Abstract
The objective of this study was to evaluate the time-series relationships between stress, sleep duration, and headache pain among patients with chronic headaches. Sleep and stress have long been recognized as potential triggers of episodic headache (< 15 headache days/month), though prospective evidence is inconsistent and absent in patients diagnosed with chronic headaches (≥ 15 days/month). We reanalyzed data from a 28-day observational study of chronic migraine (n = 33) and chronic tension-type headache (n = 22) sufferers. Patients completed the Daily Stress Inventory and recorded headache and sleep variables using a daily sleep/headache diary. Stress ratings, duration of previous nights' sleep, and headache severity were modeled using a series of linear mixed models with random effects to account for individual differences in observed associations. Models were displayed using contour plots. Two consecutive days of either high stress or low sleep were strongly predictive of headache, whereas two days of low stress or adequate sleep were protective. When patterns of stress or sleep were divergent across days, headache risk was increased only when the earlier day was characterized by high stress or poor sleep. As predicted, headache activity in the combined model was highest when high stress and low sleep occurred concurrently during the prior 2 days denoting an additive effect. Future research is needed to expand on current findings among chronic headache patients and to develop individualized models that account for multiple simultaneous influences of headache trigger factors.
Keywords: Stress, Sleep, Headache, Time-series, Headache trigger factors, Headache precipitants
1.0 Introduction
Migraine and tension-type headache (TTH) are among the most common medical complaints and causes of disability [5,27,31,40]. Approximately 1.4% to 2.2% [30] of the population sufferers from chronic migraine and 2.2% [37] from chronic TTH (CTTH), in which sufferers experience 15 or more days per month with headache [21]. Individuals with these chronic headache subforms comprise the majority of treatment-seeking patients and have significantly higher rates of medical and psychiatric comorbidities, as well as greater headache-related disability and functional impairment, than do their episodic counterparts [15,30,37,46].
High stress and inadequate sleep are two of the most commonly reported triggers of headache identified in retrospective studies [28]. Upwards of 80% of migraine patients identify stress as a headache precipitant [22,26,28,38]. However, prospective time-series analyses have inconsistently supported earlier retrospective associations between headache and stress [10,17,38,45]. In an ambitious prospective study of headache triggers, Wöber and colleagues found that stress was a significant precipitant of headache but was less potent than suggested by earlier retrospective reports [45]; similar findings in prospective versus retrospective reports have been recently noted elsewhere [14]. A small number of studies have attempted to use physiological markers of stress to predict subsequent headache episodes [2,25,35]. However, prospective studies have not always supported the notion that biological markers of stress are rally related to migraine [11,13,36,42].
Similarly, “lack of sleep” is endorsed as a trigger among 48% to 74% of migraineurs and 26% to 72% of tension-type headache sufferers, and sleep disturbance has been consistently identified as a headache trigger in retrospective studies [3,6]. Kelman and Rains assessed relations between sleep and migraine and found that approximately half of patients reported atleast occasional symptoms of insomnia, 38% reported sleeping less than 6 hours per night, and 50% of patients reported that sleep disturbance triggered their migraines [23]. The severity and prevalence of sleep problems increase proportionally to headache frequency, such that the majority of chronic migraineurs (68-84%) suffer from insomnia on a near-daily basis [8,34]. Prospective data pertaining to sleep as a headache trigger are lacking, however, and existing retrospective studies have failed to account for strong interrelationships between sleep and stress.
Stress and sleep are of particular clinical interest because they represent modifiable risk factors and have been implicated in the progression of episodic headache into more chronic subforms [4,18,32]. The present study was designed to examine individual and combined influences of perceived stress and sleep duration in predicting headache severity among chronic migraine and TTH patients with the hypothesis that stress, sleep, and their interaction would combine to predict headache severity among patients with chronic migraine and chronic TTH. The presence of intra-individual differences in perceived stress and the frequency of sleep disturbance among chronic headache sufferers likely obscures associations uncovered in previous studies and provided a strong rationale for using daily diary recordings [41] and corresponding within-subjects data-analytic approaches [20].
2.0 Methods
2.1 Participants
The present study is a reanalysis of a previously published, observational, paper-pencil diary study [20,29]. Inclusion requirements were patients between 18 and 70 years of age who had at least 4 headache days per month and had stable headache characteristics for 1 year or longer. Although the data were collected prior to their inception, sufficient information (e.g., headache intensity, duration, photophobia, phonophobia, etc.) was available to assign primary headache diagnoses adherent to 2004 International Headache Society (IHS) criteria [21] based on administration of a structured diagnostic interview. Patients were excluded if their headaches were attributable to secondary causes (eg, trauma, underlying neurologic impairment, allergies, sinus conditions), if they were unable to complete questionnaires or noncompliant with self-monitoring (e.g., returned diaries that were clearly completed in one sitting rather than daily), or if medication monitoring revealed possible medication overuse headache (ie, 10 consecutive days of medication use such as aspirin, acetaminophen, sedative, analgesic, or ergotamine tartrate). From the pool of 64 headache sufferers who were originally recruited through physician referrals and radio/newspaper advertisements to the University of Mississippi Medical Center Head Pain Center, 33 chronic migraineurs and 22 CTTH sufferers met all inclusion criteria and were retained for the present analysis. The remaining 9 patients had episodic headache subforms and were thus excluded.
