Abstract
Tumor-targeted vaccines represent a strategy to enhance the graft-versus-leukemia effect following allogeneic blood or marrow transplantation (alloBMT). We have previously shown that GVHD can negatively impact quantitative responses to vaccines. Using a minor histocompatibility antigen (mHA)-mismatched BMT (B6 → B6 × C3H.SW) followed by adoptive transfer of HY-specific T cells and HY-expressing dendritic cells we assessed whether GVHD induced by donor lymphocyte infusion (DLI) affects the persistence, proliferation and survival of vaccine-responding, nonalloantigen reactive T cells. Both CD8+ and CD4+ HY-specific T cells undergo less vaccine-driven proliferation in allogeneic recipients with GVHD. While vaccine responding CD8+ T cells show decreased gamma interferon and CD107a production, CD4+ T cells exhibit increased PD-1 and TIM3 expression. In addition, the degree of apoptosis in vaccine-responding CD8+ T cells was higher in the presence of GVHD, but there was no difference in CD4+ T cell apoptosis. Using Fas ligand-deficient or TRAIL-deficient DLI had no impact on apoptosis of HY-specific T cells. However, perforin-deficient alloreactive DLI induced significantly less apoptosis of vaccine-responding CD8+ T cells, and resulted in enhanced tumor protection. Thus, diminished vaccine responses during GVHD result from impaired proliferation of CD8+ and CD4+ T cells responding to vaccination, with an additional contribution from perforin-mediated CD8+ T cell apoptosis. These results provide important insights towards optimizing vaccine responses after alloBMT.
Introduction
Allogeneic blood and marrow transplantation (alloBMT) is associated with prolonged lymphopenia that predisposes to infection and relapse. Since thymic function is limited early after alloBMT, mature T cells in the graft or provided as a donor lymphocyte infusion (DLI) contribute substantially to immune recovery but also induce graft-versus-host-disease (GVHD), which further impairs thymic function(1). Prior studies have documented that GVHD has direct deleterious effects on mature donor T cells transferred with the graft(2), due to shortened survival in the periphery(3, 4) and activation-induced cell death(5, 6). Furthermore, GVHD may indirectly constrain the expansion of mature T cells(2, 7). The result is that, despite the contribution of alloreactivity to preventing relapse, the detrimental effects of GVHD on immune recovery could undermine strategies to manipulate the antitumor response.
Observations made by other groups clearly demonstrated that diminished proliferation and increased apoptosis contributes to T cell dysfunction in both preclinical alloBMT models and during clinical alloBMT(3-5, 7-10). One of the limitations of prior studies that have examined the impact of alloreactivity on T cell populations expanding following alloBMT has been the difficulty to identify T cells with no cross reactivity against minor histocompatibility antigens (mHA) on the host. In addition, the contribution of a competing nonalloantigen stimulus, in the form of a vaccine, to T cells present in the setting of GVHD has not been explored in depth.
Vaccines have demonstrated efficacy in the autologous setting in expanding T cells specific for tumor-associated antigens (TAA) and have the potential to enhance the graft-versus-leukemia (GVL) effect(11-18). We have previously demonstrated that even mild GVHD adversely impacts the magnitude of immune responses to a vaccine targeting antigens not expressed on normal host tissues(12) through an undefined mechanism. Although the deleterious impact of GVHD on T cell populations as a whole has been well established, few studies have carefully characterized the effect of the alloreactive environment on non-alloreactive T cells responding to a vaccine(18, 19). In this report, we studied the capacity for vaccine-responding T cells with known antigen specificity toward non-allogeneic antigens to proliferate and survive in the setting of GVHD after MHC-matched, mHA-mismatched alloBMT. We also explored the mechanism by which DLI-mediated GVHD increases apoptosis of non-alloreactive, adoptively transferred vaccine-responding T cells by examining the contribution of 3 effector pathways: Fas ligand (FasL), TRAIL and perforin.
Materials and Methods
Mice
Congenic CD45.1+ C57BL/6 (H-2b) (B6) and CD45.2+ B6 mice were purchased from the National Cancer Institute (NCI) animal production colony (Frederick, MD) and B6 Rag1-/-, B6 FasL-/-(gld), B6 Prf1-/-, C3H.SW and B6 × C3H.SW (all H-2b) (F1) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). F1 mice underwent vacuum suction removal of the thymus according to standard protocol. B6 TRAIL-/- mice were provided by Dr. Robert Wiltrout at NCI-Frederick. CD45.2+ B6 Rag2-/- Marilyn and Matahari mice obtained from the Taconic NIAID colony (Taconic, NY) with permission from Dr. Polly Matzinger(20, 21). Mice were age-matched and used between 6 and 12 weeks of age, and housed in a specific pathogen-free facility. The Animal Care and Use Committee at the NIH approved all protocols.
T cell Depleted Bone Marrow Transplantation and Donor Lymphocyte Infusion
Following lethal irradiation (10 Gy), thymectomized F1 mice were injected intravenously with 4 × 106 T cell-depleted bone marrow cells in serum-free media from CD45.1+ B6 (allogeneic) or CD45.1+/CD45.2+ F1 (syngeneic) donors as previously described(12). On day +14, recipients received a CD45.1+ DLI of 30 × 106 cells in serum-free media from pooled single cell suspensions of splenic and lymph node cells. Recipients were weighed and monitored for GVHD as previously described(22). Engraftment was confirmed by flow cytometric analysis of spleens of F1 recipients. Examination for moribund mice were performed by a veterinarian and veterinary technicians blinded to experimental design and groups, and assessed mice daily according to approved institutional protocols.
Dendritic Cell Vaccination and Adoptive Transfer of CFSE-labeled HY-reactive T cells
On day +28, 10 × 106 CFSE (Molecular Probes, Carlsbad, CA)-labeled splenocytes from CD45.2+ Marilyn or Matahari donors were injected intravenously with or without a male B6 dendritic cell (DC) vaccine. CFSE labeling was performed as previously described(23). DC vaccines were prepared from male B6 bone marrow and activated as previously described(12). DCs were injected intraperitoneally in serum-free media at 1 × 105 cells per recipient on day +28 at the time of Marilyn or Matahari transfer. Spleens and lymph nodes were harvested from BMT recipients 3, 5, and 7 days after adoptive transfer and analyzed by flow cytometry for enumeration, proliferation or apoptosis.
