Even with advances in non-invasive diagnosis of diseases such as cancer or inflammatory diseases, tissue biopsy coupled with histopathologic examination remains the gold standard in establishing an accurate diagnosis.1–2 Generally, effective tissue diagnosis is fundamentally based on biopsying lesions that are suspicious based on visual inspection. Nevertheless, numerous mucosal conditions, such as dysplasia in ulcerative colitis or Barrett’s esophagus, are not always readily recognizable visually, and thus mandate surveillance protocols that involve random biopsies.3 To effectively sample large organs such as the colon, numerous biopsies are required,4 which can be time consuming. Moreover, due to the size of current forceps and the associated mucosal trauma and to the serial operation of the forceps, the number of random biopsies that can be realistically carried out is limited. Consequently, the current standard of care for cancer surveillance in ulcerative colitis patients is performed with at least 33 sequential biopsies (4-quadrant biopsies every 10 cm), which we estimate cumulatively samples less than 0.3% of colonic mucosa. The low sampling coverage may be ineffective at detecting precancerous or cancerous lesions, especially for early, small lesions that are also the most treatable.
Description of Technology
Here, we demonstrate the use of sub-millimeter, untethered endoscopic microgrippers (μ-grippers) to retrieve tissue samples for diagnostics. Parallel fabrication, deployment and thermal actuation make the μ-grippers ideal to achieve statistical tissue sampling of large organs such as the colon. Our results suggest a new paradigm in medicine whereby large numbers of small, tether-free microsurgical tools could complement individual, large, tethered biopsying devices. We designed the μ-grippers to resemble biological appendages, such as hands, with rigid phalanges and flexible joints (Fig. 1a). The joints contain residual stress powered actuators which allow them to close and grasp without the need for tethers (Fig. 1b). The rigid phalanges of the μ-grippers are composed of nickel, and hence they respond to applied magnetic field. A thermo-sensitive trigger layer on the joints keeps the μ-grippers flat at 4 °C, but softens at 37 °C, causing the μ-grippers to close.5 It is noteworthy that the size of the μ-grippers, 1 mm tip-to-tip, is far smaller than conventional biopsy forceps currently in use (Fig. 1c).
Figure 1. Untethered, thermo-sensitive μ-grippers for tissue excision.

(A–C) Bright field microscopy images of μ-grippers in an (A) open and (B) closed state; the scale bars represent 200 μm. (C) The image showing that the μ-grippers used in our biopsy experiments were over ten times smaller than the current biopsy forceps; the scale bar represents 1mm. (D–F) Endoscopic images of deployment and retrieval of the μ-grippers in an ex vivo porcine model. (D) μ-grippers covering the colon surface, the scale bar represents 2 mm. (E) Close-up image of μ-grippers closing on the colon wall. (F) Retrieval of the μ-gripperswith a magnetic catheter.
Video Description
The μ-grippers are small enough that hundreds can be deployed at a time and actuated en masse; therefore, they can form the basis for a more statistically efficient means to screen large area organs. In a Monte Carlo model, we estimated this effectiveness of detecting a mucosal lesion of a particular size by dividing the mucosal area into bins of the lesion size and calculating the probability of getting a sample from the bin with the lesion. The probability of detecting the lesion, which is equal to the number of sampled bins divided by the total number of bins, was calculated as a function of the ratio of lesion size to the organ area (see Supp. Sec. 1). Our simulation clearly show that because large numbers of μ-grippers can sample many more bins, the sampling success of utilizing the μ-grippers is significantly higher than utilizing conventional the biopsy forceps, especially in case of small lesions. For example, for a lesion size of 6cm2, sampling success with conventional forceps is 8%, compared to 45% and 95% for 300 and 1500 μ grippers respectively. To test the feasibility of biologic tissue sampling with μ-grippers, we used a swine colon in ex vivo studies. We inserted an endoscope into the anus and advanced it under endoscopic guidance. The μ-grippers were suspended in sterile water and deployed on the colon surface using a through-the-endoscope catheter. Since the μ-grippers are free to move in the water, there is no preferred orientation when they contact the tissue surface. We uniformly spread hundreds of μ-grippers by rotating the endoscope during the deployment and visually verified the closure of the μ-grippers using the endoscopic imaging (Fig. 1d, e). To simulate the normal human temperature, the colon was submerged in a water bath kept at 37 °C. After closure, we retrieved the vast majority of μ-grippers using a magnetic catheter inserted through the endoscope (Fig. 1f). The rest of the μ-grippers were suctioned out with the endoscope into a trap bottle.
