Summary
Replication Licensing Factor (RLF) is a multiprotein complex involved in ensuring that chromosomal DNA replicates only once in a single cell cycle. It comprises two components, termed RLF-M and RLF-B. Purified RLF-M consists of a mixture of complexes containing all 6 members of the MCM/P1 family of minichromosome maintenance proteins. The precise composition of these different complexes and their contribution to RLF-M activity has been unclear. Here we show that in Xenopus extracts, MCM/P1 proteins mainly form hetero-hexamers containing each of the 6 proteins. This hetero-hexamer is readily split into subcomplexes, whose interactions and subunit composition we characterise in detail. We show for the first time an ordered multi-step assembly pathway by which the hetero-hexamer can be reformed from the subcomplexes. Importantly, this novel pathway is essential for DNA replication, as only the full hetero-hexamer can bind productively to chromatin and provide RLF-M activity.
Keywords: DNA Replication, Licensing Factor, MCM/P1, RLF-M, Xenopus, MCM2-7
Introduction
Proteins belonging to the MCM/P1 family play a central role in the control of chromosomal DNA replication in eukaryotes. Current information about the gene family suggests that it consists of 6 closely-related paralogues, termed MCM2, 3, 4, 5, 6, and 7, likely to be present in all eukaryotes (reviewed in 1,2), though developmentally-regulated variants may also exist (3). The founding members of the family, MCM2, 3 and 5, were identified in the yeast Saccharomyces cerevisiae in a screen for “Mini-Chromosome Maintenance” (MCM) mutants that showed origin-specific defects in the initiation of DNA replication (4). The first vertebrate member of the family to be identified was the P1 protein, a human Mcm3 orthologue that co-purified with DNA polymerase α (5). Since the MCM screen identified other genes such as MCMs 1, 10, 16, 17, 21 and 22 (6-10) that are not related to MCM2-7, we have suggested the name “MCM/P1” to denote the family (1). MCM/P1 family members have now been identified in a wide range of eukaryotes, including Drosophila, Xenopus and humans (reviewed in 2). Experiments in Drosophila, S .cerevisiae and Schizosaccharomyces pombe suggest that each member of the MCM/P1 family is individually required for chromosomal DNA replication. Each member of the MCM/P1 family contains a putative nucleotide binding motif (11), and a complex of Mcms 4, 6 and 7 has been shown to have weak helicase activity (12). However, the precise role of the MCM/P1 proteins in DNA replication remains unclear.
Replication Licensing Factor (RLF) was originally identified as an essential DNA replication activity that “licenses” replication origins during late mitosis or early interphase for a single initiation event, thus ensuring precise duplication of the genome (13, reviewed in 14). Fractionation of RLF activity from Xenopus egg extracts showed that it consisted of two essential components termed RLF-M and RLF-B (15,16). RLF-M consists of complexes containing all 6 members of the Xenopus MCM/P1 family (15,17-19). For licensing to occur, RLF-M and RLF-B must be incubated with chromatin that also contains bound ORC (the origin recognition complex) and Cdc6 (16,20-22). This results in the MCM/P1 proteins being assembled onto chromatin. Once licensing has occurred, ORC and Cdc6 are no longer required for DNA replication (23,24). Licensing occurs only in late mitosis and G1, but the MCM/P1 proteins are displaced from DNA as it replicates, thus ensuring that re-replication does not occur (15,17-19,25-29).
The six MCM/P1 proteins associate to form different high molecular weight complexes. The largest of these complexes, with an apparent molecular weight of ~600 kDa on gel filtration, could represent a hetero-hexamer of each of the 6 MCM/P1 proteins. The quantitative co-association of all 6 MCM/P1 family members described in Xenopus and S. pombe (18,19,27,30) is consistent with this idea. However, other complexes of a comparable size are observed that do not contain all 6 members of the MCM/P1 family and therefore cannot be simple hetero-hexamers (e.g. 12,19,31). In mammalian cells, MCM/P1 proteins are not found as hetero-hexamers, but instead mainly form smaller complexes (32-35). These include a hetero-dimer containing Mcms 3 and 5 (18,32,33,36,37) and subcomplexes containing Mcms 4, 6 and 7, plus or minus Mcm2 (31,32,34-36,38,39). The relationship between these various complexes and their precise composition has not been determined. More importantly, it has also been unclear which of these complexes is responsible for providing the essential RLF-M activity required for DNA replication.
In this paper we characterise in detail the subcomplexes of MCM/P1 proteins that are formed on chromatographic fractionation of Xenopus egg extracts. We show that MCM/P1 proteins in Xenopus egg extract exist mainly as hetero-hexamers which readily dissociate into a number of distinct subcomplexes. We characterise the composition of these subcomplexes, and show that they can be re-assembled into hetero-hexamers by a specific assembly pathway. Importantly, we show that the hetero-hexameric complex alone provides RLF-M activity.
Experimental Procedures
Preparation of egg extracts and chromatin
Xenopus egg extracts were prepared as described (40,41). 6-DMAP-treated extracts were prepared by supplementing metaphase-arrested extracts with 3 mM 6-dimethylaminopurine (6-DMAP) which blocks the activation of licensing factor that normally occurs on exit from metaphase (40,42). Interphase extracts for gel filtration were prepared by releasing metaphase extracts into interphase by the addition of 0.3 mM CaCl2. Xenopus sperm nuclei were obtained by manual dissection of testes, and were demembranated with lysolecithin as described (41). Unlicensed “6-DMAP chromatin” for the licensing assay was prepared by incubating sperm nuclei in 6-DMAP extract for 12 minutes and then isolating it through a sucrose cushion as previously described (15,41).
Antibodies
Polyclonal antibodies against XMcm proteins were raised in rabbits. Amino acids 348-892 for XMcm2, 438-625 for XMcm3, 584-859 for XMcm4, 405-821 for XMcm6 and 400-720 for XMcm7 were cloned into pQZ8 or pQE70 vectors to provide a His-tag, expressed in E. coli, affinity purified on Ni-NTA resin (Qiagen) and used as antigens. Anti-HsMcm5 antibody was kindly provided by Dr. R. Knippers. Previous analysis of the Xenopus MCM/P1 family (17,18,27,43,44) gave the following apparent and calculated molecular weights: XMcm2, 112 kDa (apparent),100.1, kDa (calculated); XMcm3, 100 kDa (apparent), 90.3 kDa (calculated); XMcm4,100 kDa (apparent), 97.1 kDa (calculated); XMcm5, 92 kDa (apparent), 84.5 kDa (calculated); XMcm6, 100 kDa (apparent), 92.6 kDa (calculated); XMcm7, 90 kDa (apparent), 81.8 kDa (calculated). Each antibody recognised only a single band on a Western blot of a size consistent with this. Consistent with previous reports XMcm7 was observed to migrate as a doublet (44). The specificity of the antibodies was confirmed as described (5).