2.2 Procedure
The original study was approved by the Institutional Review Board of the University of Mississippi Medical Center and the re-analysis of the data was conducted under the approval of the Institutional Review Board of Wake Forest School of Medicine. Participants were given an explanation of the aims and procedures of the study and provided written informed consent. Questionnaires were used to collect demographic, medical and headache history. Four times a day for 28 days, patients recorded headache severity on a 0 to 10 scale (0= no headache, 10=extremely painful) using a provided diary; these daily ratings were later summed to calculate a headache sum (HAS) for that day. Upon awakening each morning, participants recorded the duration of their previous night's sleep in hours. The Daily Stress Inventory (DSI) was completed each evening and used to determine the number of stressful events, the severity of stress, and the average stress over the previous 24 hours. The DSI is a well-validated 58-item Likert-type scale that allows a subject to identify stressful events that they have experienced in the last 24 hours [7]. For each item that is endorsed, a stressfulness impact rating is made ranging from 1 (“occurred but was not stressful”) to 7 (“caused me to panic”). Although other indices are available (e.g., sum of ratings, sum of events), for the present study the average impact rating (AIR) of the included stressful events was used in the data analytic models because it incorporates both the number of events experienced and their intensity ratings.
2.3 Statistical Analyses
All analyses were conducted using SAS 9.2 (SAS, Inc, Cary, NC). Where appropriate all inferences are two-tailed and point estimates are presented with 95% confidence intervals. There were no missing data encountered in the considered diaries of the participants. To examine the Pearson correlations between ±3 day lags and leads of the stress, sleep, and headache pain series, separate cross-correlation functions were calculated for each participant. To better ensure that the autocorrelation within each series did not impact the estimates of cross-correlation, the headache series was “pre-whitened” using a standard ARIMA (1,0,0) that was previously found to model most of the autocorrelation patterns in within-subject headache pain series [20]. In this way, the individual cross-correlations represent a depiction of the relationships between sleep, stress, and headache after controlling for the autocorrelation that is often observed in time-series data.
To model the relationships between sleep, stress, and headache severity, several linear mixed models were used. These model forms assumed that the underlying headache reports were normally distributed, an assumption previously supported among patients with frequent headaches [19,20]. To account for individual differences in both the level of pain reporting and between-subject variability in relationships between predictors, random effects were specified for the intercept (ie, allowing each subject to have their own intercept) and slopes of the various sleep and stress predictor variables. Three separate models were conducted and are reported: one for the relationship between stress and headache, one for the relationship between previous night's sleep and headache, and a final model that includes a linear composite of the stress predictors estimated from the stress model and a linear composite of sleep predictors from the sleep model. In all models curvilinear relationships were examined by squaring a predictor; interaction terms were assessed through sequential addition to the main effects. In all models, a lag of the headache sum (i.e., the previous day's sum of headache pain ratings, ranging from 0 to 40) was forced into the model to control for the previous headache pain's impact on today's headache activity. The reported models are the result of several considered models (ie, with and without quadratic terms and interactions) that minimized both the objective function (-2*Log Likelihood) and the Bayesian Information Criterion (BIC) that penalizes models having a greater number of predictors.
To visually evaluate the relationships and to display the model predictions, several 2D plots were generated. Because of the large between-subject variability in levels of pain in the relationships among sleep, stress, and headache pain, the data were smoothed prior to display in order to enhance the signal/noise ratio. To do so, the Loess smoother from Sigma Plot 11.0 was used with a 0.4 sampling proportion. The resulting smoothed data are displayed as 2D color contour plots.
3.0 Results
3.1 Patient Characteristics
Thirty-three (60%) participants from the retained sample met criteria for chronic migraine, while 22 (40%) met criteria for chronic TTH. The 13 participants meeting diagnostic criteria for both migraine and TTH were classified as chronic migraine. Most participants in the present sample were female (83.6%) and Caucasian (90.9%; 9.1% African American). The mean age of chronic migraine sufferers was 43.3 years (SD =13.5), while the mean age of TTH sufferers was 45.0 years (SD =12.4). The median number of experienced headache days (out of the 28 day/month observation period) was 26 (range: 15 to 28).
3.2 Sleep, Stress, and Headache Characteristics
Tension-type headache sufferers reported a mean (SD) headache sum (HAS) of 10.3 (6.0) with the reported means of individuals ranging from 2.2 to 27.9 (Table 1). Migraine sufferers reported a similar mean HAS of 10.8 (6.2) with a range of means from 2.2 to 27.5. Tension-type headache sufferers reported a mean sleep duration of 6.7 hours (0.82), while migraine sufferers reported mean sleep duration of 7.1 hours (0.91) hours. Mean reported average stress impact rating (AIR) was 2.3 (0.75) for tension-type headache patients and 2.5 (0.97) for migraine patients. Although differences in the diagnostic groups' daily headache intensities (d = 0.08 standard deviation difference) were not substantial, differences in sleep duration (d = 0.44), and stress levels (d = 0.24) were remarkable such that diagnostic groups were considered in post hoc sensitivity analyses for all models.