The tissue retrieved with the μ-grippers was then used for genetic diagnostics (RNA and DNA analyses), as well as for cytologic analyses. First, we extracted ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) from the retrieved tissue. We reverse transcribed the RNA into complementary DNA (cDNA), and then designed primers for several highly abundant transcripts in pigs.6 As Fig. 2a demonstrates, cDNA amplification produced bands of expected size. Similarly, we employed DNA primers designed for pig DNA.7 Fig. 2b demonstrates that we were able to amplify all three genes and that the amplified DNA had the expected size. Hence, the tissue retrieved by the μ-grippers is of sufficient quality and quantity to allow DNA and RNA extraction, as well as polymerase chain reaction (PCR) amplification in an effort to look for previously identified disease-diagnostic markers (see Supp. Sec. 2). Notable concerns are whether the cells retrieved using the μ-grippers are desquamated cells in the mucus and if conventional cytologic studies can be achieved using retrieved tissue samples. In order to address these concerns, additional in vivo experiments were also done in the esophagus, and tissue retrieved using the μ-grippers was layered on a slide and stained using hematoxylin and eosin. Cytologic results (Fig. 2c) clearly show that high quality sections and both epithelial and desquamated cells can be obtained using the μ-grippers.
Figure 2. PCR results of the excised tissue and histologic analysis from an in vivo biopsy with μ-grippers.
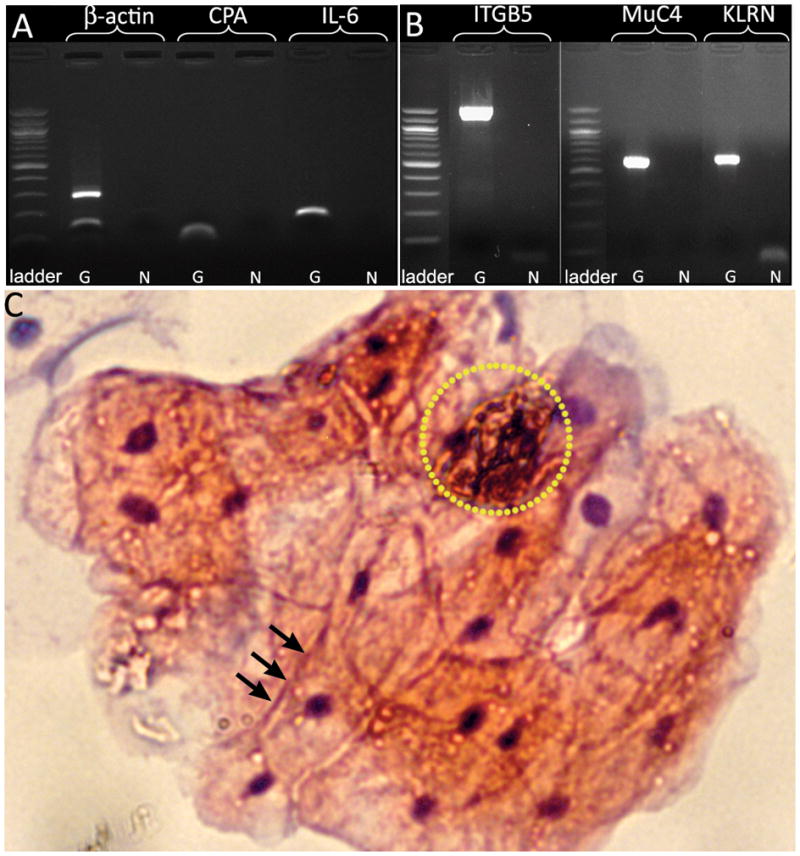
(A) cDNA (β-actin:164 bp, CPA:62 bp and IL-6:119 bp) and (B) genomic DNA (ITGB5:1300 bp, MuC4 and KLRN: 500–600 bp) from the tissue obtained with μ-grippers (G) compared to the negative control (N). (C) H&E stained section of cells retrieved with the μ-grippers from porcine esophagus in an in vivo operation. The image shows viable epithelial cells with clear, abundant cytoplasm, consistent with glycogenated cells originating from the superficial epithelial layer of the esophagus. The black arrows point to junctions between intercellular membranes. The nuclei of these cells are clearly stained and the cells appear healthy, with large cytoplasm to nucleus ratios. Also seen and highlighted in the yellow dotted circle is a separate group of cells with pyknotic nuclei, small cytoplasm-nucleus ratio and deeply stained keratin, indicative of desquamated cells. These cells are probably obtained from the mucus layer overlying the esophageal mucosa.