For immunoprecipitation, 2 volumes of serum were incubated with 1 volume of pre-swollen protein A Sepharose (Amersham-Pharmacia) for 30 min at 23°C as described (41); beads were then washed extensively in LFB1 (40 mM Hepes-KOH, 20 mM K2HPO4/KH2PO4, 2 mM MgCl2, 1 mM EGTA, 2mM DTT, 10% sucrose, 1 μg/ml leupeptin, pepstatin and aprotinin, 0.5 mM PMSF, pH 8.0) before use. Immunodepletion of Xenopus egg extracts was performed as described (41). Precipitated proteins were separated on 7.5% SDS-polyacrylamide gels, blotted onto Immobilon-P (Millipore) and detected using Enhanced Chemi-Luminescence (Amersham-Pharmacia). Quantification of Western blots and Coomassie stained gels was performed with ImageGauge software (Fuji).
Chromatography
All chromatographic procedures were performed at 4°C. A 4 - 9.5% polyethylene glycol precipitate of “Licensing Factor Extract” (LFE) was prepared as described (15,41). The pellet was resuspended in 1 volume (with respect to undiluted egg extract) of LFB1 and 4 ml were applied to 10 ml High Performance Q Sepharose in an HR10/10 column (Amersham-Pharmacia) equilibrated in LFB1. Protein was eluted with a linear gradient to LFB1/400 (LFB1 supplemented with 400 mM KCl) over 10 column volumes with a flow rate of 0.5 ml/min; 2ml fractions were collected. Fractions containing separate peaks of XMcm (Q1, Q2 or Q3) were pooled, precipitated with 17.5% PEG and resuspended in LFB1 at a concentration of 20x relative to neat extract. 200 μl of each peak was applied to 2 × 1 ml Hi-trap Heparin columns connected in series (Amersham-Pharmacia) and eluted with a linear gradient to LFB1/350 over 5 column volumes at 1 ml/min; 0.5 ml fractions were collected. Peak fractions were pooled, precipitated with 17.5% PEG, resuspended in LFB1 to a concentration of 20x relative to neat extract and frozen in liquid nitrogen. An additional purification step on phenyl Sepharose was performed as originally described for the whole RLF-M complex (15,41).
Gel filtration and glycerol gradient sedimentation
Gel filtration was performed on a 2.4 ml Superose 6 column, using a SMART system (Amersham-Pharmacia) at 30 μl/min. After centrifugation at 10,000 × g for 15 min, 10 μl samples were loaded onto the column, which had been pre-equilibrated in LFB1 supplemented with KCl; 50 μl fractions were collected. Prior to gel filtration, interphase extract was diluted 1:1 with LFB1 supplemented with appropriate amounts of KCl. Gel filtration of partly purified MCM/P1 protein sub-complexes was performed in LFB1. Gel filtration was calibrated with thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa) and aldolase (158 kDa). For glycerol gradient sedimentation, 200 μl molecular weight marker proteins (thyroglobulin, apoferritin, catalase) or LFE diluted 1:1 with H20 were layered on top of pre-formed 4ml 10-30% glycerol gradients and centrifuged in an SW 60 rotor (Beckman) at 58,000 rpm for 12 hours at 4°C; 300 μl fractions were collected.
Licensing assay and Chromatin Binding
Licensing assays were performed as described (15,41). Frozen PEG-cut RLF-B was diluted to 1 × (relative to neat extract) in LFB1 containing 2.5 mM Mg-ATP. In the “licensing reaction”, 1μl of 1 × RLF-B was incubated for 15 mins at 23°C with 0.3 μl 6-DMAP chromatin and 1 μl fractions being tested for RLF-M activity. 5.7 μl 6-DMAP-treated extract containing α32P-dATP was then added and incubated for a further 90 min at 23°C (the “replication reaction”); total DNA synthesised was measured by TCA precipitation.
To assess chromatin binding, the “licensing reaction” was scaled up 5-fold, and after 15 min was diluted with 1 ml NIB (50 mM KCl; 50 mM Hepes KOH, pH 7.6; 5 mM MgCl2; 5 mM EGTA; 2 mM ß-mercaptoethanol; 0.5 mM spermidine; 0.15 mM spermine; 1 μg/ml each leupeptin, aprotinin and pepstatin) supplemented with 0.1% NP-40. Chromatin was then centrifuged through a 15% sucrose cushion made up in the same buffer for 5 min at 700 × g in a swing-out rotor at 4°C. Where the assay was performed on sperm nuclei, i.e. to assay the binding of XMcm protein sub-complexes to the chromatin or in the first step of the two-step licensing reaction, 1 μl of demembranated sperm nuclei (at 400 ng DNA/μl) was incubated with 10 μl XMcm-depleted extract supplemented with ATP, 1 μl crude RLF-B and 3 μl of either Q1, Q3, QH3a or RLF-M for 30 min. The chromatin was isolated in NIB as described above, and resuspended in NIB at 80 ng DNA/μl.
Results
MCM/P1 proteins form hetero-hexameric complexes in Xenopus egg extract
We first analyzed the molecular weight of the native MCM/P1 complexes in Xenopus egg extract. To obtain molecular weights that are not biased by shape, a combination of gel filtration (which overestimates the size of elongated molecules) and glycerol gradient sedimentation (which underestimates the size of elongated molecules) can be used (45). Most of the 6 MCM/P1 proteins migrated on gel filtration with an apparent molecular weight of ~600 kDa (Stokes radius ~77 Å; Fig 1A), consistent with previous reports (15,19). Previous attempts to estimate the size of this complex by glycerol gradient centrifugation had given an anomalously low figure of ~13.5S, either because the complex was an elongated molecule of ~400 kDa or because the complex was unstable on the glycerol gradients (19). To improve stability on the glycerol gradient, the salt concentration was lowered to 50 mM and the glycerol concentration was reduced. This resulted in a more homogeneous peak of MCM/P1 proteins at an apparent molecular weight of ~440 kDa (~17.5 S; Fig 1B), suggesting that the previous low value had been due to the instability of the complex. Using the formula of Siegel and Monty (45), the new values suggest a slightly elongated complex of ~568 kDa. This figure is consistent with the 547 kDa calculated for the combined molecular weights of all 6 Xenopus MCM/P1 proteins (see Experimental Procedures).
Figure 1.
MCM/P1 proteins in Xenopus egg extracts are predominantly in the form of hetero-hexamers. A, B, Crude Xenopus extracts were fractionated by gel filtration (A) or glycerol gradient sedimentation (B). Fractions were analysed by PAGE, immunoblotted and probed with all six anti-MCM/P1 antibodies. Molecular weight markers (in kDa) are shown above. C, Crude Xenopus extract was immunoprecipitated with antibodies against XMcm3 or XMcm7. Samples were immunoblotted with all six anti-MCM/P1 antibodies: ‘start’ - starting extract; ‘s/n’ - unprecipitated proteins; ‘0.3M wash’ and ‘1M wash’ - proteins remaining bound to antibody after washing in 0.3 M and 1M KCl.
