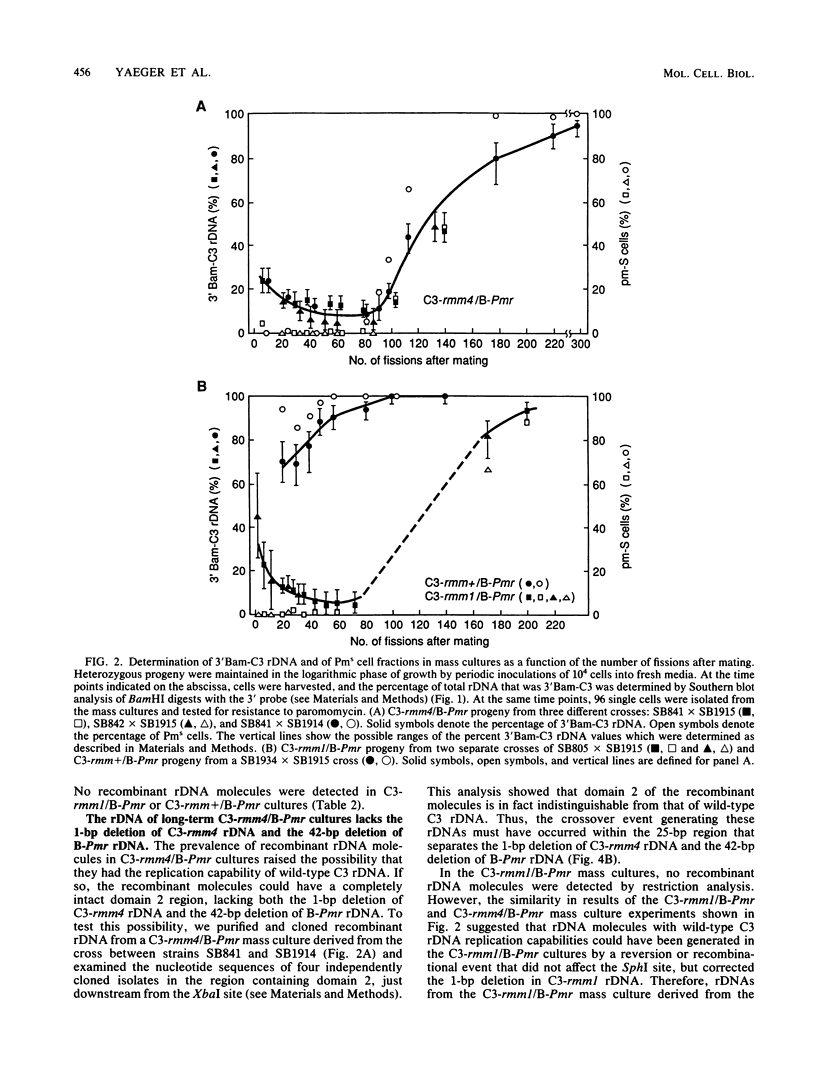
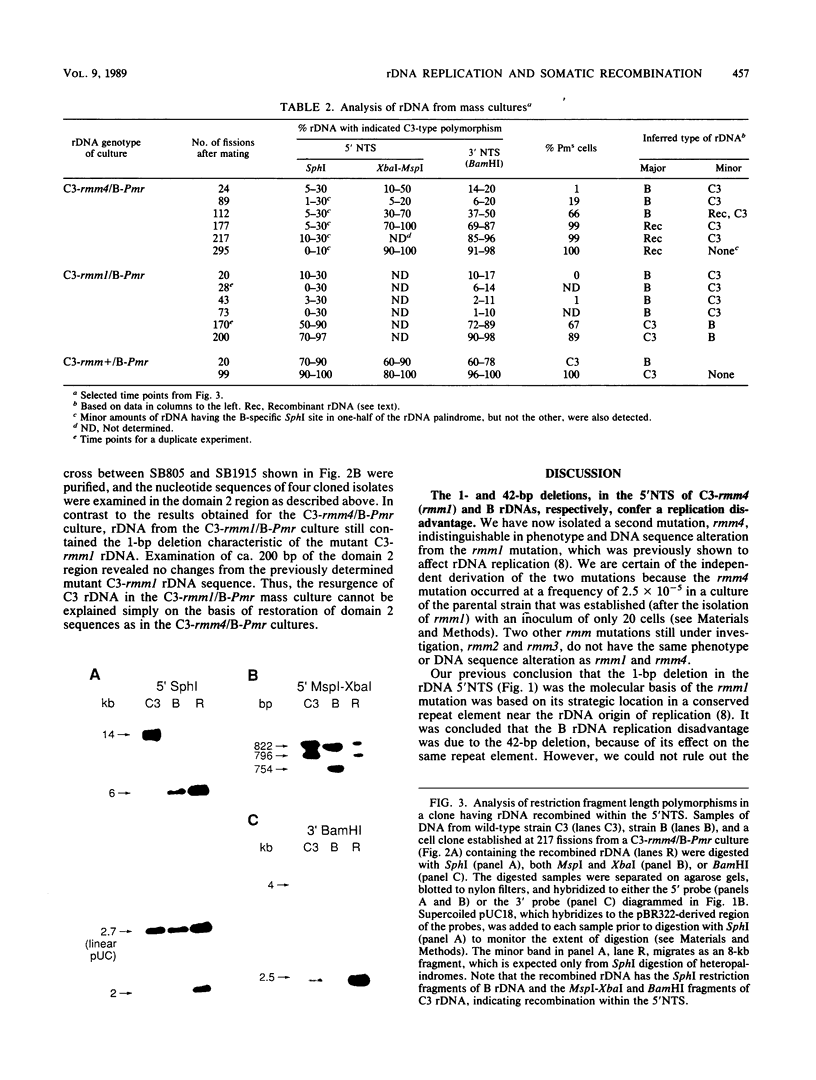
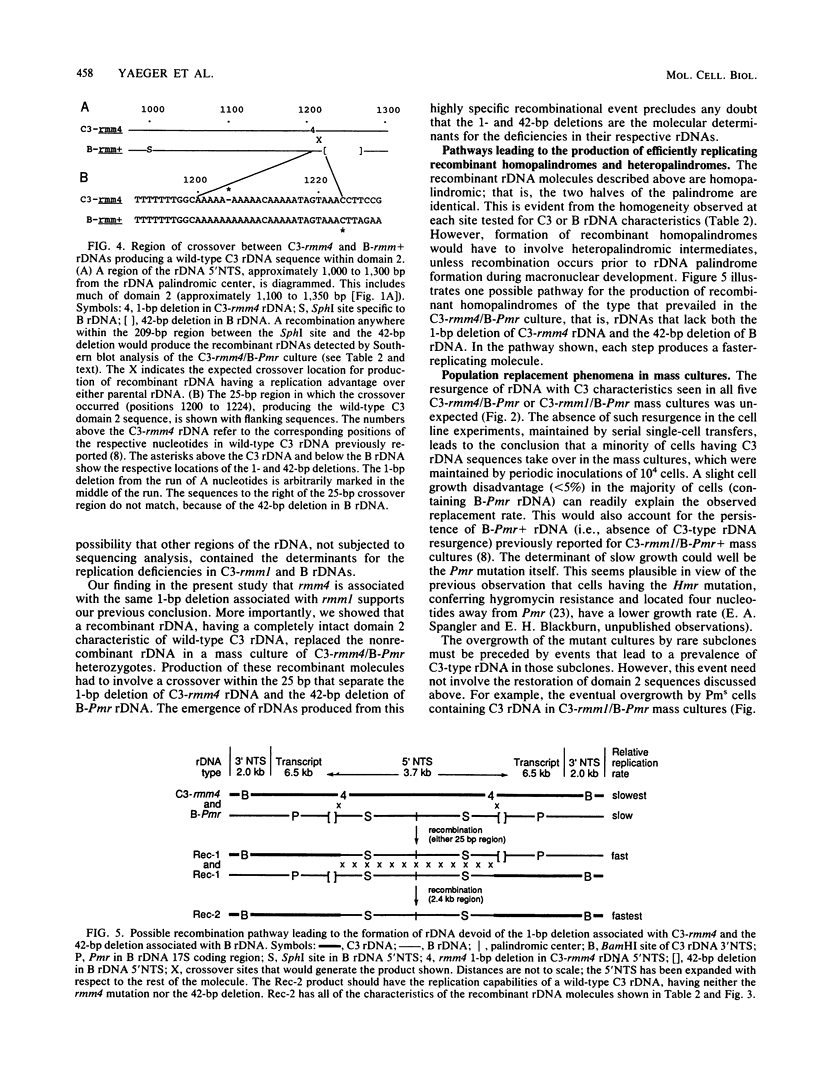
Abstract
The autonomously replicating rRNA genes (rDNA) in the somatic nucleus of Tetrahymena thermophila are maintained at a copy number of approximately 10(4) per nucleus. A mutant in which the replication properties of this molecule were altered was isolated and characterized. This mutation of inbred strain C3, named rmm4, was shown to have the same effect on rDNA replication and to be associated with the same 1-base-pair (bp) deletion as the previously reported, independently derived rmm1 mutation (D. L. Larson, E. H. Blackburn, P. C. Yaeger, and E. Orias, Cell 47:229-240, 1986). The rDNA of inbred strain B, which is at a replicational disadvantage compared with wild-type C3 rDNA, has a 42-bp deletion. This deletion is separated by 25 bp from the 1-bp deletion of rmm4 or rmm1. Southern blot analysis and DNA sequencing revealed that during prolonged vegetative divisions of C3-rmm4/B-rmm heterozygotes, somatic recombination produced rDNAs lacking both the rmm4-associated deletion and the 42-bp deletion. In somatic nuclei in which this rare recombinational event had occurred, all 10(4) copies of nonrecombinant rDNA were eventually replaced by the recombinant rDNA. The results prove that each of the two deletions is the genetic determinant of the observed replication disadvantage. We propose that the analysis of somatically recombinant rDNAs can be used as