Flow Cytometry
Spleens and lymph nodes were harvested on days +31, 33 or 35 post-BMT and flow cytometric-based enumeration of lymphocytes was accomplished using a FACS Calibur equipped with CellQuest software version 5.2.1 (Becton Dickinson, San Jose, CA). Briefly, 1 × 106 freshly isolated, erythrocyte-depleted splenocytes or lymph node cells were treated with anti-FcγIII/II receptors monoclonal antibody (clone 2.4G2) and then stained at 4°C for 20 minutes with a monoclonal antibody cocktail containing Vβ6 (for Marilyn) or Vβ8.3 (for Matahari)-PE, CD45.2-PerCP Cy5.5 and CD45.1-APC (BD Pharmingen, San Jose, CA), then washed in FACS buffer (Phosphate buffered salt solution with 0.2% fetal calf serum and 0.1% sodium azide). To examine for apoptosis, Annexin-V-PE staining was performed according to the manufacturer's instructions (Becton Dickinson, San Jose, CA). For phenotypic analysis of Marilyn and Matahari T cells in vivo, the following antibodies were used: anti-CD107a-PE (clone 1D4B, BD Pharmingen), anti-PD1-APC (clone J43, eBiosciences), and anti-Tim3 (clone RMT3-23, eBiosciences). For intracellular staining of interferon gamma (IFNγ), cells were fixed and permeabilized according to the manufacturer's instructions using Fix and Perm buffer (BD Pharmingen) and stained with anti-IFNγ-PE (clone XMG1.2, eBiosciences).
Tumor challenge
The MB49 tumor cell line is derived from a chemically-induced urothelial carcinoma in a male B6 mouse (H-2b), and expresses the male-specific minor histocompatibility antigen H-Y(24). MB49 cells were maintained in culture at 37°C in 5% CO2 in complete mouse media (CMM). Exponentially growing tumor cells were prepared as a single cell suspension in serum-free media and injected at into the subcutaneous fat of the flank at a dose of 1 × 106 tumor cells on day +35 post-BMT. Tumors were measured as previously described(12). Mice were euthanized with CO2 when tumor diameters reached 2 cm, in accordance with animal protocols. If a mouse was found dead, the previously recorded tumor measurement was carried for the rest of the experiment.
T cell/DC co-culture experiment
DCs were generated from splenocytes of male or female congenic CD45.1+ B6 and C3H.SW mice as described above. Magnetic-bead purified CD8+ T cells from CD45.2+ Matahari mice were then cultured in a 24-well plate at a 1:1 ratio with splenocyte-derived DCs for 72 hours. Wells were then harvested, counted and analyzed by flow cytometry. Matahari cells were identified by Vβ 8.3 and CD45.2 expression.
Statistical Analysis
Statistical tests were performed using GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA). Significant differences when comparing two groups were determined by two-tailed Mann-Whitney test. Kruskal-Wallis with Dunn's multiple comparison posttest was used to assess statistical differences between three or more groups. Log-rank analysis was done for survival experiments. A p value less than 0.05 was considered significant.
Results
GVHD decreases recovery of nonalloreactive, vaccine responding T cells
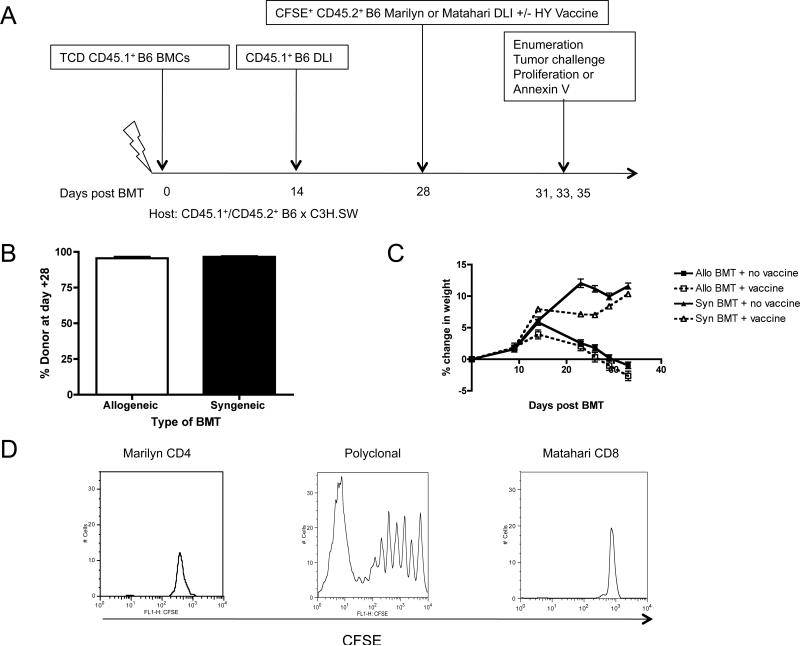
Irradiated female mice were transplanted with TCD mHA-mismatched bone marrow (CD45.1+ B6→CD45.1+/CD45.2+ B6 × C3H.SW) followed by infusion of congenic CD45.1+ polyclonal donor T cells as a delayed DLI to induce GVHD. This platform was used to study vaccine-induced proliferation and apoptosis of CD8+ and CD4+ T cells with known specificity for the male HY antigen (Figure 1A). Thymectomized recipients were chosen to mimic poor thymic function observed in humans early after alloBMT and to restrict T cell recovery to those contained in the adoptively transferred inocula. Since female donors and recipients were used, antigens causing GVHD could not contribute to the proliferation of vaccine-responding HY specific T cells. In this model, donor chimerism is nearly 100% (Figure 1B), and a delayed DLI given on day +14 induces weight loss in allogeneic recipients (Figure 1C) without lethality. Also, as expected, the donor-derived DC vaccine does not exacerbate GVHD since HY is not expressed by the female recipients. Furthermore, HY-specific T cells do not undergo lymphopenia induced proliferation (LIP)(25) (Figure 1D). Thus, proliferation could be interpreted as being either vaccine-driven or a non-specific effect of the allogeneic environment.
Figure 1. DLI-induced, mHA-mismatched non-lethal GVHD impairs efficacy of TCRTg CD8+ T cells.
(A) AlloBMT consisted of CD45.1+ T cell depleted (TCD) B6 bone marrow infused into lethally irradiated recipients (B6→B6 × C3H.SW [F1]), followed by an alloreactive CD45.1+ B6 DLI at day +14 and adoptive transfer of CD4+ CD45.2+ or CD8+ CD45.2+ B6 TCRTg cells +/- male B6 DC vaccine at day +28. F1 recipients were CD45.1+CD45.2+ double positive. Syngeneic BMT was F1→F1 on day +0 followed by F1 DLI on day +14 and adoptive transfer of CD4+ CD45.2+ or CD8+ CD45.2+ B6 TCRTg cells +/- male B6 DC vaccine at day +28. (B) Donor chimerism assessed by flow cytometric analysis for % donor cells in BMT recipient spleens on day +14, 5 mice/group, representative of two independent experiments. (C) Weights were recorded twice weekly per mouse, averaged for the group, and then compared as a % change from the day +0 weight for syngeneic and alloBMT recipients, 5-6 mice/group, representative of three independent experiments. (D) B6 Rag-/- hosts were injected with CFSE-labeled T cells obtained from CD4+ (Marilyn) or CD8+ (Matahari) TCRTg splenocytes, and after 7 days, the spleens of the Rag-/- hosts were analyzed for T cell proliferation. A representative mouse from each group is depicted, n = 3, representative of three independent experiments.