Table 1. Demographics and Group Variation of Tension-Type Headache and Migraine Sufferers.
| Tension-Type Headache n = 22 | Migraine Headache n = 33 | |||||
|---|---|---|---|---|---|---|
| n; Mean | %; SD | Range; Range of means | n; Mean | %; SD | Range; Range of means | |
| Demographic | ||||||
| Gender | ||||||
| Female | 17 | 77.3% | ---- | 29 | 87.9% | ---- |
| Male | 5 | 22.7% | ---- | 4 | 12.1% | ---- |
| Ethnicity | ||||||
| Caucasian | 21 | 95.5% | ---- | 29 | 87.9% | ---- |
| African-American | 1 | 4.5% | ---- | 4 | 12.1% | ---- |
| Age | 45.0 | 12.4 | 30 to 69 | 43.3 | 13.5 | 24 to 70 |
| Group Variation | ||||||
| HAS | ||||||
| Means | 10.3 | 6.0 | 2.2 to 27.9 | 10.8 | 6.2 | 2.2 to 27.5 |
| SDs† | 5.7 | 2.2 | 3.1 to 10.8 | 5.9 | 2.1 | 2.1 to 10.0 |
| Sleep | ||||||
| Means | 6.7 | 0.82 | 5.7 to 8.1 | 7.1 | 0.91 | 4.8 to 8.5 |
| SDs† | 1.2 | 0.46 | 0.53 to 2.1 | 1.4 | 0.51 | 0.31 to 2.4 |
| AIR | ||||||
| Means | 2.3 | 0.75 | 1.3 to 3.8 | 2.5 | 0.97 | 0.7 to 4.4 |
| SDs† | 0.67 | 0.32 | 0.3 to 1.5 | 0.82 | 0.37 | 0.3 to 1.5 |
Reflects the aggregate of individual participants' standard deviations
3.3 Preliminary Analyses – Cross-correlation functions
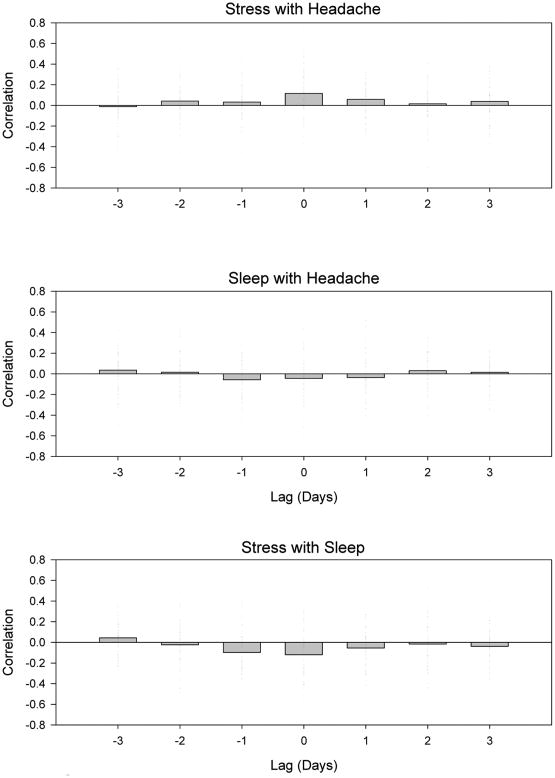
Figure 1 displays the individual associations for all of the temporally lagged cross-correlations between stress, sleep, and headache sum. The individual relationships varied substantially across individuals for each of the time lags. For example, in the stress and headache associations, the same day associations (lag 0) averaged r = 0.12 but individually varied between r = -0.36 to 0.73. This large degree of variability in the individual relationships included several statistically significant within-person associations for both the direct (ie, high stress with high headache) and inverse (ie, high stress with low headache) relationships. This indicates that when viewed in this manner, several of the individuals in the sample had opposite responses to stress. In the aggregate, yesterday's stress (lag -1: r = 0.03) and two day's previous stress (lag -2: r = 0.04) were only slightly associated with increased headache sums. Conversely, today's headache sums were only slightly associated with tomorrow's stress (lag 1: r = 0.05) and stress ratings two days from present (lag 2: r = 0.01). Similarly small aggregate correlations were observed for the temporal relationships between sleep and headache and between stress and sleep. As might be expected, each of these associations tended to be negative, with higher levels of sleep being associated with lower levels of stress and headache.
Figure 1.
Cross-correlation between stress (AIR) and headache SUM (top panel), sleep quantity and headache SUM (middle panel) and stress (AIR) and sleep quantity (lower panel). For each plot, individual tick marks represent a single subject with the y-axis displaying the Pearson correlation as a function of time lag in days. The bar plot reflects the average of individual associations.
These cross-correlations informed subsequent model building in several ways. First, because of the large degree of variability in the individual associations, a fixed effects model attempting to force one coefficient on the relationship was likely to fit the data poorly and result in only modest predictive utility. For that reason, random effects models that allow these coefficients to vary by participant were selected. Second, there were some individuals for whom recent prior experiences with stress and sleep had a potent impact on headache activity. To account for this, lagged stress and sleep predictors (up to ±2 days) were included in the final models. Next, it was very likely that the large between-person variability was being influenced by unique combinations of these predictors (eg, high levels of stress and sleep), unique relationships between them (eg, linear vs. nonlinear), or perhaps from a non-measured variable. For these reasons, interaction terms and curvilinear terms were also included in the models. Finally, because there are at least modest associations between stress and sleep, separate models were initially built for each of these two variables (ie, to avoid multicollinearity), followed by a combined final model aggregating both variables.