Take Home Message
In contrast to the dominant paradigm of “one task by one tool” used in conventional surgery, the concept of utilizing large numbers of miniaturized and untethered devices suggests a statistical approach to surgery. We have shown that it is possible with tether-free μ-grippers to retrieve high quality tissue samples which are suitable for either conventional cytologic analysis or genetic analysis. Further, the use of alternate polymer triggers could enable responsiveness to alternate stimuli, such as enzymes,8 and other biochemicals, to enable autonomous responses at diseased sites.
Supplementary Material
Acknowledgments
We would like to thank Anirban Maitra from The Sol Goldman Pancreatic Cancer Research Center, Department of Pathology, The Johns Hopkins University School of Medicine for his help on the tissue imaging. This work was funded in part by the NSF grant NSF CBET-1066898 (to D.H.G.), and the NIH Director’s New Innovator Award Program through grant DP2-OD004346-01 (to D.H.G.), in part by a Flight Attendants Medical Research Institute (FAMRI) grant (072119_YCSA to F.M.S.), by a Broad Medical Research Institute grant (IBD-0336 to F.M.S.) and by a K08 Award (DK090154-01) from the NIH (to F.M.S.).
Footnotes
Author Contributions
DHG, FMS, EG and ANK conceived the study and designed the experiments. EG, KEL and YSS fabricated and characterized the μ-grippers. DHG and EG designed the statistical model and EG developed it. FMS, MK, EG, AVO and KEL performed the ex vivo colonoscopy and in vivo esophagus sampling. FMS, SY and AVO performed the genetic analyses. FMS, SY, EG and SK performed tissue analyses. DHG, FMS, EG, ANK wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michalet X, Pinaud FF, Bentolila LA, et al. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins RH, Jr, Feldman M, Fordtran JS. Colon cancer, dysplasia, and surveillance in patients with ulcerative colitis A critical review. N Engl J Med. 1987;316:1654–1658. doi: 10.1056/NEJM198706253162609. [DOI] [PubMed] [Google Scholar]
- 3.Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–321. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 4.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 5.Gultepe E, Randhawa JR, Kadam S, et al. Biopsy with thermally-responsive untethered microtools. Advanced Materials. 2013;25:514–519. doi: 10.1002/adma.201203348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collado-Romero M, Arce C, Ramirez-Boo M, et al. Quantitative analysis of the immune response upon Salmonella typhimurium infection along the porcine intestinal gut. Vet Res. 2010;41:23. doi: 10.1051/vetres/2009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren J, Tang H, Yan X, et al. A pig-human comparative RH map comprising 20 genes on pig chromosome 13q41 that harbours the ETEC F4ac receptor locus. J Anim Breed Genet. 2009;126:30–36. doi: 10.1111/j.1439-0388.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 8.Bassik N, Brafman A, Zarafshar AM, et al. Enzymatically Triggered Actuation of Miniaturized Tools. J Am Chem Soc. 2010;132:16314–16317. doi: 10.1021/ja106218s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