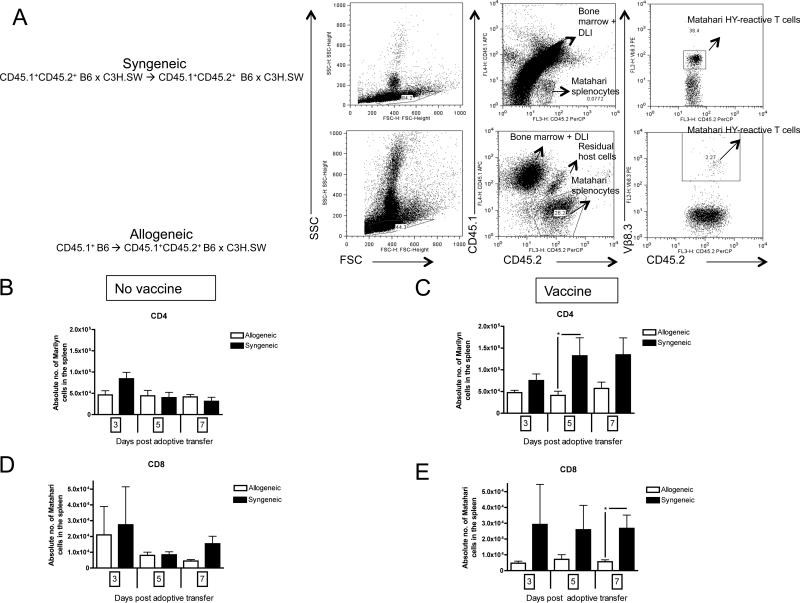
On day +28, CD8+ TCR transgenic (TCRTg) T cells (Matahari, CD45.2+, Vβ8.3+) specific for the immunodominant class I HY antigen (UTY) or CD4+ TCRTg T cells (Marilyn, CD45.2+, Vβ6+) specific for the immunodominant class II HY antigen (DBY) were given in conjunction with a male DC vaccine. To examine the impact of the allogeneic environment on recovery of vaccine responding T cells, we compared the quantity of Marilyn and Matahari cells in the spleens of unvaccinated and vaccinated allogeneic recipients with GVHD compared to syngeneic recipients. By gating on congenic markers and the associated TCR V-beta molecule, we were able to identify the vaccine-responding T cells (Figure 2A). There was no difference in recovery of CD4+ Marilyn T cells between syngeneic and allogeneic transplant recipients in the absence of vaccine (Figure 2B). However, there was decreased recovery of CD4+ Marilyn T cells in the spleens of mice with GVHD following vaccination (Figure 2C). There was an identical pattern with respect to CD8+ Matahari T cells (Figure 2D and E). These findings are also consistent with our prior observation of decreased HY-specific responses expanded from polyclonal T cells by HY vaccination(12). To exclude that the allogeneic environment did not change the trafficking pattern of HY-specific T cells to lymphoid organs, we also analyzed pooled lymph nodes and found similar results (Supplemental Fig. 1A-D).
Figure 2. GVHD decreases recovery of vaccine-responding T cells.
Mice were transplanted per Figure 1A. (A) A sample flow cytometry gating schema on (top) a F1 recipient transplanted with syngeneic CD45.1+CD45.2+ F1 bone marrow and DLI, followed by CD45.1-CD45.2+ Matahari splenocyte infusion, as well as (bottom) a F1 recipient transplanted with allogeneic CD45.1+CD45.2- B6 bone marrow and DLI, followed by CD45.1-CD45.2+ Matahari splenocyte infusion. The CD45.2+ Vβ8.3+ fraction in both BMTs represents the HY-reactive T cells from the Matahari infusion in the recipient spleen. (B) Absolute numbers of CD4+Vβ6+CD45.2+ Marilyn T cells in the host spleen were enumerated by flow cytometry 3, 5 and 7 days (days +31, 33 and 35 post-BMT) after adoptive transfer without concurrent male DC vaccination, or (C) with male DC vaccination, 4-9 mice/group, pooled from three independent experiments. (D) Absolute numbers of CD8+Vβ8.3+CD45.2+ Matahari T cells in the host spleen were enumerated by flow cytometry 3, 5 and 7 days (days +31, 33 and 35 post-BMT) after adoptive transfer without concurrent male DC vaccination, or (E) with male DC vaccination, 3-9 mice/group. * = p<0.05, pooled from three independent experiments.
GVHD prevents proliferation of antigen-specific CD4+ and CD8+ T cells from DC vaccination to a nonalloantigen
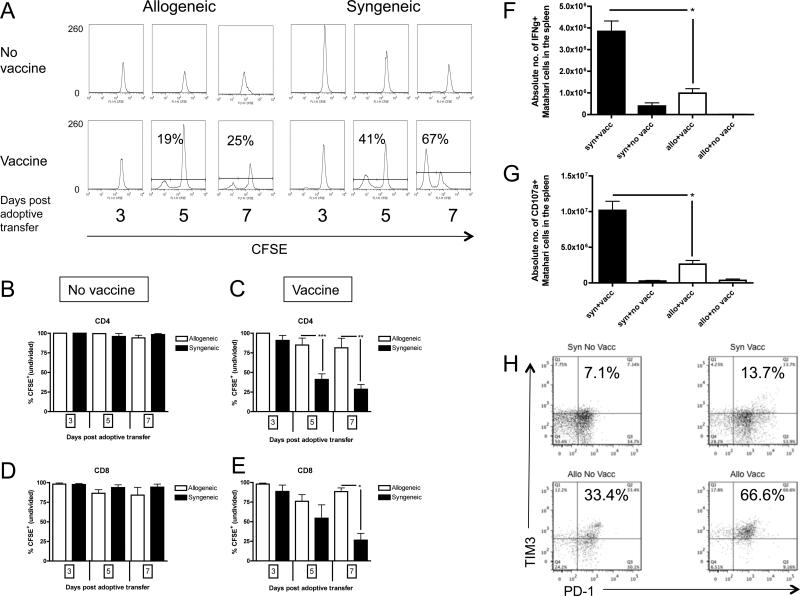
To determine the mechanism of diminished recovery of vaccine-responding T cells observed during GVHD, proliferation of CFSE-labeled Marilyn and Matahari T cells were measured in separate experiments. Neither Marilyn nor Matahari T cells proliferated in the absence of a vaccine (Figure 3A, 3B and 3D), confirming earlier reports that LIP is insufficient to drive T cell division and demonstrating that the allogeneic environment does not induce proliferation. In syngeneic BMT recipients, Marilyn T cells (Figure 3A and 3C), and Matahari T cells showed robust proliferation after vaccination (Figure 3E). As expected with a single vaccine, proliferation was synchronized, resulting in two distinct peaks, representing antigen responding and non-responding cells, rather than the typical multiple divisions noted in polyclonal T cells during LIP (Figure 1D) (26). However, GVHD significantly impaired proliferation of both Marilyn and Matahari vaccine-responding cells (Figure 3A, 3C and 3E).
