Abstract
Introduction
The objective of this study was to determine if a synthetic bone substitute would provide results similar to bone from osteoporotic femoral heads during in vitro testing with orthopaedic implants. If the synthetic material could produce results similar to those of the osteoporotic bone, it could reduce or eliminate the need for testing of implants on bone.
Methods
Pushout studies were performed with the dynamic hip screw (DHS) and the DHS Blade in both cadaveric femoral heads and artificial bone substitutes in the form of polyurethane foam blocks of different density. The pushout studies were performed as a means of comparing the force displacement curves produced by each implant within each material.
Results
The results demonstrated that test material with a density of 0.16 g/cm3 (block A) produced qualitatively similar force displacement curves for the DHS and qualitatively and quantitatively similar force displacement curves for the DHS Blade, whereas the test material with a density of 0.08 g/cm3 (block B) did not produce results that were predictive of those recorded within the osteoporotic cadaveric femoral heads.
Conclusion
This study demonstrates that synthetic material with a density of 0.16 g/cm3 can provide a good substitute for cadaveric osteoporotic femoral heads in the testing of implants. However we do recognise that no synthetic material can be considered as a definitive substitute for bone, therefore studies performed with artificial bone substrates may need to be validated by further testing with a small bone sample in order to produce conclusive results.
Keywords: Intertrochanteric fracture, Biomechanical study, Artificial bone substitute, DHS, DHS Blade, Osteoporotic Bone
Article focus
This study attempted to determine if readily available commercial artificial bone substrates produce results similar to those from osteoporotic cadaveric femoral heads in the testing of orthopaedic implants, by testing an artificial bone substrate suggested in the literature to demonstrate mechanical properties in the range of osteoporotic cancellous bone
Key messages
Artificial bone substrate with a density of 0.16 g/cm3 produces similar results to those that were observed with the osteoporotic cadaveric femoral heads, with similar force displacement curves and peak forces. Therefore this material can be considered as a good substitute for osteoporotic bone when testing orthopaedic implants
Although the artificial bone substrate with a density of 0.16 g/cm3 may be a good substitute for bone, we do recognise that studies conducted with it may need to be validated by further tests with cadaveric bone to produce indisputable results. However, by conducting the initial aspect of a study with the 0.16 g/cm3 material, the samples of cadaveric bone required may be greatly reduced
Strengths and limitations
One of the strengths of this study is that it provides evidence that the artificial bone substrate tested produces results that are consistent with those achieved with a cadaveric bone sample
Literature on the simulation of osteoporotic bone for mechanical testing is sparse and this study may offer guidance to researchers planning biomechanical studies using osteoporotic bone
One of the limitations of this study was in the sourcing of the femoral heads, as it proved difficult to get sufficient numbers of suitable osteoporotic femoral heads, due to a variety of reasons
Introduction
Biomechanical testing of implants plays a vital role in the evaluation of any new implant technology.1 Obtaining fresh disease-free cadaveric bone to be used in mechanical testing of orthopaedic implants is difficult and can be extremely expensive.2 Another problem is that cadaveric specimens are not uniform, resulting in the inclusion of specimens with vastly heterogeneous bone quality and strength.3-5 Large variations have been noted in the shape and material properties of cadaveric bone, with sds in excess of 100%.2 Ranges in the age and degree of osteoporosis of cadaver specimens can also partially account for the variability in mechanical properties.4,6 Therefore, this variability in the geometric and material properties of cadaveric specimens often requires prohibitively large sample sizes in order to detect statistically significant differences in implant performance.