3.4 Stress and Headache Model
Table 2 displays the best fitting model of how stress predicts headache. This model was the end result of many conducted inferences considering linear and quadratic versions of the various lags of stress, and several higher order interaction terms. This model minimized the BIC function such that larger models with more predictors (which could reduce error variance in headache scores) did not result in substantially better fit when penalized for using more information. Because of the many unreported inferences, the confidence intervals reported in the table are narrower than they might be if all inferences were considered and must be interpreted with caution.
Table 2. Final Sleep and Stress Models.
| Stress Predicts Headache Model | ||||
|---|---|---|---|---|
| Predictor | Estimate | 95% CI | p | |
| LB | UB | |||
| Intercept | 5.76 | 3.74 | 7.79 | <.0001 |
| Yesterday's Headache | 0.39 | 0.31 | 0.46 | <.0001 |
| Today's Stress | -1.28 | -2.55 | -0.01 | 0.048 |
| Today's Stressˆ2 | 0.58 | 0.32 | 0.83 | <.0001 |
| Yesterday's Stressˆ2 | 0.16 | -0.01 | 0.32 | 0.058 |
| Today's Stress * Yesterday's Stressˆ2 | -0.10 | -0.15 | -0.04 | 0.001 |
| Day Before Yesterday's Stressˆ2 | -0.09 | -0.20 | 0.02 | 0.097 |
| Yesterday's Stressˆ2 * Day Before Yesterday's Stressˆ2 | 0.008 | 0.002 | 0.015 | 0.013 |
| Sleep Predicts Headache Model | ||||
| Predictor | Estimate | 95% CI | p | |
| LB | UB | |||
| Intercept | 11.92 | 6.70 | 17.14 | <.0001 |
| Yesterday's Headache | 0.33 | 0.26 | 0.40 | <.0001 |
| Today's Sleep | -1.70 | -2.97 | -0.43 | 0.009 |
| Today's Sleepˆ2 | 0.14 | 0.05 | 0.24 | 0.004 |
| Yesterday's Sleepˆ2 | 0.05 | -0.02 | 0.12 | 0.172 |
| Today's Sleep * Yesterday's Sleepˆ2 | -0.009 | -0.018 | 0.001 | 0.065 |
| Final Stress and Sleep Predicting Headache Model | ||||
| Predictor | Estimate | 95% CI | p | |
| LB | UB | |||
| Intercept | 6.13 | 5.24 | 7.03 | <.0001 |
| HAS_1 | 0.38 | 0.31 | 0.45 | <.0001 |
| Stress predictor*† | 2.01 | 1.34 | 2.69 | <.0001 |
| Stressˆ2 | -0.15 | -0.26 | -0.04 | 0.006 |
| Sleep predictor*† | 0.75 | 0.24 | 1.26 | 0.004 |
| Sleepˆ2 | -0.02 | -0.14 | 0.10 | 0.795 |
Calculated using a linear composite of the parameter weights from the stress/sleep models
Converted to a z-score metric (mean = 0, SD = 1)
LB = Lower bound, UB = Upper bound
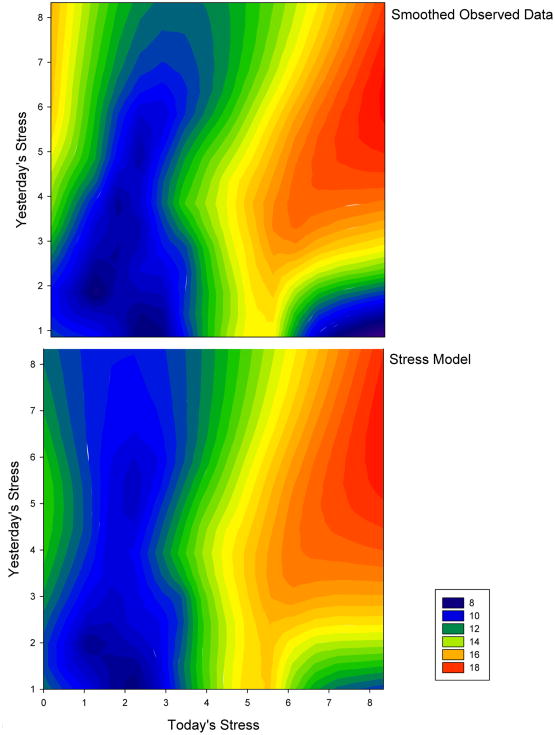
The stress model reflects a complicated impact of the role of stress in precipitating headache, describing how the two most recent days' (ie, today and yesterday) stress levels combine to predict current headache intensity (SUM). Several interaction terms in the model indicate that recent stress levels combine in unique ways to influence headache (i.e., that stress impacts headache severity differently when different patterns of stressful days have been encountered). The squared predictors indicate a non-linear pattern of relationships best depicted graphically. Figure 2 depicts the actual data (top pane) and the model predictions (bottom pane). The similarity of the two plots reflects the good fit of the model predictions to the actual data. Several interesting trends between stress and headache were noted. First, if yesterday's stress was moderate or greater (i.e., > 3 on the AIR scale) and today's stress is moderate or greater (i.e., > 3 on the AIR scale), headache activity is predicted to be very high (e.g., two bad days results in increased headache). Similarly, if both yesterday and today's stress is low, headache activity is predicted to be low (e.g., two good days results in decreased headache). However, if yesterday's stress is low and today's stress is high, headache activity is predicted to be low (eg, when under new stress, the sufferer is not likely to experience high headache activity). Finally, when yesterday's stress was high, but today's stress is low, the sufferer is likely to experience increased activity (e.g., the “let down” or “Saturday” headache).