To determine whether this complex mainly represented a hetero-hexamer of all 6 proteins, crude Xenopus extract was immunoprecipitated with antibodies against XMcm3 or XMcm7. Consistent with previous studies (18,19,27,46), virtually all of the MCM/P1 proteins co-precipitated with either anti-XMcm3 or anti-XMcm7 antibodies, suggesting the presence of an XMcm(2-7) hetero-hexamer (Fig 1C). When the immunoprecipitates were washed in 0.3 M and 1 M salt, a separation of the MCM/P1 proteins was observed. XMcm5 remained associated with XMcm3 in 1M salt, whereas most of the XMcms 2, 4, and 6 and 7 were displaced. Conversely, XMcms 4 and 6 remained associated with the XMcm7 immunoprecipitate in 1 M salt, whilst all of XMcm 2 and most of XMcms 3 and 5 were removed. This suggests that stable subcomplexes containing different MCM/P1 proteins can be formed from the hexamers.
MCM/P1 protein complexes can be separated into distinct subcomplexes chromatographically
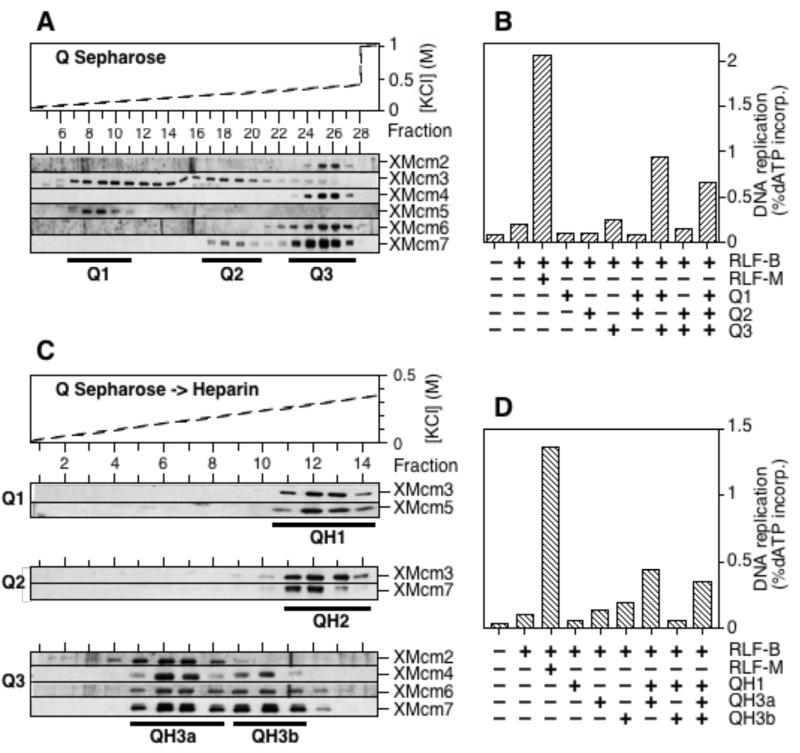
We next investigated whether the MCM/P1 subcomplexes could be resolved by chromatography. A crude fraction of interphase Xenopus extract containing all of the MCM/P1 proteins (15,19) was applied to a Q Sepharose column. No MCM/P1 proteins appeared in the flow-through or in the 1 M salt wash of the column. Upon elution with a shallow KCl gradient they separated into three distinct peaks, designated Q1, Q2 and Q3 (Fig. 2A). Q1 contained XMcms 3 and 5, Q2 contained XMcms 3 and 7, whilst Q3 contained XMcms 2, 4, 6 and 7. A small amount of XMcm3 trailed out of the Q1 peak unassociated with other MCM/P1 proteins. When used in a functional licensing assay, no individual fraction alone provided significant RLF-M activity (Fig 2B). However, a mixture of Q1 and Q3 could reconstitute RLF-M activity to ~50% of the input level. No significant stimulatory activity was found in the flow-through fraction or the 1 M salt wash of the column. Each of the 3 fractions was then applied to a heparin column and eluted with a KCl gradient (Fig. 2C). The Q1 fraction eluted as a single peak containing XMcms 3 and 5, designated QH1. The Q2 fraction eluted essentially as a single peak containing XMcms 3 and 7, designated QH2. The Q3 fraction separated into two peaks: the first peak, designated QH3a, contained XMcms 2, 4, 6 and 7, whilst the second peak, designated QH3b, contained only XMcms 4, 6 and 7; in addition some XMcm2 appeared in the flow-through. Fractions were again assayed for their ability to reconstitute RLF-M activity (Fig 2D). After the Q3 fraction had been split into the QH3a and QH3b fractions, only the QH3a fraction provided significant activity when mixed with QH1. These results mean that consistent with previous reports (18), combinations of complexes that provided significant quantities of RLF-M activity were also those that provided each of the 6 MCM/P1 proteins.
Figure 2.
Fractionation of MCM/P1 proteins by Q Sepharose and heparin chromatography. A, Crude RLF-M was applied to a Q Sepharose column, and eluted with a KCl concentration gradient. Individual fractions were immunoblotted with all six anti-MCM/P1 antibodies. B, Q1, Q2 and Q3 fractions from the Q Sepharose column were pooled as indicated and assayed for RLF-M activity. C, Q1, Q2 and Q3 fractions were applied to a heparin column and eluted with a KCl concentration gradient. Fractions were immunoblotted with relevant anti-MCM/P1 antibodies. D, QH1, QH3a and QH3b fractions from the heparin column were pooled as indicated and assayed for RLF-M activity.
We next investigated the composition of these subcomplexes by gel filtration and immunoprecipitation. On gel filtration of fraction Q1, both XMcms 3 and 5 co-migrated with an apparent molecular weight of ~230 kDa (Fig 3A). XMcm3 in this fraction sedimented on glycerol gradients with an apparent molecular weight of ~170 kDa (data not shown), suggesting a molecular weight of ~ 200 kDa. Immunoprecipitation of XMcm3 from the Q1 fraction co-precipitated all of the XMcm5 (Fig 3F), suggesting that the complex mainly consists of XMcm(3+5) hetero-dimers. Consistent with this, further purification of QH1 to apparent homogeneity by hydrophobic chromatography yielded approximately equal quantities of XMcm3 and 5 as judged by Coomassie staining (Fig 3E, lane 1). Densitometry of the bands gave the ratio XMcm3: XMcm5 as 1.0: 1.2. The slight excess of XMcm5 might possibly be due to the presence of some XMcm5 homo-dimer. Similar results were obtained with the Q2 fraction, where XMcms 3 and 7 co-migrated on gel filtration with an apparent molecular weight of ~230 kDa (Fig 3B). The majority of the XMcm7 was co-precipitated with anti-XMcm3 antibodies (Fig 3F), again suggesting a hetero-dimer, this time of XMcm(3+7).
Figure 3.
