Abstract
During mitosis, duplicated sister chromatids are properly aligned at the metaphase plate of the mitotic spindle before being segregated into two daughter cells. This requires a complex process to ensure proper interactions between chromosomes and spindle microtubules. The kinetochore, the proteinaceous complex assembled at the centromere region on each chromosome, serves as the microtubule attachment site and powers chromosome movement in mitosis. Numerous proteins/protein complexes have been implicated in the connection between kinetochores and dynamic microtubules. Recent studies have advanced our understanding on the nature of the interface between kinetochores and microtubule plus ends in promoting and maintaining their stable attachment. These efforts have demonstrated the importance of this process to ensure accurate chromosome segregation, an issue which has great significance for understanding and controlling abnormal chromosome segregation (aneuploidy) in human genetic diseases and in cancer progression.
1. INTRODUCTION
For an accurate chromosome segregation during mitosis, each pair of sister chromatids duplicated in S phase captures spindle microtubules (MTs) and aligns at the metaphase plate of the mitotic spindle prior to anaphase onset. The kinetochore, the protein complex assembled at the centromere of each mitotic chromosome, serves as the attachment site for the spindle MTs. A combination of forces generated by kinetochores and microtubule dynamics is thought to contribute to kinetochore-MT attachment and chromosome movement in achieving metaphase chromosome alignment. Unattached kinetochores also generate the mitotic checkpoint signal to inhibit premature anaphase onset until every chromosome has been successfully attached to spindle MTs and aligned at the metaphase plate. The mitotic checkpoint has been reviewed elsewhere (Cleveland et al., 2003; Musacchio, 2011; Musacchio and Salmon, 2007). In the last decade, many proteins and protein complexes have been identified and many models have been proposed for metaphase chromosome alignment. This article will review the current state of the research and attempt to summarize our current understanding of different mechanisms involved in this process.
2. INITIAL SPINDLE MICROTUBULE CAPTURE BY KINETOCHORE
2.1. Classic “Search-and-Capture” Model
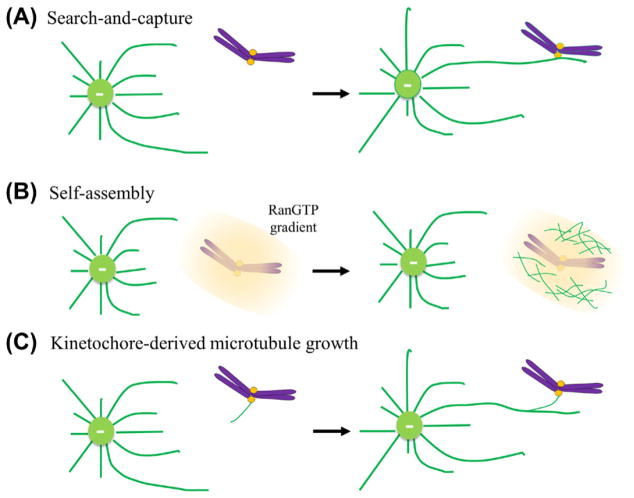
Metazoan cells progress through cell division with “open mitosis”. After nuclear envelope breakdown, centrosome-nucleated MTs undergo repeated growth and shrinkage in various directions until they are captured and stabilized by kinetochores – “search and capture” (Kirschner and Mitchison, 1986) (Fig. 6.1A). In this random process, the capture is initiated by lateral binding of a single MT with one of the sister kinetochores (Rieder and Alexander, 1990). Upon the MT capture, the sister chromatid pair exhibits a rapid poleward movement that is believed to be mediated by the kinetochore-associated minus end-directed motor, cytoplasmic dynein. The dynein-dependent poleward movement could be counted by the kinetochore-associated plus-end directed motor, CENP-E. Chromosomes are left at the spindle poles without CENP-E function (McEwen et al., 2001; Putkey et al., 2002). When the other sister kinetochore captures MTs from the opposite pole, the bi-oriented sister chromosome pair will then move toward the spindle equator. The “search-and-capture” model remains attractive; however, mathematical modeling analysis has shown that this mechanism alone is not efficient enough to allow the mitotic spindle to capture realistic number of chromosomes within characteristic mitotic time-scales (Wollman et al., 2005).