Figure 2.
A contour plot depicting the smoothed headache SUM using color as a function of today's stress (x-axis) and yesterday's stress (y-axis). The top panel displays the smoothed observed data, and the bottom panel displays the smoothed stress model predictions. The similarity between plots reflects a good fit of the model to the data.
3.5 Sleep and Headache Model
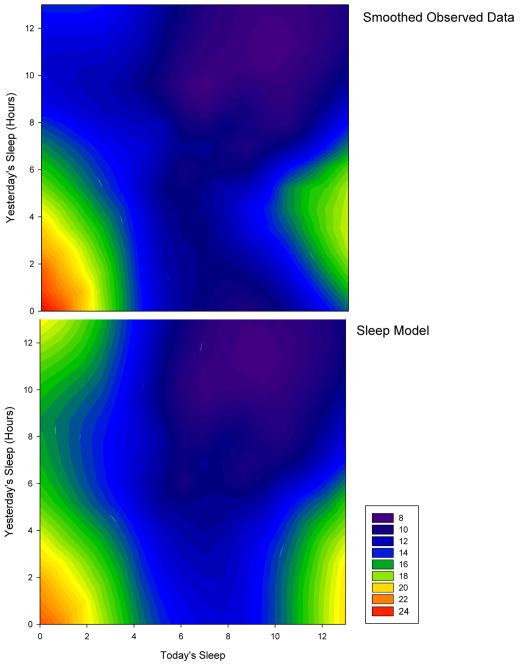
Table 2 displays the best fitting model of how sleep the previous two days predicts headache, which was derived using the same inferences and BIC function minimization of the stress and headache model. The sleep model reflects a complicated impact of sleep duration's role in influencing headache. Unlike the stress model, the sleep model only utilizes “yesterday's” (ie, 2 days prior) and “today's” sleep (ie, the night before) duration to combine to predict current headache intensity (SUM). As with stress, the interaction terms were used to examine how different combinations of sleep on subsequent days combined to predict headache severity. The squared predictors reveal a non-linear relationships across levels of sleep duration. Figure 3 depicts the actual data (top pane) and the model predictions (bottom pane). The similarity between the two plots again reflects strong fit between predicted and observed data. The plots display several notable relationships.
Figure 3.
A contour plot depicting the smoothed headache SUM using color as a function of last night's sleep (x-axis) and the previous night's sleep (y-axis). The top panel displays the smoothed observed data, and the bottom panel displays the smoothed sleep model predictions. The similarity between plots reflects a good fit of the model to the data.
First, if yesterday's sleep was low (i.e., < 4 hours duration) and today's sleep is also low (i.e., < 4 hours duration), headache activity is predicted to be very high (e.g., two bad days results in increased headache). Conversely, if yesterday's sleep duration was low but today's sleep was high, headache activity is predicted to be high (e.g., the “sleep-in” headache). Finally, if a sufferer gets approximately 8 hours of sleep on consecutive days, headache activity is predicted to be low.
3.6 Combined Final Model of Stress and Sleep
Table 2 displays the combined final model of stress and sleep. This model utilized what was learned about the relationships between stress, sleep, and headache in the previous two models to create a single stress and sleep predictor. To calculate the omnibus predictor, each of the stress model coefficients was used to weight the stress predictors to combine them into one “stress risk” score. In this way, a single dimension of stress could be used to reflect a single day's stress risk in the model, thus reducing the complexity of the predictors. This approach does present some interpretation difficulties because, for example, a low stress score could be obtained from two good stress days or from a high stress day today combined with a low stress yesterday, as both of these combinations were associated with low headache activity in the stress model. The same approach was taken with the sleep predictors.
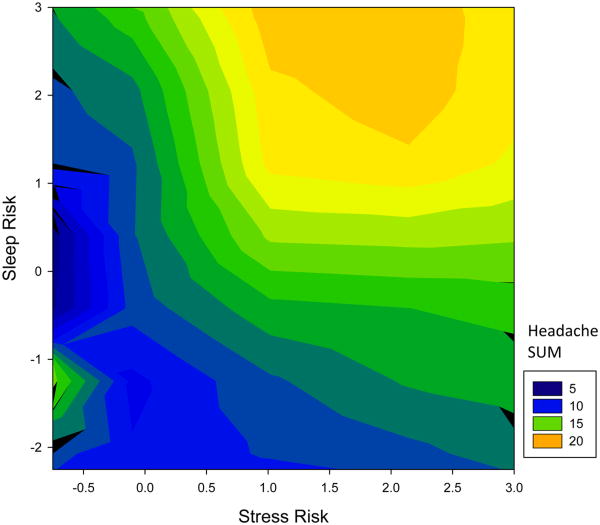
The coefficients for this model are displayed in Table 2. Figure 4 depicts the model predictions from this final combined model. The model fit best when the stress and sleep scores (the “risk scores”) were additive and stress was modeled with a quadratic term (though the sleep term was more linear). In Figure 4, the zero-zero coordinate reflects the average stress and sleep risks in the model. As can be seen in Figure 4, the lowest headache activity was associated with low stress and low sleep risk scores.