Figure 3. Vaccine-induced proliferation is impaired for TCRTg CD4+ and CD8+ T cells during GVHD.
Mice were transplanted per Figure 1A and received CFSE-labeled CD4+ Marilyn or CD8+ Matahari splenocytes with or without a male B6 DC vaccine. Proliferation was measured by flow cytometry at 3, 5 and 7 days (days +31, 33 and 35 post-BMT) after transfer. (A) Results from a representative recipient of CD4+ Marilyn T cells after allogeneic and syngeneic BMT analyzed at each timepoint are depicted, with unvaccinated recipient (top), and male DC vaccinated recipient (bottom). (B) Pooled data from three independent experiments depicted for CD4+ Marilyn T cells in unvaccinated, and (C) male DC vaccinated recipients, as well as from three independent experiments for (D) CD8+ Matahari T cells in unvaccinated, and (E) male DC vaccinated recipients, 3-9 mice/group. CD8+ Matahari T cells from syngeneic and allogeneic recipients were analyzed 3 days following vaccination with male DC vaccine for intracellular IFNγ (F) or CD107a (G) (n=3 mice/group). (H) Representative FACS plots of PD-1 and TIM3 on CD4+ Marilyn T cells from syngeneic and allogeneic recipients 3 days after male DC vaccination (total of n=3 mice per group analyzed). * p<0.05, ** p<0.01, *** p<0.001.
To assess additional functional characteristics of vaccine-responding T cells, cytokine production and lytic potential of Matahari T cells was analyzed following vaccination of allogeneic and syngeneic recipients. As shown in Figure 3F, the absolute number of Matahari cells producing IFNγ in response to HY DC vaccination was significantly reduced in the setting of GVHD as was the absolute number CD107a+ Matahari T cells (Figure 3G). Thus, in addition to reduced proliferation and total HY-specific T cell recovery, GVHD also affected the number of functional vaccine responding CD8+ T cells.
To begin to address the mechanism for the reduced proliferation of Marilyn T cells in response to vaccination, we assessed the expression of markers of T cell dysfunction in both allogeneic and syngeneic recipients. As shown in Figure 3H, both programmed death 1 (PD-1) receptor and T cell immunoglobulin mucin-like domain 3 (TIM3) was more highly expressed on Marilyn T cells in allogeneic recipients as compared to syngeneic recipients. Interestingly, although the expression of these markers was increased by DC vaccination, there was higher PD-1 and TIM3 on Marilyn T cells in allogeneic recipients in the absence of HY vaccination. Since HY is not an allogeneic antigen in this system, these results suggest a non-specific “bystander effect” induced by GVHD that does not require the presence of cognate antigen.
GVHD increases apoptosis of CD8+, but not CD4+, T cells responding to vaccination
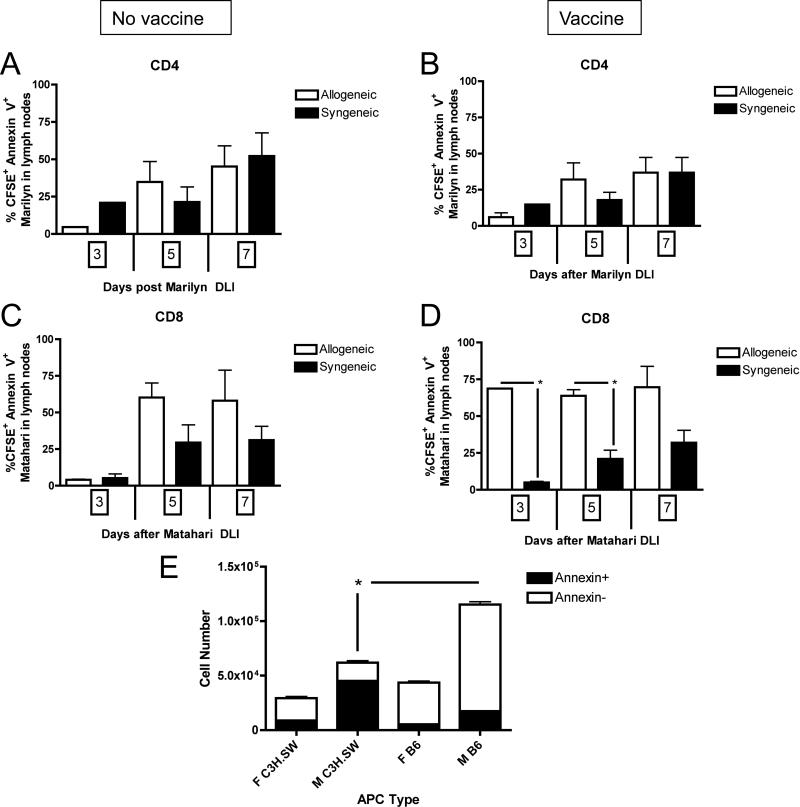
We next tested whether increased apoptosis also contributed to diminished recovery of vaccine-responding T cells. Because there was a large proportion of undivided (CFSE+) T cells present in mice with GVHD, we were particularly interested in assessing whether these cells were undergoing apoptosis and thus unable to proliferate to the vaccine. Whereas there were equivalent numbers of undivided, CFSE+ Annexin V+ Marilyn T cells after both syngeneic and alloBMT (Figure 4A and 4B) with or without a vaccine, there was a significant increase in apoptosis of vaccine-responding Matahari T cells in the lymph nodes of alloBMT recipients (Figure 4D). Interestingly, no differences in the % CFSE+ Annexin V+ T cells were observed in the spleen (data not shown). Although a reduction in CD4 and CD8 vaccine-induced proliferation was seen in both spleen and lymph nodes of mice with GVHD, there was no increase in CD4 apoptosis in splenic T cells. We hypothesized that, in this model, simultaneous presentation of alloantigens to alloreactive T cells and HY antigens to HY specific T cells may partially explain the increased bystander apoptosis observed in HY-specific T cells. To test this, we performed in vitro co-culture experiments with HY TCRTg T cells and male DCs in the presence or absence of alloantigen presentation. Indeed, as shown in Figure 4E, the recovery of Matahari T cells was reduced when cultured with allogeneic T cells plus DCs presenting both HY and alloantigens, with an increased fraction of Annexin V+ Matahari T cells noted.
