main agonist molecule. This was further supported by our finding that the response was blocked by suramin, a broad spectrum P2 receptor antagonist. Agonist dose–response curves and inhibition by selective antagonists demonstrate that P2XRs are main candidates for involvement in this process. However, we have not ruled out minor participation of the G-coupled protein receptors, the P2Y receptors, in the transmission of the ATP signal.
With regard to ATP release, the results obtained in this study indicate that the opening of Panx1 channels by voltage can lead to the release of ATP from the stimulated cell and activation of P2 receptors in adjacent cells. It has long been known that purine and pyrimidine nucleotides are messenger molecules involved in neurotransmission and autocrine/paracrine regulation of cellular functions [21, 22]. Panx1 is widely expressed in most tissues, including both brain and spinal cord, where it is found in both neurons and glia [23–25]. A role for this channel in glial biology likely involves transmission of intercellular calcium waves which are increases in cytosolic Ca2+ levels mediated both by direct flux through gap junctions of molecules such as IP3 and subsequent release of Ca2+ from the ER from the neighboring cells and by stimulation of one cell’s P2 receptors by extracellular ATP released from the other cell [26, 27]. ATP is released by many cell types through both vesicular mechanisms and ion channels [reviewed in 2]. Although the channel most favored for this role in astrocytes and other cell types has been the Cx43 “hemichannel” [28–31], supporting evidence for this role has primarily consisted of pharmacological blockade by agents that also affect Panx1 channels.
Panx1 expressed in neurons has been proposed as the molecular substrate of “hemichannels” that are activated in ischemic neuronal death [32, 33] and through interaction with the P2X7 receptor, in oligodendrocyte excitotoxicity as well [34]. The findings that Panx1 is also expressed in postsynaptic density of neurons by electron microscopy [35] and that N-methyl-d-aspartate receptors (NMDARs) evoke pannexon currents in the hippocampus [33] suggests that Panx1 is likely to be involved in diverse physiological and pathophysiological nervous system functions.
Supplementary Material
Acknowledgments
We appreciate the technical support of Ms. Marcia Urban-Maldonado with Panx1 constructs, and the original gifts of hPanx1 and mPnx1 constructs from Dr. Gerhard Dahl (Miami) and Dr. George Zoidl (formerly Bochum, now Toronto). Supported in part by the National Institute of Neurological Disorders (NINDS) of the National Institutes of Health (NIH), NS041282 (to DCS). Studies described here were performed on the Neuro2A cell line to which DCS was introduced by Dr. Robert Ledeen and has been widely used as an exogenous expression system to characterize gap junction channels formed of various connexins (16).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11064-012-0720-6) contains supplementary material, which is available to authorized users.
References
- 1.Scemes E, Spray DC. Connexin expression (gap junctions and hemichannels) in astrocytes. In: Parpura V, Haydon HG, editors. Astrocytes in pathophysiology of the nervous system. Berlin: Springer Verlag; 2008. pp. 107–150. [Google Scholar]
- 2.Spray DC, Ye ZC, Ransom BR. Functional connexin ‘‘hemichannels’’ a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 3.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology. 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1 beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of ‘‘hemi-channels’’. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell–cell communication. Neuron Glia Biol. 2007;3(3):199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman WR, de Rivero Vaccari SP, Locovei S, Qui F, Carlsson SK, Scemes E, Kean RW, Dahl G. The Pannexin 1 channel activates the inflamassome in nomas and astrocytes. JBC. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:752–760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iglesias R, Dahl G, Qui F, Spray DC, Scemes E. Pannexin1: the molecular substrate of astrocyte ‘‘hemichannels’’. J Neurosci. 2009;29(21):7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328(2):409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Corsso C, Srinivas M, Urban-Maldonado M, Moreno AP, Fort AG, Fishman GI, Spray DC. Transfection of mammalian cells with connexins and measurement of voltage sensitivity of their gap junctions. Nat Protoc. 2006;1:1799–1809. doi: 10.1038/nprot.2006.266. [DOI] [PubMed] [Google Scholar]
- 17.Prochnow N, Hoffmann S, Dermietzel R, Zoidl G. Replacement of a single cysteine in the fourth transmembrane domain of the zebrafish Panx1 alters hemichannel gating behavior. Exp Br Res. doi: 10.1007/s00221-009-1957-4. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63(3):641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CP, Beckberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin in C6 glioma cells. Cancer Res. 2007;67:1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 20.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnstock G. Purinergic signaling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 22.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 24.Ray A, Zoidl G, Wahle P, Dermietzel R. Pannexin expression in the cerebellum. Cerebellum. 2006;5:189–192. doi: 10.1080/14734220500530082. [DOI] [PubMed] [Google Scholar]
- 25.Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;41:113–120. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 34.Matute C. P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol. 2008;38:123–128. doi: 10.1007/s12035-008-8028-x. [DOI] [PubMed] [Google Scholar]
- 35.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.