With constraints regarding availability, handling and reproducibility of cadaveric specimens, bone surrogate models have been introduced for mechanical testing of fracture fixation implants.3,4,7,8 These specimens, such as Sawbones composite bones (Malmö, Sweden) exhibit known mechanical characteristics with minimal variation, allowing statistically valid comparisons to be made with much smaller sample sizes. It is widely accepted that the performance of fracture fixation, including mechanisms of failure, differ between strong and weak bone.9-11 Several studies have confirmed that current bone surrogates possess mechanical properties adequate to evaluate the performance of implants in normal bone.3,4,7,8 The mechanical properties of composite femora have been shown to fall well within the range for cadaveric specimens, with no significant differences being detected between the synthetic femurs and cadaveric femurs.3 The inter-femur variability for the composite femurs was between 20 and 200 times lower than that for the cadaveric specimens, thus allowing smaller differences to be characterised as significant using the same sample size, with the use of composite femora.3
With the aim of providing a uniform test base, artificial bone substrates in the form of polyurethane foam blocks have also been developed.2 These materials can be sourced in different densities with varying associated predetermined mechanical properties. These materials are manufactured to provide consistent and uniform -materials with properties in the range of human cancellous bone.
The objective of this study was to determine if a synthetic bone substitute could provide results similar to those from bone from cadaveric osteoporotic femoral heads when testing orthopaedic implants. The implants tested for the purpose of this study were the dynamic hip screw (DHS) and the DHS Blade (both Synthes GmbH, Zuchwil, Switzerland), which are used in the treatment of intertrochanteric fractures.
Materials and Methods
Ethical approval for this study was obtained from the -ethics committee within the hospital and the university.
The lag screw elements of the DHS screw and the DHS Blade were chosen as the test implants, as these are inserted into osteoporotic femoral heads during the fixation of intertrochanteric fractures. A factor in choosing these devices was that a supply of osteoporotic femoral heads should be available as these can be obtained after hemiarthroplasty for intracapsular fracture of the femoral neck.
Polyurethane foam blocks (Sawbones) were chosen to be the artificial bone substrates. These blocks were 180 mm in length, 130 mm in width and 40 mm in depth. Blocks of two different densities were used (0.16 g/cm3 (block A) and 0.08 g/cm3 (block B)), as these were two densities described as having properties in the range of osteoporotic bone.12 Patel et al12 concluded that the material with a density of 0.08 g/cm3 was more brittle than osteoporotic bone and that the 0.16 g/cm3 material was a good alternative for in vitro testing of osteoporotic bone. The mechanical properties of both these materials are documented in Table I.
Table I.
Mechanical properties of the polyurethane test blocks
| Compressive | Tensile | Shear | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Block | Density (g/cm3) | Strength (MPa) | Modulus (MPa) | Strength (MPa) | Modulus (MPa) | Strength (MPa) | Modulus (MPa) | ||
| A | 0.16 | 2.2 | 58 | 2.1 | 86 | 1.6 | 19 | ||
| B | 0.08 | 0.60 | 16 | 1.0 | 32 | 0.59 | 7.1 | ||
The femoral heads used in our study were harvested from patients who had undergone a hemiarthroplasty at Midwestern Regional Hospital, Limerick, for a fracture of the femoral neck and were classified as osteoporotic on that basis. The 12 femoral heads were taken from 12 patients (nine female and three male) with a mean age at operation of 75 years (sd 9.66). Protocol for tissue -storage was followed after harvesting the femoral heads and they were stored in a bone bank at -80° until required for testing. The femoral heads also underwent dual--emission X-ray absorptiometry (DEXA) scanning prior to testing, in order to determine their bone mineral density (BMD, g/cm2). An equal number of femoral heads were initially obtained for testing with each implant group and were assigned to each implant after DEXA scanning, so that an equal number of femoral heads of similar density would be tested with each implant. However, difficulties encountered during testing and the limited supply of specimens of bone resulted in unequal numbers of femoral heads in each group at the end of testing. Some femo-ral heads being damaged at time of extraction and were therefore unsuitable for testing, and on other occasions problems were encountered when securing the femoral heads in the customised testing rig. If a problem was encountered that was considered to affect the results of testing, the femoral head was discarded.