Figure 4. GVHD increases apoptosis 7 days after adoptive transfer of TCRTg CD8+, but not CD4+, T cells.
Mice were transplanted per Figure 1A and given either an infusion of Marilyn or Matahari splenocytes on day +28 with or without a male B6 DC vaccine. Apoptosis of nonproliferated, CFSE+, TCRTg T cells was enumerated by flow cytometric analysis of Annexin V at 3, 5 and 7 days (days +31, 33 and 35 post-BMT) after transfer of CD4+ Marilyn T cells in (A) unvaccinated and (B) male DC vaccinated recipient lymph nodes, and after transfer of CD8+ Matahari T cells in (C) unvaccinated and (D) male DC vaccinated recipient lymph nodes, 3-7 mice/group, pooled from three independent experiments. (E) Matahari T cells were cultured with male or female DCs from either allogeneic (C3H.SW) or syngeneic (B6 CD45.1+) donors at a 1:1 ratio for 72 hours. Cells were then counted and analyzed for expression of Annexin V on CD45.2+CD8+Vβ 8.3+7AAD- cells. Triplicate co-culture wells were set up for each condition. * p<0.05.
Vaccine-induced apoptosis in the setting of GVHD can be reversed through disruption of the perforin pathway resulting in restoration of vaccine-mediated tumor protection
Because the TCRTg T cells in our model do not recognize an alloantigen and do not undergo expansion in either syngeneic or allogeneic recipients without a vaccine, alloantigen could not directly mediate apoptosis. Rather we hypothesized that alloreactive T cells present in the DLI were mediating bystander apoptosis of vaccine responding CD8+ T cells as has been reported for polyclonal T cells of unknown specificity in the absence of a vaccine(5). To determine which molecules were regulating bystander apoptosis, we infused DLIs deficient in specific cytolytic pathways that could be potentially utilized by the DLI to induce apoptosis in the vaccine-responding CD8+ T cells (Figure 5A). Prior work has implicated both Fas-Fas ligand(3, 5, 27-37) and perforin(29, 33-35, 37-39) in alloantigen-reactive T cell apoptosis during GVHD. In our model, DLI deficient in Fas ligand or TRAIL did not result in reduced apoptosis of vaccine-responding CD8+ T cells. However, infusing DLI deficient in perforin resulted in a modest but significantly reduced percentage of undivided, apoptotic Matahari in spleen (Figure 5B) and greater than 50% decrease in the lymph nodes (Figure 5C) of alloBMT recipients. To determine if this had functional implications, we challenged alloBMT recipients that were treated with perforin-deficient DLI followed by Matahari and DC vaccine with MB49 tumor, and observed mildly attenuated weight loss (Figure 6A) and enhanced tumor protection by Matahari T cells when perforin was absent from the DLI (Figure 6B).
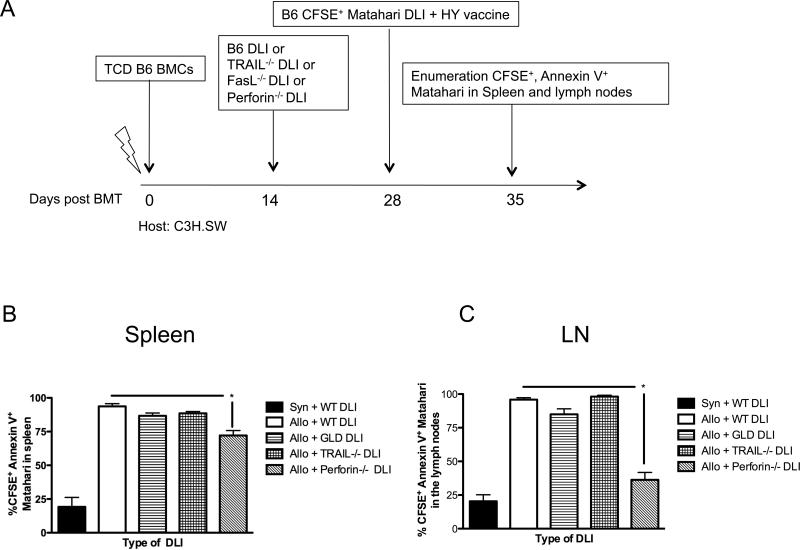
Figure 5. Administration of perforin-deficient DLI attenuates GVHD-induced apoptosis of TCRTg CD8+ T cells in spleens and lymph nodes of alloBMT recipients.
(A) C3H.SW recipients received TCD B6 bone marrow on day +0 followed by 30 × 106 DLI from B6 (wild type, WT), B6 FasL-/- (GLD), B6 TRAIL-/-, or B6 prf1-/- (perforin) donors on day +14. On day +28, 10 × 106 CFSE-labeled Matahari T cells were adoptively transferred and all recipients concurrently received B6 male DC vaccine. After 7 days (day +35), nonproliferated CFSE+ Annexin V+ cells were enumerated in (B) spleens and (C) pooled axillary, inguinal and cervical lymph nodes from alloBMT recipients, 5-10 mice/group, * = p < 0.05, data pooled from two independent experiments.
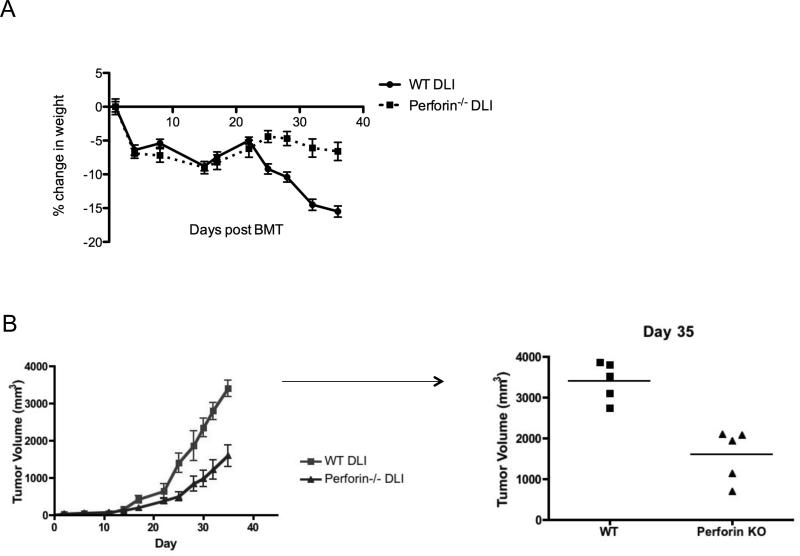
Figure 6. Infusion of perforin-deficient DLI enhances tumor protection by TCRTg CD8+ T cells expanded in vivo with a tumor-specific vaccine.