Figure 6.1. Initial interaction between kinetochores and microtubules.
(A) “Search-and-capture” model. Centrosome-nucleate microtubules undergo repeated growth and shrinkage in various directions until they are captured and stabilized by kinetochores. (B) A Ran-GTP gradient dependent “self-assembly” model. The chromatin association of the guanine nucleotide exchange factor (GEF) RCC1 produces a Ran-GTP gradient around mitotic chromosomes to simulate centrosome-independent microtubule nucleation. (C) Kinetochore-derived microtubule growth. Microtubules grow at or near kinetochore regions and later incorporate into the mitotic spindle. (For color version of this figure, the reader is referred to the online version of this book.)
2.2. “Self-Assembly” Model – a Ran-GTP Gradient-Dependent Process
MTs can be nucleated around chromosomes in mitotic cells (McKim and Hawley, 1995; Schmit et al., 1994). Addition of DNA-coated beads in CSF-arrested Xenopus meiotic egg extracts induces bi-polar mitotic structures in the absence of centrosomes and kinetochores (Heald et al., 1996). This “self-assembly” mechanism (Fig. 6.1B), by which chromosome-generated activities contribute to centrosome-independent MT nucleation, relies primarily upon a RanGTP gradient around mitotic chromosomes (Carazo-Salas et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999) that is established by the chromatin association with the guanine nucleotide exchange factor (GEF) RCC1 (Li et al., 2003).
The relative levels of contribution from the “search-and-capture” and “self-assembly” pathways vary in different systems. Compared to mammalian cells, Xenopus egg extracts have a larger area around the metaphase plate covered by the RanGTP gradient (Kalab et al., 2006). Abolishing the RanGTP gradient by adding excess RanGTP in egg extracts results in a substantial decrease in chromosome-MT interactions and metaphase chromosome misalignment (Caudron et al., 2005). In contrast, the consequences induced by perturbations of the Ran pathway in mammalian cells are much less severe, in which the most prominent phenotype is a metaphase delay (Kalab et al., 2006). In human cells undergoing mitosis with unreplicated genomes (MUG) in which kinetochores are spatially separated from the bulk of chromatin due to the minimal amount of centromeric DNA, mitotic spindles are robustly formed outside of the RanGTP gradient peak induced by MUG chromatin, supporting the predominance of the centrosome-kinetochore driven mechanism in mammalian cells (O’Connell et al., 2009).
2.3. Kinetochore-Derived Microtubule Growth
Kinetochore-derived MT growth has been proposed to enhance the encounter of kinetochores and spindle MTs (Fig. 6.1C). MTs have been observed to grow at or near kinetochore regions and later incorporate into the mitotic spindle (Khodjakov et al., 2003). The mechanism of MTs emerging directly from/around kinetochores is not completely understood. The chromosomal passenger complex (CPC) at the centromere has been shown to stimulate the pathway, possibly through Aurora B-mediated MT stabilization (Sampath et al., 2004). Ran-GTP is also found to be required for kinetochore-mediated MT organization (Tulu et al., 2006).
3. CONVERSION FROM LATERAL BINDING TO END-ON ATTACHMENT
By electron microscopy, MT ends appear to terminate at kinetochores, leading to the view that kinetochores capture MTs by end-on binding instead of lateral binding. How the initial lateral kinetochore-MT interaction is converted into an end-on attachment upon bi-orientation of the sister kinetochores remains an unresolved question in mitosis research. Recent studies on cytoplasmic dynein and its kinetochore-targeting components have shed some lights on this question.