a general method in locating other mutations which affect rDNA propagation in T. thermophilia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns P. J., Katzen A. L., Martin L., Blackburn E. H. A drug-resistant mutation in the ribosomal DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1985 May;82(9):2844–2846. doi: 10.1073/pnas.82.9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B. C., Brussard T. B., Bruns P. J. Induced resistance to 6-methylpurine and cycloheximide in tetrahymena. I. Germ line mutants of T. thermophila. Genetics. 1978 Aug;89(4):695–702. doi: 10.1093/genetics/89.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challoner P. B., Amin A. A., Pearlman R. E., Blackburn E. H. Conserved arrangements of repeated DNA sequences in nontranscribed spacers of ciliate ribosomal RNA genes: evidence for molecular coevolution. Nucleic Acids Res. 1985 Apr 11;13(7):2661–2680. doi: 10.1093/nar/13.7.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din N., Engberg J. Extrachromosomal ribosomal RNA genes in Tetrahymena: structure and evolution. J Mol Biol. 1979 Nov 5;134(3):555–574. doi: 10.1016/0022-2836(79)90367-x. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Løvlie A., Haller B. L., Orias E. Molecular evidence for somatic recombination in the ribosomal DNA of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5156–5160. doi: 10.1073/pnas.85.14.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles E. G., Cunningham K., Jain R. Structure of the Tetrahymena pyriformis rRNA gene. Nucleotide sequence of the transcription termination region. J Biol Chem. 1981 Dec 25;256(24):12857–12860. [PubMed] [Google Scholar]