Pushout studies were chosen as the experimental protocol for this study as they are a good representation of the most common mode of failure with these implants in clinical practice, which is ‘cut-out’ or migration of the implants through the femoral heads until failure.13 This has been documented in the literature with previous studies using pushout tests to investigate implant performance in relation to the treatment of intertrochanteric fractures.14-16 Pushout studies involve pushing each implant through the test material and recording the corresponding force-displacement curves produced.
Pushout studies were performed in the osteoporotic femoral heads and in the polyurethane blocks A and B. With the cadaveric femoral heads, both implants were inserted as per clinical practice, with the femoral heads being reamed and tapped to the correct depth for the DHS and the DHS Blades being tapped into the correct position. They were both placed within the bone to a target tip apex distance of 25 mm.
All the polyurethane test blocks were 40 mm in depth and each implant was inserted to a depth of 30 mm. For the DHS each test block was reamed and tapped to 30 mm with the DHS inserted to the same depth. The DHS blade was tapped into each test block to a depth of 30 mm as per manufacturers’ instructions.
In order to perform the pushout tests, the implants with either the synthetic bone blocks or the femoral heads were mounted on a Tinius Olsen testing machine (Tinius Olsen Ltd, Redhill, United Kingdom), as shown in Figure 1. Testing was standardised and conducted at a rate of 2 mm/min. Tests were stopped after either dramatic failure or when displacement > 7 mm was measured. Individual force displacement curves were recorded for each test. For comparative purposes, trendlines were created for each group tested by fitting a sixth-order polynomial curve to the force displacement curves for each group tested.
Fig. 1.

Photograph showing the experimental set-up for pushout testing of the implant in a synthetic bone construct.
Results
The results from testing using the DHS implant are shown in Table II. The mean peak forces reached in block A and in the femoral heads were similar (1035 N (sd 46) and 1377 N (sd 406.5), respectively), while the mean peak force reached in block B was much lower (305 N (sd 28)). The force displacement curves for all three materials tested with the DHS reached their peak forces at similar mean displacements of 1.48 mm (sd 0.12), 1.2 mm (sd 0.14) and 1.5 mm (sd 0.41) for block A, block B and the femoral heads, respectively. The force displacement curves for the three materials is shown in Figure 2a. The osteoporotic cadaveric femoral heads and block A produce similar curves, whereas block B produces a curve depicting substantially lower values than those achieved with the cadaveric femoral heads.
Figs. 2a - 2b.
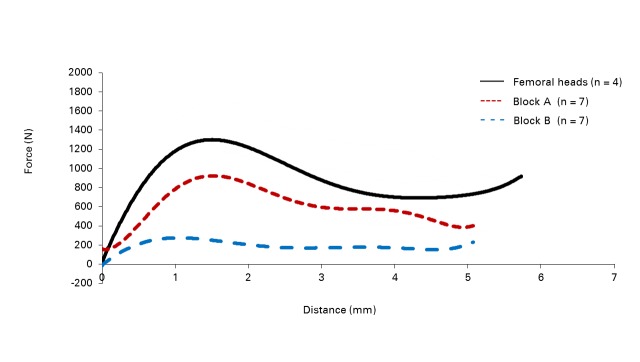
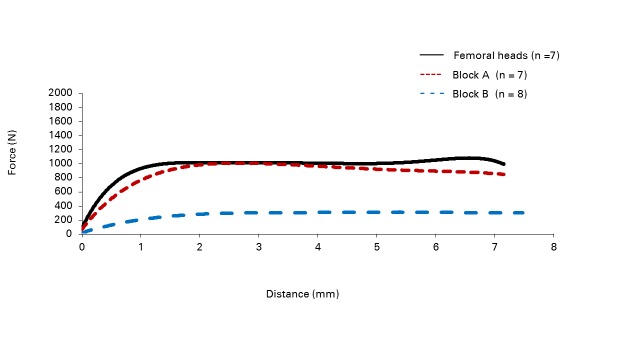
Force–displacement curves for the pushout tests involving a) the dynamic hip screw (DHS) and b) the DHS Blade in cadaveric femoral heads and -synthetic bone constructs with densities of 0.16 g/cm3 (block A) and 0.08 g/cm3 (block B).