The conversion of kinetochores from lateral binding to end-on MT attachment seems to correlate with the reduced level of dynein associated with kinetochores (King et al., 2000), though some dynein molecules continue to remain at kinetochores (Whyte et al., 2008). Inhibition of cytoplasmic dynein function by antibody injection or expressing dynein tail constructs without the motor domain impairs the end-on kinetochore-MT attachment to produce mis-oriented sister kinetochores relative to the spindle equator (Varma et al., 2008). This unexpected role of cytoplasmic dynein, a minus-end motor protein, in contributing to end-on attachment could be attributed to controlling the activity of the Rod/Zwilch/Zw10 (RZZ) complex at kinetochores (Gassmann et al., 2008). In Caenorhabditis elegans, depletion of Spindly by RNAi prevents dynein/dynactin targeting to kinetochores without perturbing RZZ kinetochore localization and reduces the formation of the load-bearing (end-on) attachments. In contrast, RZZ inhibition, which abolishes both dynein/dynactin and Spindly recruitment onto kinetochores, does not substantially affect end-on attachments. Therefore, the RZZ complex can inhibit the formation of end-on attachments, and this activity is controlled by the kinetochore dynein “cycle” involving dynein turnover through a combination of recruitment mediated by Spindly (Griffis et al., 2007) and self-removal (Whyte et al., 2008), along with the RZZ complex (Basto et al., 2004), upon the end-on attachment.
Another model to explain the contribution of cytoplasmic dynein to end-on attachment is pulling the bi-oriented sister kinetochore pairs to balance the pushing force from the plus-end motor, CENP-E, resulting in end-on attachment as a metastable compromise (Mao et al., 2010). This motor-mediated activity could facilitate the interactions of MT lattice at or close to the plus ends with kinetochore-associated MT-binding proteins, such as the KMN network (see below for details).
4. STABLE END-ON KINETOCHORE-MICROTUBULE ATTACHMENT
Upon bi-orientation and switching to the end-on attachment, the sister kinetochores maintain stable interactions with dynamic MT plus-ends and power chromosome movement coupled with MT polymerization and depolymerization. Many proteins and protein complexes, including motors and non-motor MT binding proteins, are necessary for this stable attachment, as well as the processive MT plus-end tracking.
4.1. Hill-Sleeve Model
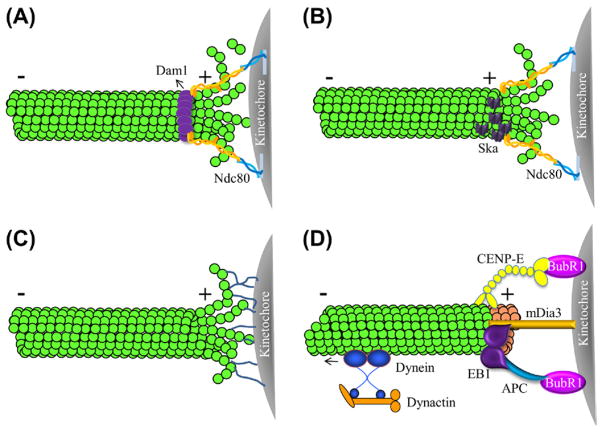
In 1985, Terrell Hill proposed a sleeve model to describe the interaction between the kinetochores and spindle MTs (Hill, 1985). The kinetochore-associated components surround MTs near the plus-ends and create a rigid sleeve at the outer surface of MTs. With many weak binding sites, the sleeve can slide along the polymerizing or depolymerizing MTs and continuously associate with the dynamic plus ends (Fig. 6.2A).
Figure 6.2. The connection between kinetochores and microtubule plus ends.
(A) The Hill-sleeve model suggests that kinetochores connect with microtubules near the plus-ends through a rigid sleeve, such as the Dam1 complex (purple), at the outer surface of micro-tubules. (B) The biased-diffusion model has been proposed in which the Ndc80 complex serves as the major microtubule binding sites at the kinetochore for stabilizing end-on microtubule attachment. Ndc80 is able to detect the curving of protofilaments at depolymerizing microtubule ends (or through its interaction with the Ska complex) and diffuse along the lattice of shrinking microtubules. (C) The fibril-connector model proposes that kinetochores bind to the luminal side of peeling protofilaments through fibril-like attachments. (D) Microtubule attachment and plus-end tracking mediated by motor and microtubule plus-end binding proteins. The plus-end motor CENP-E, a binding partner of a kinetochore-associated kinase BubR1, captures spindle microtubules. The minus-end directed motor cytoplasmic dynein pulls itself, as well as other kinetochore components associated with it such as dynactin, out of the kinetochore and stream along the kinetochore microtubules. Microtubule plus-end binding proteins (such as EB1 and APC) track microtubule plus ends. The interaction between EB1/APC and the kinetochore-associated formin mDia3 provides another connection between kinetochores and microtubule ends. The stable accumulation of EB1 and APC on kinetochore microtubule ends can be influenced by the mitotic kinases, e.g., BubR1 and Bub1. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this book.)