Analysis of MCM/P1 protein sub-complexes by gel filtration and immunoprecipitation. A-D, Q1 (A), Q2 (B), QH3a (C) and QH3b (D) were subjected to gel filtration. Fractions were immunoblotted with relevant anti-MCM/P1 antibodies. Molecular weight markers (in kDa) are shown above. E, Coomassie-stained gel: lane 1, QH1 after purification to homogeneity on phenyl Sepharose; lane 2, QH3a; lane 3, QH3b after purification to homogeneity on phenyl Sepharose. Molecular weight markers (in kDa) are shown to the left. F,G Q1 (F, left-hand panel) and Q2 (F, right-hand panel) were precipitated with anti-XMcm3 antibody whilst QH3a (G, left-hand panel) and QH3b (G, right-hand panel) were precipitated with anti-XMcm7 antibody. Each panel shows starting material, supernatant after the immunoprecipitation and the immunoprecipitate probed with relevant anti-MCM/P1 antibodies.
On gel filtration of the QH3a fraction, XMcms 2, 4, 6 and 7 co-migrated as a single complex with an apparent molecular weight of ~500 kDa (Fig 3C). Immunoprecipitation of XMcm7 from the QH3a fraction co-precipitated all of the XMcm2, XMcm4 and XMcm6 (Fig 3G), suggesting that they form a single complex. Coomassie staining of the QH3a fraction (Fig 3E, lane 2) showed that the complex is essentially pure at this stage, containing only XMcms 2, 4, 6 and 7. Densitometry of the Coomassie-stained bands separated further on a 6% gel gave the ratio of XMcm2: XMcm4: XMcm6: XMcm7 as 1.0: 1.1: 1.1: 1.1, suggesting that this complex is a tetramer of XMcm(2+4+6+7).
Gel filtration of the QH3b fraction showed XMcms 4, 6 and 7 co-migrating with an apparent molecular weight of ~600 kDa (Fig 3D). Immunoprecipitation of XMcm7 from the QH3b fraction co-precipitated all of the XMcm4 and XMcm6 (Fig 3G), suggesting that these proteins form a single complex. Further purification of QH3b to apparent homogeneity by hydrophobic chromatography yielded approximately equal quantities of XMcm4, 6 and 7 as judged by Coomassie staining (Fig 3E, lane 3). Using the QH3a fraction as a standard, quantitative Western blotting of the QH3b fraction gave the ratio of XMcm4: XMcm6: XMcm7 as 1.0: 1.0: 1.1. Together with the gel filtration and immunoprecipitation results this suggests that the complex largely comprises a XMcm(4+6+7)2 hexamer. However we cannot rule out the presence of minor forms that deviate slightly from this stoichiometry, such as XMcm(4+6+6+7+7+7).
Because the immunoprecipitation experiment shown in Fig 1C suggested that XMcm2 can be separated from XMcm7 by treatment with high salt, we performed gel filtration on the QH3a fraction in 1 M KCl (Fig 4A). Most of the XMcm2 was displaced from the XMcm(2+4+6+7) complex, whilst the XMcms 4, 6 and 7 that had separated from XMcm2 shifted from an apparent molecular weight of ~500 kDa (Fig 4A, fraction 5) up to an apparent molecular weight of ~600 kDa (Fig 4A, fraction 3), keeping an approximately constant stoichiometry. When this high molecular weight fraction was gel filtered again in low salt (Fig 4B), its migration at ~600 kDa was unchanged (XMcms 4, 6 and 7 peaking at fractions 3-4). This pattern was identical to the behaviour of the XMcm(4+6+7)2 hexamer (Fig 3D), suggesting that on removal of XMcm2 from XMcm(2+4+6+7), the XMcm(4+6+7)2 hexamer was reformed. Consistent with this interpretation, when XMcm7 immunoprecipitates were washed in 1M salt, XMcms 4 and 6 remained tightly associated (Fig 1C and data not shown). In contrast, the XMcm2 displaced from the XMcm(2+4+6+7) complex shifted down to an apparent molecular weight of ~270 kDa under high salt gel filtration (Fig 4A, fraction 9), and remained there when gel-filtered again in low salt (Fig 4D). Given that the starting material for the gel filtration was essentially purified XMcm(2+4+6+7) (Fig 3E, lane 2), this suggests that the ~ 270 kDa XMcm2 complex is likely to be an elongated homo-dimer of XMcm2. The QH3a that continued to migrate at its original position in 1 M KCl (Fig 4A, fraction 5) behaved similarly when gel filtered again in low salt (Fig 4C, XMcms 2, 4, 6 and 7 peaking at fractions 5-6), suggesting that it was persisting XMcm(2+4+6+7). Consistent with this, the XMcm2 in this fraction was co-precipitated with anti-XMcm7 antibodies, even in 1M salt (data not shown; see Fig 6B below).
Figure 4.
QH3a sub-complex can be separated further by 1 M KCl treatment. The QH3a fraction was gel filtered in buffer containing 1 M KCl. A, Fractions were immunoblotted with relevant antibodies. B, C, D, Aliquots of fractions 3 (B), 5 (C) and 9 (D) were transferred to a low salt buffer and gel filtered again in low salt. Fractions were immunoblotted with relevant anti-MCM/P1 antibodies. Molecular weight markers (in kDa) are shown above.
Figure 6.
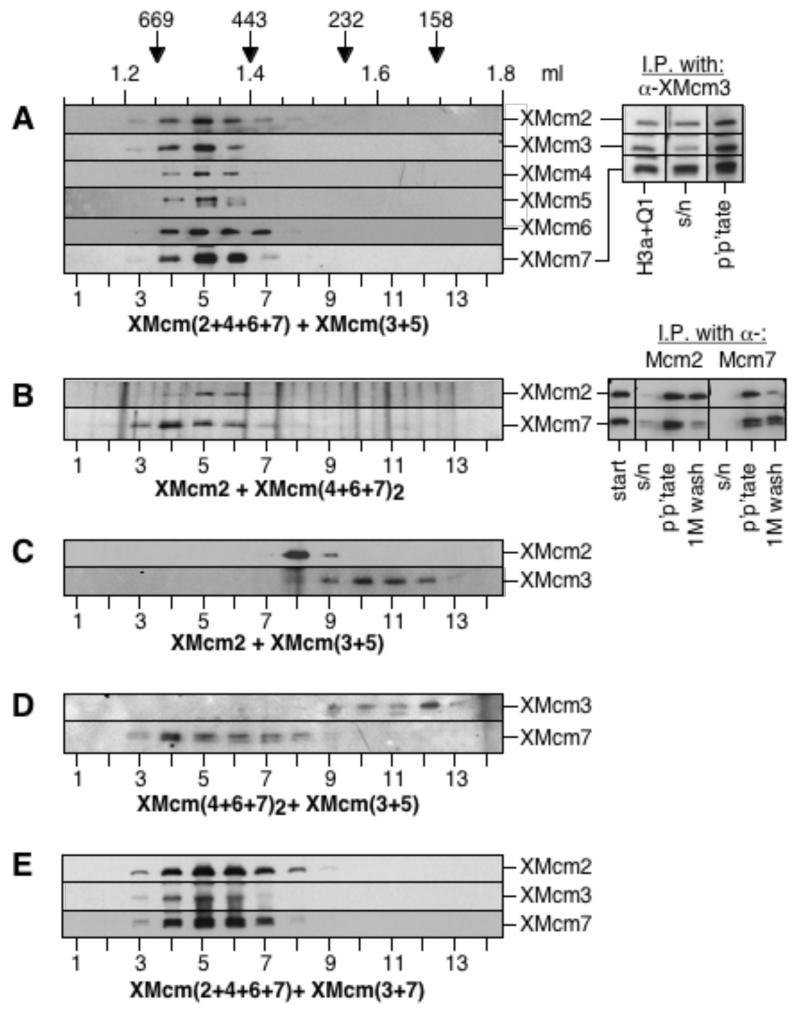
High molecular weight complexes can be recreated by mixing separated sub-complexes. Different sub-complexes were mixed together as follows: A, XMcm(2+4+6+7) and XMcm(3+5); B, XMcm2 and XMcm(4+6+7)2; C, XMcm2 and XMcm(3+5); D, XMcm(4+6+7)2 and XMcm(3+5); E, XMcm(2+4+6+7) and XMcm(3+7). They were gel filtered in low salt buffer and fractions were immunoblotted with relevant anti-MCM/P1 antibodies. For A, and B (right-hand panels), samples were also immunoprecipitated with antibodies as indicated; different lanes show starting fractions, supernatants, immunoprecipitates and immunoprecipitates washed in buffer containing 1M KCl.