Table II.
Peak forces and distance to peak force for the dynamic hip screw (DHS) and DHS Blade in test blocks A and B and in the femoral heads
| Mean (sd) peak force (N) | Mean (sd) distance to peak force (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Test material | Density (g/cm3) | Mean (sd) BMD* (g/cm2) | DHS | DHS Blade | DHS | DHS Blade | |
| Test blocks | |||||||
| A (n = 7) | 0.16 | - | 1035 (46) | 1020 (14) | 1.48 (0.12) | 3.17 (0.63) | |
| B (n = 7) | 0.08 | - | 305.6 (28) | 297 (16) | 1.2 (0.14) | 3.19 (0.52) | |
| Femoral heads | |||||||
| DHS (n = 4) | - | 0.912 (0.15) | 1377 (406.6) | - | 1.5 (0.41) | - | |
| DHS Blade (n = 8) | - | 0.784 (0.18) | - | 1302 (513) | - | 5.89 (2.14) | |
* BMD, bone mineral density (as measured on dual-emission X-ray absorptiometry)
The results from testing using the DHS Blade are shown in Table II. The mean peak forces reached in block A and in the cadaveric femoral heads were again similar (1020 N (sd 14) and 1302 N (sd 512.96), respectively), and the mean peak force achieved by block B was again much lower, at 297 N (sd 16). However, as shown by the force displacement curves in Figure 2b, the block A and block B test materials reached their peak forces at mean displacements of 3.17 mm (sd 0.63) and 3.19 mm (sd 0.52), respectively, while the cadaveric femoral heads reached their peak forces at a mean of 5.89 mm (sd 2.14). The force displacement curves for the osteoporotic femoral heads and block A can be seen to be very similar, with block B providing a much shallower curve (Fig. 2b).
Discussion
The objective of this study was to determine if a synthetic bone substitute could provide results similar to those from cadaveric osteoporotic femoral heads when testing orthopaedic implants. The results suggest that block A, with a density of 0.16 g/cm3, provides a good alternative test material to osteoporotic cadaveric femoral heads, as it produced qualitatively similar force displacement curves for the DHS (Fig. 2a) and qualitatively and quantitatively similar force displacement curves for the DHS Blade (Fig. 2b). This same is true for the DHS Blade, although it reaches its peak force at a different point with the polyurethane test materials (means of 3.17 mm (sd 0.63) for block A and 3.19 mm (sd 0.52) for block B) than with the cadaveric femoral heads (mean of 5.89 mm (sd 2.14)). This may be due to the fact that peak force is not well defined with the DHS Blade, as the force displacement curve reaches a plateau early on in the curve and then continues along this plateau until failure. Where the peak force occurs along this curve can be therefore influenced by a small variation in the resistance of the test material.
Block B produced lower results than those recorded with the cadaveric femoral heads (Fig. 2). It is recognised that osteoporotic bone does not have one absolute value, but instead exists within a spectrum of values. However, the results achieved with block B were consistently below those achieved with the cadaveric femoral heads. This material is therefore a less suitable substitute for osteoporotic bone than the higher density block A (0.16 g/cm3). The most important finding in this study is that in using these artificial test materials, both implants produced force displacement curves that had a similar pattern of failure as those produced with the femoral heads. As the values of the forces reached before failure within block A were similar to those achieved within the cadaveric femoral heads, it proved to be a good substitute.
Another point of discussion is that it is recognised that patients who suffer an intertrochanteric fracture often are more osteoporotic than those who suffer an intracapsular fracture.17 As all the femoral heads that were harvested for this study were collected after hemiarthroplasty for intracapsular fractures, it is possible that the femoral heads that these implants (the DHS and DHS Blade) will be used in will be more osteoporotic than those used in this study. So the forces achieved with these theoretically more osteoporotic femoral heads may even more closely resemble those that were achieved with block A.