C3H.SW recipients received TCD B6 bone marrow on day +0 followed by 30 × 106 DLI from B6 (wild type, WT or B6 prf1-/-(perforin) donors on day +14. On day +28, 10 × 106 Matahari T cells were adoptively transferred and all recipients concurrently received B6 male DC vaccine. (A) alloBMT recipients were weighed twice weekly, and on day +35, (B) all recipients were challenged with 1 × 106 MB49 tumor cells in the flank and measured twice weekly, 5 mice/group, p < 0.01 on day 35, data representative from two independent experiments.
Discussion
Tumor-targeted vaccines are a promising approach to direct immune responses following alloBMT. Indeed, a number of preclinical studies have demonstrated the efficacy of such a strategy(11-18) and clinical trials using vaccines following alloBMT are underway. However, preventative and therapeutic interventions for GVHD, as well as GVHD itself, are globally immunosuppressive. Furthermore, the impact of the allogeneic environment on T cells expanding in response to a vaccine has not been well characterized. We have previously demonstrated that sublethal GVHD is immunosuppressive, leading to decreased vaccine responses to TAAs (12). We confirm the reduction in vaccine responses following alloBMT in the present study using CD4+ and CD8+ T cells with known specificity for a non-alloantigen. The ability to carefully track the behavior of these T cells in the allogeneic recipients allowed us to establish that this reduction in vaccine responses is due to diminished proliferation, and, for CD8+ T cells, increased bystander apoptosis mediated, at least in part, by perforin.
Previous studies have utilized proliferative rate to distinguish between alloreative and nonalloreactive T cells(8) but did not definitively identify non-alloreactive T cells based on known TCR specificity. Using this approach, cytokines have been shown to enhance global T cell reconstitution, with enhanced graft versus tumor responses demonstrated in some of these studies (40-43). However, the impact on antigen-specific T reconstitution was not analyzed. There have been a few studies that have specifically assessed the impact of alloreactive T cells on cognate antigen-driven expansion of T cells with known specificity for a non-alloantigen(18, 19). Jenq et al. performed T cell depleted alloBMT to demonstrate that recombinant keratinocyte growth factor could enhance thymic output, improve DNA vaccine responses and enhance tumor protection but did not specifically address the question as to whether GVHD affects vaccine responses. Manzo et utilized both an HY-alloreactivity model and miHA-disparate transplant model to demonstrate that early vaccine responses against non-alloantigens are preserved at one week but persistence beyond 2 weeks is impaired in the setting of GVHD, consistent with our earlier observations(12). However, the specific mechanism by which loss of vaccine-responding T cells are lost was not analyzed. We specifically found that GVHD impairs CD4+ and CD8+ T cell responses to a vaccine in part from diminished antigen-induced proliferation. These data support recent clinical data demonstrating that CD8+ T cells recognizing TAAs from acute myeloid leukemia fail to proliferate in an alloBMT environment(9). Interestingly, these authors suggest that one explanation may be that GVHD induces a state of replicative senescence, whereby the T cells are in a nonproliferative and apoptosis-resistant state from chronic stimulation.
While the studies presented here allow definite discrimination of vaccine-responding nonalloreactive T cells made possible through the use of murine transplant models and T cell receptor transgenic T cells, they must be interpreted with caution in terms of relevance to clinical alloBMT in humans. However, given the complexity of the allogeneic milieu and difficulty in developing in vitro systems using human cells or xenogeneic models, murine models represent an important tool in understanding the immunobiology of alloBMT. Although we did not use immunosuppressive medications, such as calcineurin inhibitors typically used after alloBMT in humans, post-transplant interventions would likely be most effective after these medications are discontinued. Furthermore, due to graft manipulation, alloBMT without or with limited use of immunosuppressive medications are being used with increasing frequency. Thus, we believe these results present important, and potentially clinically relevant, insights into the use of tumor-directed vaccine approaches after alloBMT.
Clinical studies have shown increased apoptosis of T cells after alloBMT(3, 4, 6, 10), but as with proliferation, these studies are not able to distinguish between T cells that recognized alloantigen and T cells with no reactivity against alloantigen. In a murine study by Alpdogan et al., rapidly proliferating T cells that acquire CD44hi expression with presumed specificity for alloantigen demonstrated increased apoptosis(8). In our study we show that known non-alloreactive CD8+ T cells, in particular non-dividing T cells with vaccine specificity, undergo increased apoptosis in the allogeneic environment. Interestingly, we did not see increased apoptosis of these T cells in absence of cognate antigen stimulation. However, the combination of cognate antigen plus the allogeneic environment resulted in marked apoptosis of CD8+ T cells. In contrast, our data indicates that vaccine specific CD4+ T cell apoptosis does not contribute to impaired responses during GVHD. While our results are consistent with a report that polyclonal CD8+ T cells from recipients of HLA-matched sibling alloBMT appear more sensitive to apoptosis than CD4+ T cells(3) they contrast with another report concluding the opposite in recipients of HLA-matched unrelated donors(4). In neither of these clinical studies and in prior murine studies, was the specificity of the T cells analyzed for apoptosis known and could have contained alloreactive T cells, non-alloreactive T cells or both. Nonetheless, the studies presented here, where T cells with known specificity for non-alloantigen were evaluated, demonstrate that the impact of GVHD on CD8+ T cell apoptosis does not require recognition of alloantigen by the vaccine-responding cells. Our results also suggest that administering a vaccine with pro-survival cytokine such as IL-7 or IL-15 may decrease CD8+ T cell apoptosis further(40, 41), particularly of nonalloreactive T cells such as those used in our alloBMT model.
There are a multitude of studies demonstrating that FasL, perforin and TRAIL can all contribute to the pathophysiology of GVHD(3, 5, 27-39). In the majority of these studies, these pathways were implicated in the effector mechanisms by which alloreactive T cells mediate tissue injury. In our model we were specifically interested in the role these molecules play in the deletion of non-alloreactive T cells responding to a vaccine. Alpdogon et al. demonstrated that apoptosis of rapidly proliferating cells resulted in impaired lymphocyte recovery via a mechanism that does not require Fas or TRAIL on donor bone marrow(8). It must be noted that these studies examined effects on polyclonal T cell populations. Brochu et al. demonstrated that alloreactive T cells can cause bystander apoptosis of infused polyclonal non-alloreactive T cells via Fas-FasL(5). In the present study we used T cells with known antigen specificity to assess the mechanism of apoptosis in vaccine responding T cells induced by the allogeneic environment. Surprisingly, this appears to be mediated, at least in part, by perforin rather than FasL. The difference in the pathway involved may reflect either vaccine-induced changes in T cell susceptibility to Fas or to trafficking. Indeed, the most marked decline in apoptosis with perforin deficient DLI was noted in the lymph nodes rather than the spleen, but led to enhanced tumor protection. Consistent with our results, human regulatory T cells have been shown to cause apoptosis of autologous, activated T cells via perforin(44). The findings presented here suggest that inhibition of the perforin pathway, such as by antibody blockade or drug inhibition, may augment CD8+ T cell responses to a DC vaccine.