Similar Subcomplexes are Obtained by Gel Filtration in High Salt
To determine whether these subcomplexes were products of a general disassembly pathway, or whether they were specific to our fractionation scheme, gel filtration of crude extract was performed in 0.3M or 1M KCl (Fig. 5). XMcms 3 and 5 completely dissociated from the high molecular weight complexes in 0.3M KCl (Fig 5A), migrating at ~250 kDa and consistent with the formation of the XMcm(3+5) dimer. Some XMcm7 also appeared at the same size, consistent with the XMcm(3+7) complex of fractions Q2. Significantly, after removal of XMcms 3 and 5 in 0.3 M salt, the migration of XMcms 2, 4, 6 and 7 was essentially unchanged, consistent with the formation of the XMcm(2+4+6+7) and XMcm(4+6+7)2 complexes. The presence of XMcm2 in the higher molecular weight fractions 3 and 4 may also suggest the formation of complexes containing more than one molecule of XMcm2. However, in 1M salt, most of the XMcm2 dissociated and migrated at about 270 kDa, whilst XMcms 4, 6 and 7 (with some residual XMcm2) migrated as expected of tetramers or hexamers, again consistent with the presence of XMcm(4+6+7)2 and XMcm(2+4+6+7) complexes. Some of the more slowly migrating XMcms 4, 6 and 7 seen in 1 M salt might also represent an XMcm(4+6+7) trimer. Formation of these subcomplexes is also consistent with the immunoprecipitation data (Fig 1C), which showed a tight interaction between XMcm 3 and 5 and between XMcms 4, 6, and 7, with XMcm2 forming a weaker interaction with the latter. In summary, we observe a similar, though not identical, set of complexes by chromatography and by high salt treatment, suggesting that these are components of a general disassembly pattern.
Figure 5.
Gel filtration of Xenopus egg extract in 0.3 M and 1M salt. Crude Xenopus egg extract was supplemented with KCl to final concentrations of 0.3 M (A) or 1 M (B) and was gel filtered in buffer containing these concentrations of KCl. Fractions were immunoblotted with all six anti-MCM/P1 antibodies. Molecular weight markers (in kDa) are shown above.
Hetero-hexameric complexes can be reformed from the subcomplexes
There are two possible explanations for the ability of the subcomplexes to reconstitute RLF-M activity when mixed together (Fig 2). One possibility is that the licensing activity defined as “RLF-M” (15,19) in fact comprises several subcomplexes that each act separately on chromatin. Alternatively, when the different subcomplexes are mixed they may reform the XMcm(2-7) hetero-hexamer, which alone is responsible for providing RLF-M activity. We therefore determined whether hetero-hexamers could re-assemble from mixtures of the subcomplexes (Fig. 6). When XMcm(3+5) was mixed with XMcm(2+4+6+7) and subjected to gel filtration, all 6 MCM/P1 proteins now appeared as a single peak with a molecular weight of 500 - 600 kDa, similar to the hetero-hexamer (Fig. 6A, left-hand panel). Re-constitution of the XMcm(2-7) hetero-hexamer was confirmed by the co-precipitation of XMcms 2 and 7 with anti-XMcm3 antibody (Fig. 6A, right-hand panel). We conclude that mixtures of subcomplexes capable of providing RLF-M activity are capable of forming hetero-hexamers.
We then mixed the XMcm2 and XMcm(4+6+7)2 complexes that had been obtained by separation of XMcm(2+4+6+7) in 1 M KCl, as shown in Fig 4. On mixing, the XMcm2 now migrated on gel filtration as expected as a component of the XMcm(2+4+6+7) complex (Fig 6B, left-hand panel). Consistent with this, XMcms 2 and 7 were co-precipitated from the mixture in low salt, but most of the XMcm2 was removed in 1 M salt (Fig 6B, right-hand panel). We next investigated whether the complexes could associate promiscuously or whether they only associate in a specific order. When XMcm2 was mixed with XMcm(3+5), or when XMcm(4+6+7)2 was mixed with XMcm(3+5), no complex formation was observed, either by gel filtration (Fig 6C and 6D) or by co-immunoprecipitation (data not shown). This suggests that there is an obligatory sequence to the assembly of the hetero-hexamer, involving first an interaction between XMcm2 and XMcm(4+6+7)2 to form XMcm(2+4+6+7), followed by an interaction between XMcm(2+4+6+7) and XMcm(3+5) to form the XMcm(2-7) hetero-hexamer.
Finally, we investigated whether the XMcm(3+7) dimer found the in the Q2 and QH2 fractions could interact with XMcm(2+4+6+7) in analogous manner to the XMcm(3+5) dimer. When XMcm(3+7) was mixed with XMcm(2+4+6+7) and then gel-filtered, all the XMcm3 and 7 shifted up to a hexameric position, consistent with the formation of an XMcm(2+3+4+6+7+7) hexamer (Fig 6E). This suggests that the XMcm(3+7) dimer is generated from XMcm(2+3+4+6+7+7) present in the extract which does not provide RLF-M activity (Fig 2).