The number of femoral heads used in this study was relatively small and it is worth mentioning that it was not attempted to statistically prove this association between the block A test material and the cadaveric femoral heads. It is widely recognised that large interbone material property variations exist and as the mechanical properties of the cadaveric femoral heads also vary according to their bone mineral density, a large number of femoral heads may be required for any results to approach statistical significance. Therefore this would be outside the capabilities of this study.
Patel et al12 had previously investigated whether low--density polyurethane foams are suitable for mimicking human osteoporotic cancellous bone. However, their study used quasi-static compression tests on polyurethane foam cylinders of different densities (0.09 g/cm3, 0.16 g/cm3 and 0.32 g/cm3) in order to determine the Young’s modulus, yield strength and energy absorbed to yield. The results from these tests with the synthetic polyurethane foam were then compared with the results of another study by Li and Aspden,18 which investigated the material properties of -cancellous bone from patients with osteoporosis. The comparison revealed that polyurethane foam of 0.09 g/cm3 was much weaker than osteoporotic bone, but that poly-urethane foam of 0.16 g/cm3 demonstrated compressive Young’s modulus and yield strength values similar to the osteoporotic bone that was tested in compression.12
Further work has also been carried out on comparing synthetic bone models with cadaveric bone, such as in studies by Cristofolini et al3 and McNamara et al.19 These studies concentrated on comparing the mechanical properties of cadaveric whole femurs against composite femurs, whereas our study focused on proving that the artificial bone substrate tested was a good substitute for osteoporotic bone in cadaveric femoral heads by comparing the pattern of failure of the implants tested within them. We are also aware that there may not be universal agreement about the use of artificial materials as a substitute for cadaveric bone. Schoenfeld et al20 looked at the pullout strength and load to failure properties of self--tapping cortical screws in synthetic and cadaveric environments representative of healthy and osteoporotic bone, and found that although trends may be similar, screw performance in synthetic models was markedly different from that in cadavers. However, we believe we have demonstrated that block A (density 0.16 g/cm3) can be substituted for osteoporotic cancellous bone and that the results produced with it provide a good basis for further testing.
Conclusion
This study demonstrates that the 0.16 g/cm3 synthetic material can provide a good substitute for osteoporotic bone within cadaveric femoral heads when testing orthopaedic implants as the force displacement curves produced are qualitatively and quantitatively -similar to those produced within the osteoporotic cadaveric bone. We do recognise however, that even though this 0.16 g/cm3 material is a good substitute for this osteo-porotic bone, no synthetic material can be considered as a definitive substitute for cadaveric osteoporotic bone as large interbone material property variations exist with cadaveric bone, therefore studies performed with artificial bone substrates may need to be validated by further testing with a small bone sample in order to produce conclusive results.
This artificial bone substrate may then have a definite and valuable role in designing, planning and initiating studies which may still require a small number of bone samples to be tested in order to produce definitive results.
Funding Statement
None declared
Footnotes
The authors would like to acknowledge the staff at the University of Limerick and the Midwestern Regional Hospital, Limerick, in particular those involved in the DEXA scanning and Radiology units, for their assistance in this study.
Author contributions:F. O’Neill: Writing of manuscript, Main author, Data collection
F. Condon: Clinical supervision
B. Lenehan: Writing of manuscript
C. Coffey: Assistance in facilitation of study
T. McGloughlin: Support and guidance during study
M. Walsh: Supervision, Data collection
ICMJE Conflict of Interest:None declared
References
- 1.Choueka J, Koval KJ, Kummer FJ, Crawford G, Zuckerman JD. Biomechanical comparison of the sliding hip screw and the dome plunger: effects of material and fixation design. J Bone Joint Surg [Br] 1995;77-B:277–283 [PubMed] [Google Scholar]
- 2.Szivek JA. Synthetic materials and structures used as models for bone. In: An YH, Draughn RA, eds. Mechanical testing of bone and the bone-implant interface. Boca Raton: CRC Press, 1999:159–175.