Strategies to enhance the efficacy and potency of the GVL effect are clearly needed. Our data demonstrates that, while vaccines represent an attractive strategy, even mild GVHD impairs vaccine responses through a combination of diminished non-alloantigen-specific CD4+ and CD8+ T cell proliferation and increased CD8+ T cell apoptosis mediated through a perforin pathway. Taken together, successful tumor-directed immunotherapy post-alloBMT may require minimization of GVHD or specific targeting of relevant pathways to improve proliferation and prevent apoptosis of tumor-specific T cells. These findings have important implications for the optimal design of clinical trials involving antigen-specific T cells and/or vaccines to selectively enhance GVL.
Supplementary Material
Acknowledgements
We thank Crystal Mackall, Rimas Orentas, Paul Sondel and Manish Patankar for critically reviewing data and providing helpful feedback.
Footnotes
This work was supported by the intramural program at the National Institutes of Health (T.J.F.), Hyundai Hope on Wheels (C.M.C.), and the Midwest Athletes Against Childhood Cancer Fund (C.M.C.). M.G. is a scholar of Fond de la Recherche du Quebec.
References
- 1.Crooks GM, Weinberg K, Mackall C. Immune Reconstitution: From Stem Cells to Lymphocytes. Biology of Blood and Marrow Transplantation. 2006;12(1)(Supplement 1):42–6. doi: 10.1016/j.bbmt.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Dulude G, Roy D-C, Perreault C. The Effect of Graft-versus-Host Disease on T Cell Production and Homeostasis. J Exp Med. 1999 Apr 19;189(8):1329–42. doi: 10.1084/jem.189.8.1329. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebib NC, Deas O, Rouleau M, Durrbach A, Charpentier B, Beaujean F, et al. Peripheral Blood T Cells Generated After Allogeneic Bone Marrow Transplantation: Lower Levels of Bcl-2 Protein and Enhanced Sensitivity to Spontaneous and CD95-Mediated Apoptosis In Vitro. Abrogation of the Apoptotic Phenotype Coincides With the Recovery of Normal Naive/Primed T-Cell Profiles. Blood. 1999 Sep 1;94(5):1803–13. 1999. [PubMed] [Google Scholar]
- 4.Lin M-T, Tseng L-H, Frangoul H, Gooley T, Pei J, Barsoukov A, et al. Increased apoptosis of peripheral blood T cells following allogeneic hematopoietic cell transplantation. Blood. 2000 Jun 15;95(12):3832–9. 2000. [PubMed] [Google Scholar]
- 5.Brochu S, Rioux-Masse B, Roy J, Roy D-C, Perreault C. Massive Activation-Induced Cell Death of Alloreactive T Cells With Apoptosis of Bystander Postthymic T Cells Prevents Immune Reconstitution in Mice With Graft-Versus-Host Disease. Blood. 1999 Jul 15;94(2):390–400. 1999. [PubMed] [Google Scholar]
- 6.Brugnoni D, Airo P, Pennacchio M, Carella G, Malagoli A, Ugazio AG, et al. Immune reconstitution after bone marrow transplantation for combined immunodeficiencies: down-modulation of Bcl-2 and high expression of CD95/Fas account for increased susceptibility to spontaneous and activation-induced lymphocyte cell death. Bone marrow transplantation. [Research Support, Non-U.S. Gov't] 1999 Mar;23(5):451–7. doi: 10.1038/sj.bmt.1701608. [DOI] [PubMed] [Google Scholar]
- 7.Gorski J, Chen X, Gendelman M, Yassai M, Krueger A, Tivol E, et al. Homeostatic expansion and repertoire regeneration of donor T cells during graft versus host disease is constrained by the host environment. Blood. 2007 Jun 15;109(12):5502–10. doi: 10.1182/blood-2006-12-061713. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpdogan SO, Lu SX, Patel N, McGoldrick S, Suh D, Budak-Alpdogan T, et al. Rapidly proliferating CD44hi peripheral T cells undergo apoptosis and delay posttransplantation T-cell reconstitution after allogeneic bone marrow transplantation. Blood. 2008 Dec 1;112(12):4755–64. doi: 10.1182/blood-2008-02-142737. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beatty GL, Smith JS, Reshef R, Patel KP, Colligon TA, Vance BA, et al. Functional Unresponsiveness and Replicative Senescence of Myeloid Leukemia Antigen-specific CD8+ T Cells After Allogeneic Stem Cell Transplantation. Clin Cancer Res. 2009 Aug 1;15(15):4944–53. doi: 10.1158/1078-0432.CCR-08-3332. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg AD, Margolick JB, Beltz LA, Donnenberg VS, Rinaldo CR., Jr Apoptosis parallels lymphopoiesis in bone marrow transplantation and HIV disease. Research in Immunology. 1995;146(1):11–21. doi: 10.1016/0923-2494(96)80236-7. [DOI] [PubMed] [Google Scholar]
- 11.Perales M-A, Diab A, Cohen AD, Huggins DW, Guevara-Patino JA, Hubbard VM, et al. DNA Immunization against Tissue-Restricted Antigens Enhances Tumor Immunity after Allogeneic Hemopoietic Stem Cell Transplantation. J Immunol. 2006 Sep 15;177(6):4159–67. doi: 10.4049/jimmunol.177.6.4159. 2006. [DOI] [PubMed] [Google Scholar]
- 12.Capitini CM, Herby S, Milliron M, Anver MR, Mackall CL, Fry TJ. Bone marrow deficient in IFN-{gamma} signaling selectively reverses GVHD-associated immunosuppression and enhances a tumor-specific GVT effect. Blood. 2009 May 14;113(20):5002–9. doi: 10.1182/blood-2008-11-187385. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson LD, Jr., Savary CA, Mullen CA. Immunization of allogeneic bone marrow transplant recipients with tumor cell vaccines enhances graft-versus-tumor activity without exacerbating graft-versus-host disease. Blood. 2000 Apr 1;95(7):2426–33. 2000. [PubMed] [Google Scholar]
- 14.Teshima T, Mach N, Hill GR, Pan L, Gillessen S, Dranoff G, et al. Tumor Cell Vaccine Elicits Potent Antitumor Immunity after Allogeneic T-Cell-depleted Bone Marrow Transplantation. Cancer Res. 2001 Jan 1;61(1):162–71. 2001. [PubMed] [Google Scholar]
- 15.Anderson LD, Jr., Mori S, Mann S, Savary CA, Mullen CA. Pretransplant Tumor Antigen-specific Immunization of Allogeneic Bone Marrow Transplant Donors Enhances Graft-versus-Tumor Activity without Exacerbation of Graft-versus-Host Disease. Cancer Res. 2000 Oct 1;60(20):5797–802. 2000. [PubMed] [Google Scholar]