Figure 4.
A contour plot depicting the smoothed headache SUM using color as a function of stress risk (x-axis) and sleep risk (y-axis). The combination of stress and sleep risks additively combine to predict increased headache activity.
As each of the individual stress and sleep risk terms increase, a corresponding increase in headache activity is observed, with the most headache activity occurring when both are high. There is a counter-intuitive level of modestly-elevated headache risk when stress and sleep risk are both extremely low (lower left quadrant), but this could be due to the model's proclivity to overestimate the importance of yesterday's sleep when today's sleep duration was low (ie, contrast the upper left quadrants of the two images in Figure 3). Taken together, the model's coefficients support the interpretation that stress and sleep are additive in predicting headache activity in chronic headache sufferers.
4.0 Discussion
The present study examined the individual and combined influences of perceived stress and sleep duration in predicting headache severity among a sample of chronic migraine and chronic TTH patients. The presented models depict a series of preliminary relationships between stress, sleep, and their combination in predicting headache. Regarding stress, the models indicate that the impact of concurrent stress is modified by an individual's recent experience with stress. High stress on the previous and current day increases risk for headache, whereas low stress on both days is associated with decreased risk. However, when patients experienced differing levels of stress across two days, the models indicate that high stress on the previous day is a risk factor for subsequent headache when new stress is low, but that novel stress alone is not associated with increased risk of headache insofar as the previous day's stress is low. Prior stress thus seems to differentially evoke headache compared to new stress. The variable impact of differential stress across subsequent days is consistent with the notion that daily hassles influence headache activity and differentiate headache patients from controls more than major stressful life events [17,18,24,44]. Considered together, these data indicate that stress can be protective, evocative, or non-informative for headache severity depending on a patient's recent history.
Regarding sleep, the modeled data indicate that two consecutive days of decreased sleep is associated with increased risk for headache; headache severity ratings were inversely related to sleep duration (ie, less sleep is associated with more severe headaches). These data support those from retrospective studies that have identified insufficient sleep as one of the most common triggers of headache [23,28]. They indicate also that adequate sleep for two consecutive days is protective against headache and that the optimal sleep duration on both nights approximates 8 hours. However, as with the stress data, inconsistency across the two modeled days was associated with differential headache outcomes: Risk for headache is increased insofar as the prior day's sleep was low and today's sleep is high, but headache risk is not as elevated when yesterday's sleep was high and today's sleep is low.
The final model assessed the interactive effects of stress and sleep in predicting headache, given the paucity of research on their additive effects despite the high frequency with which both are reported as headache triggers. As expected, stress and sleep function as headache triggers most strongly when both are elevated as risk factors, that is, when high stress and poor sleep occur concurrently in time. The risk for severe headache increases proportionally with both high perceived stress and low sleep duration. Because high stress and poor sleep are strongly correlated and occasion one another [9,16,33,43], synergistic suffering may occur for headache sufferers who do not sleep well under high stress.
Findings from the present study confirm that the headache activity of chronic headache patients can be predicted from combinations of perceived stress and sleep duration, even among individuals experiencing near-daily headache. The ability of these variables to strongly predict such a frequent occurrence speaks to the robustness of the observed findings and the strong fit between the observed and modeled data. They also comport well with time-series data from other chronic medical conditions showing that pain intensity can be reliably predicted by stress even among patients with very frequent pain [1,12].
Interestingly, the single-variable models underscore the notion that yesterday's events (ie, high stress, poor sleep) are stronger predictors of headache activity than are same-day events when the pattern of activity is divergent across days. Previous studies have indeed observed differential effects of various temporal lags on headache activity [24], although the large majority of existing studies on headache precipitants have not simultaneously considered temporal lags in the data analytic approach. Extreme variability in the observed cross-correlations further confirms the importance of taking into account individual differences and non-linear relationships to account for these differences, as was done in the present study using mixed linear models with specified random effects. It is likely that previous mixed findings in previous studies of stress and sleep as headache triggers are partly a function of the failure to consider these important issues, and future studies using time-series designs should continue to address them.
The present study is unique because of its prospective design including multiple daily data collection points, inclusion of both chronic migraine and CTTH patients, and a data analytic plan that afforded quantification of both temporal relationships and intra-individual differences. Despite these strengths, several substantial limitations exist. The limitations in the available data result in the notion that these models should be viewed as providing preliminary support of the uncovered relationships. The most notable limitations are highlighted below.
The daily diaries were collected using paper-pencil forms that have a large potential for unreliability. Stone and colleagues examined participant compliance with paper and pencil diaries and found that although participants reported 90% compliance in completing the diaries, they were actually only 11% compliant [39]. This startling finding highlights the possibility that the diary information in the present study was completed using retrospective, or even faked reports (e.g., a patient completing the entire diary immediately before turning it in). The diaries used in the present study contained a high cognitive burden that might have increased the chances of low compliance. In future studies, electronic diaries should be used to confirm compliance with the study protocol.