Active RLF-M is a hetero-hexamer containing all 6 MCM/P1 proteins
We have shown that mixtures of XMcm(3+5) and XMcm(2+4+6+7) re-assemble into hetero-hexamers (Fig 6) and provide RLF-M activity (Fig 2). We next wanted to determine whether hetero-hexamer assembly must take place before interaction with chromatin, or whether RLF-M activity can be built up on chromatin from individual subcomplexes. Figure 7 shows that subcomplexes can bind separately to chromatin. Demembranated sperm nuclei were incubated in XMcm-depleted extract supplemented with combinations of Q1 (containing XMcm(3+5)) or Q3 (containing XMcm(2+4+6+7)). Chromatin was then isolated and immuno-blotted for XMcms 3 and 7 (Fig 7A). This showed that both groups of subcomplexes bound separately to the chromatin at levels comparable to native RLF-M.
Figure 7.
Separated sub-complexes bind to chromatin but the binding is not RLF-B dependent. A, Demembranated sperm nuclei were incubated for 30 mins in XMcm-depleted extract plus crude RLF-B and combinations of Q1, Q3, and crude RLF-M. Chromatin was then isolated in buffer containing 0.1% NP-40 and was immunoblotted for XMcm3 and XMcm7. B, 6-DMAP chromatin was incubated for 15 mins with combinations of XMcm(3+5), XMcm(2+4+6+7), crude RLF-M and crude RLF-B. Chromatin was then isolated in buffer containing 0.1% NP-40 and immunoblotted for XMcm3 and XMcm7.
Previous work has shown that unlicensed chromatin can be assembled in extracts treated with the kinase inhibitor 6-DMAP (40): subsequent licensing of this “6-DMAP chromatin” requires both RLF-B and RLF-M components of the licensing system (15,16). Figure 7B shows the effect of RLF-B on the ability of different MCM/P1 subcomplexes to be assembled onto 6-DMAP chromatin. The XMcm(2-7) complex in native RLF-M was only assembled onto chromatin in the presence of RLF-B (Fig 7B, lanes 7 and 8). In contrast, both XMcm(3+5) and XMcm(2+4+6+7) bound to chromatin in the absence of RLF-B (Fig 8B, lanes 4 and 5), whilst addition of RLF-B actually decreased their binding (Fig 7B, lanes 1 and 2). When XMcm(3+5) and XMcm(2+4+6+7) were mixed together, the quantity of XMcms 3 and 7 bound to chromatin in the presence of RLF-B increased (Fig 7B, lane 6), whilst in the absence of RLF-B it decreased (Fig 7B, lane 6). This is consistent with the reformation of the active XMcm(2-7) complex (Fig 6A), whose binding to chromatin is RLF-B-dependent. Taken as a whole, these results suggest that the chromatin binding of the separate complexes is illegitimate and cannot license the chromatin for replication.
Figure 8.
Sequential incubation of chromatin with individual subcomplexes. Demembranated sperm nuclei were first incubated in XMcm-depleted extract supplemented with crude RLF-B and combinations of XMcm(3+5) or XMcm(2+4+6+7) as indicated. Chromatin was isolated and incubated again in XMcm-depleted extract supplemented with fractions as indicated. Chromatin was then incubated in 6-DMAP extract containing α32P-dATP to assess the degree of licensing that had occurred.
To test this hypothesis, we performed a two-step licensing reaction (Fig 8). XMcm-depleted extract was first supplemented with either XMcm(3+5) or XMcm(2+4+6+7) or both together. Sperm chromatin was then incubated in these extracts, under conditions similar to those used in Fig 7A. After 15 min, chromatin was isolated and a second incubation with complementary subcomplexes was performed. Figure 8 shows that only chromatin incubated simultaneously with both XMcm(3+5) and XMcm(2+4+6+7) became licensed. Sequential treatment with the two individual subcomplexes gave only background levels of licensing. This suggests that the chromatin binding of the individual subcomplexes shown in Fig 7 is unproductive, and that the MCM/P1 proteins can only provide RLF-M activity when assembled into the XMcm(2-7) hetero-hexamer.
Discussion
Subunit composition of different MCM/P1 subcomplexes
In this paper we have characterised the composition and function of MCM/P1 complexes present in Xenopus egg extracts. Since similar complexes are found in a range of other organisms, our conclusions are likely to apply to all eukaryotes. Using a combination of gel filtration, glycerol gradient sedimentation and co-immunoprecipitation we show that all 6 Xenopus MCM/P1 proteins are present mainly in the form of XMcm(2-7) hetero-hexamers, consistent with previous reports (18,19,27,46). A small proportion (~10% of the total) appears to be in the form of an XMcm(2+3+4+6+7+7) hexamer. Previous results showing an anomalously slow sedimentation value (19,47) appear to be due to the instability of these complexes.
Fractionation of extracts by a number of different techniques readily generated a reproducible set of subcomplexes. We have characterised these complexes and provide evidence that they consist mainly of an XMcm(3+5) dimer, an XMcm(3+7) dimer, an XMcm2 dimer, an XMcm(2+4+6+7) tetramer and a XMcm(4+6+7)2 hexamer. These are the predominant combinations though minor variations in sub-complex composition may occur. For example a small proportion of XMcm3 was identified that was not associated with the other MCM/P1 proteins. Further, slight deviations of the relative abundance of XMcms 4, 6 and 7 in the QH3b fractions (which vary from preparation to preparation) may suggest the presence of complexes that deviate slightly from the 2: 2: 2 composition that we propose. However, these minor variations do not affect the major conclusions that we draw. Although this is the first comprehensive analysis to be performed, complexes consistent with our proposals have been observed in other eukaryotes. An Mcm(3+5) dimer has been identified as the major form of these two proteins in human cell extracts (32-35), whilst a tight association between Mcms 3 and 5 has been observed in S. cerevisiae (48) and S. pombe (37). In mammalian cell extracts, Mcms 2, 4, 6 and 7 are predominantly found associated with one another, but substantially free of Mcms 3 and 5 (12,32,34-36,38), consistent with our XMcm(2+4+6+7) complex. In mammalian extracts, further fractionation could separate Mcm2 from this complex, leaving Mcms 4, 6, and 7 migrating on gel filtration as an apparent hexamer (12,36), whilst in S. pombe, Mcm2 was shown to interact weakly with a complex containing Mcms 4 and 6 (39), consistent with the our generation of XMcm(4+6+7)2 from XMcm(2+4+6+7). Further, there is evidence of a hexameric complex lacking Mcm5 in Drosophila (31), consistent with our XMcm(4+6+7)2 complex. In S. pombe, Mcm2 was shown to interact weakly with a complex containing Mcms 4 and 6 (39). S. pombe Mcm2 was also shown to interact with itself in a two-hybrid screen (39), consistent with our proposed XMcm2 homo-dimer. The separation of MCM/P1 hetero-hexamers into specific subcomplexes therefore appears to have been highly conserved throughout evolution, suggesting that this has functional importance.