- 16.Moyer JS, Maine G, Mule JJ. Early Vaccination with Tumor Lysate-Pulsed Dendritic Cells after Allogeneic Bone Marrow Transplantation Has Antitumor Effects. Biology of Blood and Marrow Transplantation. 2006;12(10):1010–9. doi: 10.1016/j.bbmt.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Falkenburg JHF, Heslop HE, Barrett AJ. T Cell Therapy in Allogeneic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2008;14(1, Supplement 1):136–41. doi: 10.1016/j.bbmt.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzo T, Hess Michelini R, Basso V, Ricupito A, Chai J-G, Simpson E, et al. Concurrent Allorecognition Has a Limited Impact on Posttransplant Vaccination. The Journal of Immunology. 2011 Feb 1;186(3):1361–8. doi: 10.4049/jimmunol.1002030. 2011. [DOI] [PubMed] [Google Scholar]
- 19.Jenq RR, King CG, Volk C, Suh D, Smith OM, Rao UK, et al. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009 Feb 12;113(7):1574–80. doi: 10.1182/blood-2008-05-155697. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8+ T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3(9):844–51. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 21.Lantz O, Grandjean I, Matzinger P, Di Santo JP. [gamma] chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1(1):54–8. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 22.Capitini CM, Davis JPE, Larabee SM, Herby S, Nasholm NM, Fry TJ. Extracorporeal Photopheresis Attenuates Murine Graft-versus-Host Disease via Bone Marrow Derived Interleukin-10 and Preserves Responses to Dendritic Cell Vaccination. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(6):790–9. doi: 10.1016/j.bbmt.2010.12.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10(2):149–57. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005 May 1;115(5):1177–87. doi: 10.1172/JCI23134. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Diez A, Joncker NT, Choi K, Chan WFN, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007 Jun 15;109(12):5346–54. doi: 10.1182/blood-2006-10-051318. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the Frequency of Alloreactive T Cells In Vivo: New Answers to an Old Question. J Immunol. 2001 Jan 15;166(2):973–81. doi: 10.4049/jimmunol.166.2.973. 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Chong S, Lee J, Kim S, Min Y, Hahn J, et al. Difference in the expression of Fas/Fas-ligand and the lymphocyte subset reconstitution according to the occurrence of acute GVHD. Bone Marrow Transplantation. 1997;20(10):883–8. doi: 10.1038/sj.bmt.1700986. [DOI] [PubMed] [Google Scholar]
- 28.Via C, Nguyen P, Shustov A, Drappa J, Elkon K. A major role for the Fas pathway in acute graft-versus-host disease. The Journal of Immunology. 1996 Dec 15;157(12):5387–93. 1996. [PubMed] [Google Scholar]
- 29.Shustov A, Nguyen P, Finkelman F, Elkon KB, Via CS. Differential Expression of Fas and Fas Ligand in Acute and Chronic Graft-Versus-Host Disease: Up-Regulation of Fas and Fas Ligand Requires CD8+ T Cell Activation and IFN-[gamma] Production. The Journal of Immunology. 1998 Sep 15;161(6):2848–55. 1998. [PubMed] [Google Scholar]
- 30.Wasem C, Frutschi C, Arnold D, Vallan C, Lin T, Green DR, et al. Accumulation and Activation-Induced Release of Preformed Fas (CD95) Ligand During the Pathogenesis of Experimental Graft-Versus-Host Disease. The Journal of Immunology. 2001 Sep 1;167(5):2936–41. doi: 10.4049/jimmunol.167.5.2936. 2001. [DOI] [PubMed] [Google Scholar]
- 31.Schmaltz C, Alpdogan O, Horndasch KJ, Muriglan SJ, Kappel BJ, Teshima T, et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood. 2001 May 1;97(9):2886–95. doi: 10.1182/blood.v97.9.2886. 2001. [DOI] [PubMed] [Google Scholar]
- 32.Baker M, Riley R, Podack E, Levy R. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proceedings of the National Academy of Sciences. 1997 Feb 18;94(4):1366–71. doi: 10.1073/pnas.94.4.1366. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker M, Altman N, Podack E, Levy R. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med. 1996;183(6):2645–56. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun M, Lowin B, French L, Acha-Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med. 1996;183(2):657–61. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda Y, Levy RB, Reddy P, Liu C, Clouthier SG, Teshima T, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005 Mar 1;105(5):2023–7. doi: 10.1182/blood-2004-08-3036. 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hattori K, Hirano T, Miyajima H, Yamakawa N, Tateno M, Oshimi K, et al. Differential Effects of Anti-Fas Ligand and Anti-Tumor Necrosis Factor Antibodies on Acute Graft-Versus-Host Disease Pathologies. Blood. 1998 Jun 1;91(11):4051–5. 1998. [PubMed] [Google Scholar]
- 37.Tsukada N, Kobata T, Aizawa Y, Yagita H, Okumura K. Graft-Versus-Leukemia Effect and Graft-Versus-Host Disease Can Be Differentiated by Cytotoxic Mechanisms in a Murine Model of Allogeneic Bone Marrow Transplantation. Blood. 1999 Apr 15;93(8):2738–47. 1999. [PubMed] [Google Scholar]
- 38.Graubert TA, DiPersio JF, Russell JH, Ley TJ. Perforin/granzyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. The Journal of Clinical Investigation. 1997;100(4):904–11. doi: 10.1172/JCI119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blazar B, Taylor P, Vallera D. CD4+ and CD8+ T cells each can utilize a perforin-dependent pathway to mediate lethal graft-versus-host disease in major histocompatibility complex-disparate recipients. Transplantation. 1997;64(4):571–6. doi: 10.1097/00007890-199708270-00004. [DOI] [PubMed] [Google Scholar]
- 40.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005 Jan 15;105(2):865–73. doi: 10.1182/blood-2003-09-3344. 2005. [DOI] [PubMed] [Google Scholar]
- 41.Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. The Journal of Clinical Investigation. 2003;112(7):1095–107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capitini C, Fry T, Mackall C. Cytokines as adjuvants for vaccine and cellular therapies for cancer. American Journal of Immunology. 2009;5(3):65–83. doi: 10.3844/ajisp.2009.65.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002 Sep 18;100(7):2642–9. doi: 10.1182/blood-2002-04-1082. 2002. [DOI] [PubMed] [Google Scholar]
- 44.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T Regulatory Cells Can Use the Perforin Pathway to Cause Autologous Target Cell Death. Immunity. 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.