There were limitations on the nature of assessments. Stress was only assessed once daily so intra-day temporal relationships with sleep could not be evaluated. The impact of daily medications to prevent headache, or the attenuating influence of abortive medications was also not considered in the models. Participants were instructed to maintain a stable medication regimen, reducing variability in these predictors, but future studies could include this behavior as a potential confounder. A range of other potential effect modifiers might impact the uncovered associations (eg, ovarian hormone levels, psychiatric comorbidities), but these predictors were not available for analysis in the present study. The quantification of the sleep predictor was based on duration of sleep only; it is conceivable that sleep quality or sleep efficiency would have a substantially different role in predicting headache activity. Future studies should consider assessment of stress and sleep at multiple time points throughout the day and in quantifying sleep based on perceived quality or using a validated sleep instrument.
The present analysis evaluated only a modest sample of individuals. The extent to which these individuals represent chronic headache sufferers in general, or to which these results generalize to episodic sufferers is unknown. These results must be replicated in future samples in a more diverse population to evaluate their generalizability.
Finally, we attempted to reduce the probability of obtaining spurious associations from considering multiple models by applying rigorous model selection criteria (objective function, BIC). Despite this, we did have to consider multiple models that were relatively large in size compared with each individual's provided data set (ie, using approximately 25% of each individual's provided data). To verify that the observed models did not “over-fit” each patient's experience, the reported models should be replicated in an external data set.
Given the significant impact of perceived stress, sleep duration, and their combination in robustly predicting headache severity, these preliminary findings can be used to inform future research on headache precipitants among patients with chronic headache disorders. Future research is needed to build on current evidence and develop individualized models that account for multiple simultaneous influences.
Footnotes
Conflicts of Interest: Timothy T. Houle: Dr. Houle receives research support from GlaxoSmithKline and Merck and Co., Inc. and is a consultant for Allergan.
Ross A. Butschek: Mr. Butschek reports no conflicts of interest
Dana P. Turner: Ms. Turner receives research support from Merck and Co., Inc.
Todd A. Smitherman: Dr. Smitherman reports no conflicts of interest.
Jeanetta C. Rains: Dr. Rains reports no conflicts of interest.
Donald B. Penzien: Dr. Penzien receives research support from Merck and Co., Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Affleck G, Tennen H, Urrows S, Higgins P. Person and contextual features of daily stress reactivity: individual-differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. J Pers Soc Psychol. 1994;66:329–340. doi: 10.1037//0022-3514.66.2.329. [DOI] [PubMed] [Google Scholar]
- 2.Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86:133–138. doi: 10.1016/s0304-3959(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 3.Barbanti P, Fabbrini G, Aurilia C, Vanacore N, Cruccu G. A case-control study on excessive daytime sleepiness in episodic migraine. Cephalalgia. 2007;27:1115–1119. doi: 10.1111/j.1468-2982.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- 4.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46:1334–1343. doi: 10.1111/j.1526-4610.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, Goadsby PJ, Lipton RB. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301–315. doi: 10.1177/0333102410381145. [DOI] [PubMed] [Google Scholar]
- 6.Boardman HF, Thomas E, Millson DS, Croft PR. Psychological, sleep, lifestyle, and comorbid associations with headache. Headache. 2005;45:657–669. doi: 10.1111/j.1526-4610.2005.05133.x. [DOI] [PubMed] [Google Scholar]
- 7.Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A daily stress inventory: develpment, reliability, and validity. J Behav Med. 1987;10:61–74. doi: 10.1007/BF00845128. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun AH, Ford S, Finkel AG, Kahn KA, Mann JD. The prevalence and spectrum of sleep problems in women with transformed migraine. Headache. 2006;46:604–610. doi: 10.1111/j.1526-4610.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effects of a major stressful event and its resolution. Psychiat Res. 1991;39:199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- 10.Chabriat H, Danchot J, Michel P, Joire JE, Henry P. Precipitating factors of headache: a prospective study in a national control-matched survey in migraineurs and nonmigraineurs. Headache. 1999;39:335–338. doi: 10.1046/j.1526-4610.1999.3905335.x. [DOI] [PubMed] [Google Scholar]
- 11.Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128:209–214. doi: 10.1016/j.pain.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol. 2004;23:267–274. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Ahmed T, Banerjee B, Bhatia M. Plasma calcitonin gene-related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter-ictal period. J Headache Pain. 2009;10:161–166. doi: 10.1007/s10194-009-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume M, Yamada U, Sato A, Hayashi K, Amano Y, Makino M, Yoshiuchi K, Tsuboi K. Stress and psychological factors before a migraine attack: a time-based analysis. Biopsychosoc Med. 2008;2:14. doi: 10.1186/1751-0759-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckman BD, Holroyd KA. Tension-type headache and psychiatric comorbidity. Curr Pain Headache Rep. 2006;10:439–447. doi: 10.1007/s11916-006-0075-2. [DOI] [PubMed] [Google Scholar]