A Sequential Assembly Pathway for MCM/P1 Proteins
One obvious explanation for the presence of distinct subcomplexes is that they are intermediates in the assembly pathway for the hetero-hexamer. As summarised in Figure 9, we show here for the first time that the subcomplexes can combine to reform the hetero-hexamer, and that they do this only in a defined order. XMcms 4, 6 and 7 are first assembled into a stable hexamer, mainly comprising XMcm(4+6+7)2. XMcm2, probably in the form of a homo-dimer, can then associate with the XMcm(4+6+7)2 hexamer to form two XMcm(2+4+6+7) tetramers. A hetero-dimer of XMcm(3+5) can then interact with these complexes to form the XMcm(2-7) hetero-hexamer. A small proportion (~10%) of XMcms 3 and 7 present as an XMcm(3+7) dimer can also interact with the XMcm(2+4+6+7) tetramer to form a XMcm(2+3+4+6+7+7) hexamer which does not contribute to RLF-M activity. All the different subcomplexes are likely to be in equilibrium with one another, and the scheme shown in Figure 9 appears to be both an assembly and a disassembly pathway. Although there is no direct evidence for disassembly occurring in vivo, Kubota et al (1997) have described the preferential release of XMcms 3 and 5 from chromatin when replication forks are stalled with aphidicolin.
Figure 9.
Proposed model for the assembly of the MCM/P1 protein complexes. Cartoon showing assembly of the Mcm(2-7) hetero-hexamer (“RLF-M”) from Mcm(4+6+7)2, Mcm(2+2) and Mcm(3+5) via the Mcm(2+4+6+7) intermediate. Assembly of Mcm(2+3+4+6+7+7) from Mcm(2+4+6+7) and Mcm(3+7) is also shown. Large arrows show the forward reaction occurring in Xenopus egg extract.
The assembly of precursor complexes as intermediates in multi-protein complex assembly was first noted for haemoglobin, where β-globin can associate into homo-tetramers before being assembled into the active α2β2 tetramer (49). The assembly pathway for snRNP complexes has striking similarity to the one we propose here. The snRNP core proteins eventually form a hetero-heptamer (50) but first assemble into a hexamer of two identical trimers, one of which is replaced by two dimers of other core proteins (51). As with the snRNP core proteins, the close similarity between all members of the MCM/P1 family may necessitate a defined assembly pathway involving high molecular weight intermediates.
In Xenopus eggs, the equilibrium between the different subcomplexes appears to favour formation of the hetero-hexamer, since most of the MCM/P1 proteins in crude extract can be co-immunoprecipitated (18,19,27,46, and this paper). The hetero-hexamer may also predominate in the Drosophila early embryo and in S. pombe (30,31). In contrast, in mammalian cells (33,34,36) and in S. cerevisiae (48,52) the hetero-hexamer is apparently not abundant, with XMcm(2+4+6+7) and XMcm(3+5) being the predominant forms. The cause of this difference in relative abundance of the subcomplexes is unclear, but given the weak association between the subcomplexes and the ease with which the hetero-hexamer disassembles, additional co-factors may be required to maintain the hetero-hexamer. Indeed, there is evidence for hetero-hexamer assembly factors in Xenopus, as the reconstitution of RLF-M activity from subcomplexes can be enhanced by the addition of XMcm-depleted extract (Fig 8 and T.A.P., unpublished data). This hetero-hexamer assembly factor may plausibly be a chaperone-like protein that prevents illegitimate association between the subunits and favours formation of the hetero-hexamer. Given that only the hetero-hexamer can provide RLF-M licensing activity, regulation of such a hetero-hexamer assembly factor could provide a powerful way of regulating DNA replication, particularly in mammalian cells where hetero-hexamer assembly is apparently not favoured.
RLF-M activity can only be provided by the hetero-hexamer
We show here that only mixtures of subcomplexes capable of reforming the XMcm(2-7) hetero-hexamer are capable of providing RLF-M activity. This is consistent with immunoprecipitation studies in Xenopus (18) and genetic studies in yeast, showing that each of the 6 MCM/P1 proteins are essential for DNA replication (reviewed in 2). We have also addressed the question of whether hetero-hexamer formation is required prior to interaction with chromatin, or whether licensing can occur by subcomplexes binding separately to chromatin. Our results showed that although individual subcomplexes were capable of binding to chromatin, they could not contribute to RLF-M activity once they had bound. The essential licensing activity RLF-B (15,16) was required for the chromatin binding of the hetero-hexamer but not of the individual subcomplexes. Once individual subcomplexes had associated with chromatin, they could not be used for generation of RLF-M activity when re-incubated with complementary complexes. This suggests that only after the hetero-hexamer has been assembled in solution can the MCM/P1 proteins provide RLF-M activity and associate productively with chromatin.
The identification of RLF-M as a hetero-hexamer supports the idea that the MCM/P1 proteins function as DNA helicases, since a range of known DNA helicases form hexameric rings with the DNA passing through the centre (reviewed in 53). When MCM/P1 proteins purified from S. pombe were viewed by electron microscopy, they had a globular shape consistent with a ring structure (30). Each member of the MCM/P1 family has a putative ATPase domain reminiscent of those in known DNA helicases (11), whilst a purified Mcm(4+6+7)2 complex from mammalian cells has been reported to show weak helicase activity (12). Further, the structure of the Xenopus MCM/P1 proteins appears to change as a consequence of their being loaded onto chromatin during the licensing reaction: the free hetero-hexamer is highly salt-sensitive, but once licensing has occurred it interacts tightly with chromatin and resists elution with salt (24 and T.A.P, unpublished data). This is plausibly explained by the MCM/P1 proteins forming a ring around the DNA when licensing occurs, which could also explain why individual subcomplexes cannot bind productively to DNA. With the ability to re-assemble the active MCM/P1 hetero-hexamer, we are now in a position to test these ideas biochemically.
Acknowledgements
Thanks to Angus Lamond, Tom Owen-Hughes and Neil Perkins for comments on the manuscript. This work was supported by Cancer Research Campaign grant SP2385. TAP is a Darwin Trust Fellow.