- 3.Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech 1996;29:525–535 [DOI] [PubMed] [Google Scholar]
- 4.Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech 2001;34:773–771 [DOI] [PubMed] [Google Scholar]
- 5.Marti A, Fankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma 2001;15:482–487 [DOI] [PubMed] [Google Scholar]
- 6.Bolliger Neto R, Rossi JD, Leivas TP. Experimental determination of bone cortex holding power of orthopedic screw. Rev Hosp Clin Fac Med Sao Paulo 1999;54:181–186 [DOI] [PubMed] [Google Scholar]
- 7.Agneskirchner JD, Freiling D, Hurschler C, Lobenhoffer P. Primary stability of four different implants for opening wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 2006;14:291–300 [DOI] [PubMed] [Google Scholar]
- 8.Peindl RD, Zura RD, Vincent A, et al. Unstable proximal extraarticular tibia fractures: a biomechanical evaluation of four methods of fixation. J Orthop Trauma 2004;18:540–545 [DOI] [PubMed] [Google Scholar]
- 9.Battula S, Schoenfeld A, Vrabec G, Njus GO. Experimental evaluation of the holding power/stiffness of the self-tapping bone screws in normal and osteoporotic bone material. Clin Biomech (Bristol, Avon) 2006;21:533–537 [DOI] [PubMed] [Google Scholar]
- 10.Gardner MJ, Demetrakopoulos D, Shindle MK, Griffith MH, Lane JM. Osteoporosis and skeletal fractures. HSS J 2006;2:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seebeck J, Goldhahn J, Morlock MM, Schneider E. Mechanical behavior of screws in normal and osteoporotic bone. Osteoporos Int 2005;16(Suppl 2):S107–S111 [DOI] [PubMed] [Google Scholar]
- 12.Patel PS, Shepherd DE, Hukins DW. Compressive properties of commercially available polyurethane foams as mechanical models for osteoporotic human cancellous bone. BMC Musculoskelet Disord 2008;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgaertner MR, Curtin SL, Lindskog DM, Keggi JM. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg [Am] 1995;77-A:1058–1064 [DOI] [PubMed] [Google Scholar]
- 14.Richards RH, Evans G, Egan J, Shearer JR. The AO dynamic hip screw and the Pugh sliding nail in femoral head fixation. J Bone Joint Surg [Br] 1990;72-B:794–796 [DOI] [PubMed] [Google Scholar]
- 15.Rosenblum SF, Zuckerman JD, Kummer FJ, Tam BS. A biomechanical evaluation of the Gamma nail. J Bone Joint Surg [Br] 1992;74-B:352–357 [DOI] [PubMed] [Google Scholar]
- 16.Aminian A, Gao F, Fedoriw WW, et al. Vertically oriented femoral neck fractures: mechanical analysis of four fixation techniques. J Orthop Trauma 2007;21:544–548 [DOI] [PubMed] [Google Scholar]
- 17.Koval KJ, Zuckerman JD. Epidemiology and mechanism of injury. In: Hip fractures: a practical guide to management. New York: Springer-Verlag, 2000:9–25.
- 18.Li B, Aspden RM. Material properties of bone from the femoral neck and calcar femoral of patients with osteoporosis or osteoarthritis. Osteoporos Int 1997;7:450–456 [DOI] [PubMed] [Google Scholar]
- 19.McNamara BP, Cristofolini L, Toni A, Taylor D. Evaluation of experimental and finite element models of synthetic and cadaveric femora for pre-clinical design-analysis. Clin Mater 1994;17:131–140 [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld AJ, Battula S, Sahai V, et al. Pullout strength and load to failure properties of self-tapping cortical screws in synthetic and cadaveric environments representative of healthy and osteoporotic bone. J Trauma 2008;64:1302–1307 [DOI] [PubMed] [Google Scholar]