- 16.Hicks RA, Garcia ER. Level of stress and sleep duration. Percept Motor Skill. 1987;64:44–46. doi: 10.2466/pms.1987.64.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Holm JE, Lokken C, Myers TC. Migraine and stress: a daily examination of temporal relationships in women migraineurs. Headache. 1997;37:553–558. doi: 10.1046/j.1526-4610.1997.3709553.x. [DOI] [PubMed] [Google Scholar]
- 18.Houle T, Nash JM. Stress and headache chronification. Headache. 2008;48:40–44. doi: 10.1111/j.1526-4610.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 19.Houle TT, Penzien DB, Rains JC. Time-series features of headache: individual distributions, patterns, and predictability of pain. Headache. 2005;45:445–458. doi: 10.1111/j.1526-4610.2005.05096.x. [DOI] [PubMed] [Google Scholar]
- 20.Houle TT, Remble TA, Houle TA. The examination of headache activity using time-series research designs. Headache. 2005;45:438–444. doi: 10.1111/j.1526-4610.2005.05095.x. [DOI] [PubMed] [Google Scholar]
- 21.International Headache Society. The international classification of headache disorders. Cephalalgia. (2nd) 2004;24(suppl 1):S1–S151. [Google Scholar]
- 22.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 23.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904–910. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- 24.Kohler T, Haimerl C. Daily stress as a trigger of migraine attacks: results of thirteen single-subject studies. J Consult Clin Psychol. 1990;58:870–872. doi: 10.1037//0022-006x.58.6.870. [DOI] [PubMed] [Google Scholar]
- 25.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin KC, Huang CC, Wu CC. Association between stress at work and primary headache among nursing staff in Taiwan. Headache. 2007;47:576–584. doi: 10.1111/j.1526-4610.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF the AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 28.Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483–495. doi: 10.1016/j.cpr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Mosley TH, Penzien DB, Johnson CA, Brantley PJ. Time-series analysis of stress and headache. Cephalalgia. 1991;11(suppl 1):306–307. [Google Scholar]
- 30.Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, Lipton RB. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609. doi: 10.1111/j.1468-2982.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 31.Pryse-Phillips W, Findlay H, Tugwell P, Edmeads J, Murray TJ, Nelson RF. A Canadian population survey on the clinical, epidemiologic and societal impact of migraine and tension-type headache. Can J Neurol Sci. 1992;19:333–339. [PubMed] [Google Scholar]
- 32.Rains JC. Chronic headache and potentially modifiable risk factors: screening and behavioral management of sleep disorders. Headache. 2008;48:32–39. doi: 10.1111/j.1526-4610.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 33.Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychol. 2004;23:542–545. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- 34.Sancisi E, Cevoli S, Vignatelli L, Nicodemo M, Pierangeli G, Zanigni S, Grimaldi D, Cortelli P, Montagna P. Increased prevalence of sleep disorders in chronic headache: a case-control study. Headache. 2010;50:1464–1472. doi: 10.1111/j.1526-4610.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 35.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 36.Schoonman GG, Evers DJ, Ballieux BE, de Geus EJ, de Kloet ER, Terwindt GM, van Dijk JG, Ferrari MD. Is stress a trigger factor for migraine? Psychoneuroendocrino. 2007;32:532–538. doi: 10.1016/j.psyneuen.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–383. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 38.Spierings ELH, Sorbi M, Maassen GH, Honkoop PC. Psychophysical precedents of migraine in relation to the time of onset of the headache: the migraine time line. Headache. 1997;37:217–220. doi: 10.1046/j.1526-4610.1997.3704217.x. [DOI] [PubMed] [Google Scholar]
- 39.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003 Apr;24(2):182–99. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 40.Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher AI, Steiner TJ, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 41.Tennen H, Affleck G, Armeli S, Carney MA. A daily process approach to coping: linking theory, research, and practice. Am Psychol. 2000;55:626–636. doi: 10.1037//0003-066x.55.6.626. [DOI] [PubMed] [Google Scholar]
- 42.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- 43.Waters WF, Adams SG, Binks P, Varnado P. Attention, stress and negative emotion in persistent sleep-onset and sleep-maintenance insomnia. Sleep. 1993;16:128–136. doi: 10.1093/sleep/16.2.128. [DOI] [PubMed] [Google Scholar]
- 44.Wittrock DA, Myers TC. The comparison of individuals with recurrent tension-type headache and headache-free controls in physiological response, appraisal, and coping with stressors: a review of the literature. Ann Behav Med. 1998;20:118–134. doi: 10.1007/BF02884458. [DOI] [PubMed] [Google Scholar]
- 45.Wöber C, Brannath W, Schmidt K, Kapitan M, Rudel E, Wessely P, Wöber-Bingol C, the PAMINA Study Group. Prospective analysis of factors related to migraine attacks: the PAMINA study. Cephalalgia. 2007;27:304–314. doi: 10.1111/j.1468-2982.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 46.Zwart JA, Dyb G, Hagen K, Odegard KJ, Dahl AA, Bovim G, Stovner LJ. Depression and anxiety disorders associated with headache frequency: the Nord-Trondelag Health Study. Eur J Neurol. 2003;10:147–152. doi: 10.1046/j.1468-1331.2003.00551.x. [DOI] [PubMed] [Google Scholar]