References
- 1.Chong JP, Thommes P, Blow JJ. Trends Biochem. Sci. 1996;21:102–106. [PubMed] [Google Scholar]
- 2.Kearsey SE, Labib K. Biochim. Biophys. Acta. 1998;1398:113–136. doi: 10.1016/s0167-4781(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 3.Sible JC, Erikson E, Hendrickson M, Maller JL, Gautier J. Curr. Biol. 1998;8:347–350. doi: 10.1016/s0960-9822(98)70136-8. [DOI] [PubMed] [Google Scholar]
- 4.Maine GT, Sinha P, Tye BK. Genetics. 1984;106:365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thömmes P, Fett R, Schray B, Burkhart R, Barnes M, Kennedy C, Brown NC, Knippers R. Nucleic Acids Res. 1992;20:1069–1074. doi: 10.1093/nar/20.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passmore S, Maine GT, Elble R, Christ C, Tye BK. J. Mol. Biol. 1988;204:593–606. doi: 10.1016/0022-2836(88)90358-0. [DOI] [PubMed] [Google Scholar]
- 7.Merchant AM, Kawasaki Y, Chen Y, Lei M, Tye BK. Mol. Cell. Biol. 1997;17:3261–3271. doi: 10.1128/mcb.17.6.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanyal K, Ghosh SK, Sinha P. Mol. Gen. Genet. 1998;260:242–250. doi: 10.1007/s004380050892. [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Poddar A, Lohia A, Sinha P. Current Genetics. 1997;32:182–189. doi: 10.1007/s002940050264. [DOI] [PubMed] [Google Scholar]
- 10.Poddar A, Roy N, Sinha P. Mol. Microbiol. 1999;31:349–360. doi: 10.1046/j.1365-2958.1999.01179.x. [DOI] [PubMed] [Google Scholar]
- 11.Koonin EV. Nucleic Acids Res. 1993;21:2541–2547. doi: 10.1093/nar/21.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishimi Y. J. Biol. Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 13.Blow JJ, Laskey RA. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 14.Tada S, Blow JJ. Biol. Chem. 1998;379:941–949. [PMC free article] [PubMed] [Google Scholar]
- 15.Chong JP, Mahbubani HM, Khoo CY, Blow JJ. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- 16.Tada S, Chong JPJ, Mahbubani HM, Blow JJ. Curr. Biol. 1999;9:211–214. doi: 10.1016/s0960-9822(99)80092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Cell. 1995;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 18.Kubota Y, Mimura S, Nishimoto S, Masuda T, Nojima H, Takisawa H. EMBO J. 1997;16:3320–3331. doi: 10.1093/emboj/16.11.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thömmes P, Kubota Y, Takisawa H, Blow JJ. EMBO J. 1997;16:3312–3319. doi: 10.1093/emboj/16.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman TR, Carpenter PB, Dunphy WG. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 21.Rowles A, Chong JP, Brown L, Howell M, Evan GI, Blow JJ. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- 22.Romanowski P, Madine MA, Rowles A, Blow JJ, Laskey RA. Curr. Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- 23.Hua XH, Newport J. J. Cell Biol. 1998;140:271–281. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowles A, Tada S, Blow JJ. J. Cell Sci. 1999;112:2011–2018. doi: 10.1242/jcs.112.12.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura H, Nozaki N, Sugimoto K. EMBO J. 1994;13:4311–4320. doi: 10.1002/j.1460-2075.1994.tb06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todorov IT, Attaran A, Kearsey SE. J. Cell Biol. 1995;129:1433–1445. doi: 10.1083/jcb.129.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madine MA, Khoo CY, Mills AD, Laskey RA. Nature. 1995;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- 28.Krude T, Musahl C, Laskey RA, Knippers R. J. Cell Sci. 1996;109:309–318. doi: 10.1242/jcs.109.2.309. [DOI] [PubMed] [Google Scholar]
- 29.Su TT, O’Farrell PH. J. Cell Biol. 1997;139:13–21. doi: 10.1083/jcb.139.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi Y, Usukura J, Yanagida M. Genes Cells. 1997;2:467–479. doi: 10.1046/j.1365-2443.1997.1350333.x. [DOI] [PubMed] [Google Scholar]
- 31.Su TT, Feger G, O’Farrell PH. Mol. Biol. Cell. 1996;7:319–329. doi: 10.1091/mbc.7.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulte D, Richter A, Burkhart R, Musahl C, Knippers R. Eur. J. Biochem. 1996;235:144–151. doi: 10.1111/j.1432-1033.1996.00144.x. [DOI] [PubMed] [Google Scholar]
- 33.Burkhart R, Schulte D, Hu D, Musahl C, Gohring F, Knippers R. Eur. J. Biochem. 1995;228:431–438. [PubMed] [Google Scholar]
- 34.Musahl C, Schulte D, Burkhart R, Knippers R. Eur. J. Biochem. 1995;230:1096–1101. doi: 10.1111/j.1432-1033.1995.tb20660.x. [DOI] [PubMed] [Google Scholar]
- 35.Richter A, Knippers R. Eur. J. Biochem. 1997;247:136–141. doi: 10.1111/j.1432-1033.1997.00136.x. [DOI] [PubMed] [Google Scholar]
- 36.Ishimi Y, Ichinose S, Omori A, Sato K, Kimura H. J. Biol. Chem. 1996;271:24115–24122. doi: 10.1074/jbc.271.39.24115. [DOI] [PubMed] [Google Scholar]
- 37.Sherman DA, Forsburg SL. Nucleic Acids Res. 1998;26:3955–3960. doi: 10.1093/nar/26.17.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura H, Ohtomo T, Yamaguchi M, Ishii A, Sugimoto K. Genes Cells. 1996;1:977–993. doi: 10.1046/j.1365-2443.1996.840284.x. [DOI] [PubMed] [Google Scholar]
- 39.Sherman DA, Pasion SG, Forsburg SL. Mol. Biol. Cell. 1998;9:1833–1845. doi: 10.1091/mbc.9.7.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blow JJ. J. Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong JP, Thommes P, Rowles A, Mahbubani HM, Blow JJ. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]
- 42.Mahbubani HM, Chong JP, Chevalier S, Thommes P, Blow JJ. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coué M, Kearsey SE, Mechali M. EMBO J. 1996;15:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- 44.Romanowski P, Madine MA, Laskey RA. Proc. Natl. Acad. Sci. USA. 1996;93:10189–10194. doi: 10.1073/pnas.93.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel LM, Monty KJ. Biochim. Biophys. Acta. 1966;112:346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- 46.Madine MA, Khoo CY, Mills AD, Mushal C, Laskey RA. Curr. Biol. 1995;5:1270–1279. doi: 10.1016/s0960-9822(95)00253-3. [DOI] [PubMed] [Google Scholar]
- 47.Coué M, Amariglio F, Maiorano D, Bocquet S, Mechali M. Exp. Cell Res. 1998;245:282–289. doi: 10.1006/excr.1998.4271. [DOI] [PubMed] [Google Scholar]
- 48.Lei M, Kawasaki Y, Tye BK. Mol. Cell. Biol. 1996;16:5081–5090. doi: 10.1128/mcb.16.9.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucci E, Fronticelli C, Chiancone E, Wyman J, Antonini E, Rossi-Fanelli A. J. Mol. Biol. 1965;12:183–192. doi: 10.1016/s0022-2836(65)80292-3. [DOI] [PubMed] [Google Scholar]
- 50.Kambach C, Walke S, Young R, Avis JM, de la Fortelle E, Raker VA, Luhrmann R, Li J, Nagai K. Cell. 1999;96:375–387. doi: 10.1016/s0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 51.Raker VA, Plessel G, Luhrmann R. EMBO J. 1996;15:2256–2269. [PMC free article] [PubMed] [Google Scholar]
- 52.Yan H, Merchant AM, Tye BK. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]
- 53.West SC. Cell. 1996;86:177–180. doi: 10.1016/s0092-8674(00)80088-4. [DOI] [PubMed] [Google Scholar]