- Orias E., Bruns P. J. Induction and isolation of mutants in Tetrahymena. Methods Cell Biol. 1976;13:247–282. [PubMed] [Google Scholar]
- Orias E., Hamilton E. P. Cytogamy: An Inducible, Alternate Pathway of Conjugation in TETRAHYMENA THERMOPHILA. Genetics. 1979 Apr;91(4):657–671. doi: 10.1093/genetics/91.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palen T. E., Cech T. R. Chromatin structure at the replication origins and transcription-initiation regions of the ribosomal RNA genes of Tetrahymena. Cell. 1984 Apr;36(4):933–942. doi: 10.1016/0092-8674(84)90043-6. [DOI] [PubMed] [Google Scholar]
- Pan W. C., Orias E., Flacks M., Blackburn E. H. Allele-specific, selective amplification of a ribosomal RNA gene in Tetrahymena thermophila. Cell. 1982 Mar;28(3):595–604. doi: 10.1016/0092-8674(82)90214-8. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Zeff R. A., Frankel W., Rajan T. V. Mitotic recombination between homologous chromosomes generates H-2 somatic cell variants in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1634–1637. doi: 10.1073/pnas.84.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. T., Jr, Orias E. A cycloheximide-resistant mutant of Tetrahymena pyriformis. Exp Cell Res. 1973 Oct;81(2):312–316. doi: 10.1016/0014-4827(73)90520-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]