The discovery of the formation of ring-like structures around MTs by the oligomeric Dam1/DASH complex makes the Hill-Sleeve model more attractive (Miranda et al., 2005; Westermann et al., 2005). In vitro studies with purified components show that the Dam1/DASH complex containing 16 monomers can form a ring with an insider diameter of ~35 nm around the MT, which has a 25 nm outside diameter (Wang et al., 2007; Westermann et al., 2005). The interaction is mediated by the C-termini of the Dam1/DASH that extends to MT lattice (Westermann et al., 2005). The Dam1/DASH rings exhibit lateral mobility on MTs (Westermann et al., 2005) and can track the curling ends of depolymerizing MTs (Wang et al., 2007). We should note here that the Dam1/DASH ring structure has not been demonstrated in vivo.
Although it is well conserved in fungi, the Dam1/DASH complex is essential for survival only in budding yeast, but not in fission yeast (Sanchez-Perez et al., 2005). Budding yeast requires the Dam1/DASH complex for viability, probably because it has only one MT bound to each kinetochore. In contrast, every kinetochore captures several MTs in fission yeast. The Dam1/DASH complex is also essential in Candida albicans, another yeast with kinetochores that only attach to a single MT (Joglekar et al., 2008). The need of Dam1/DASH complex for growth can, however, be partially bypassed upon increasing the expression level of CENP-A (a centromere specific histone H3 variant) to recruit more kinetochore proteins to the centromere region and to increase the numbers of MTs bound to each kinetochore (Burrack et al., 2011). In vertebrates, kinetochores are generally attached to 20–30 MTs. Up to date, no homologs of the Dam1/DASH complex have been identified.
4.2. Biased-Diffusion Model
Recent studies have suggested that the conserved Ndc80 complex serves as the major MT-binding site at the kinetochore for stabilizing the end-on MT attachment. This topic has been reviewed in great detail elsewhere (Joglekar et al., 2010; Santaguida and Musacchio, 2009). Structural analysis (Ciferri et al., 2008), in vitro single molecule assays (Powers et al., 2009), and super resolution microscopy analysis in cells (Wan et al., 2009) all support a biased-diffusion mechanism for force generation by Ndc80 molecules acting along the MT axis (Fig. 6.2B).
The Ndc80 complex is composed of four subunits, comprising Ndc80 (Hec1), Nuf2, Spc24, and Spc25. This complex has a rod-like structure with two globular ends: one binds to MTs and the other anchors to the kinetochores (Ciferri et al., 2008; Wei et al., 2005). The flexible hinged coiled coil (Wang et al., 2008) and the weak affinity to MTs (Cheeseman et al., 2006) make the Ndc80 complex ideal for biased diffusion. By a subnanometer-resolution cryo-electron microscopy, the Ndc80 complex has been shown to bind to MTs with a tubulin monomer repeat recognizing α- and β-tubulin at both intra- and inter-tubulin dimmer interfaces in a manner that is sensitive to tubulin conformation and to self-associate along protofilaments (Alushin et al., 2010), arguing that Ndc80 could detect the curving protofilaments at depolymerizing MT ends and, consequently, diffuse along shrinking MTs.
In vitro motility assays with purified components have also supported the biased-diffusion model. By total internal reflection fluorescence (TIRF) microscopy, the Ndc80 complex at single molecule level exhibits transient one-dimensional diffusion along the MT lattice (Powers et al., 2009). On the other hand, the Ndc80-coated microbeads with 6–30 complexes, similar to what have been found per MT at kinetochores in vivo (Emanuele et al., 2005; Joglekar et al., 2008), are able to track MT tips that permit assembly- and disassembly-coupled movement (Powers et al., 2009). Furthermore, the yeast Dam1 complex can enhance the ability of the Ndc80 complex to form a load-bearing MT attachment and mediate the continuous association of Ndc80 with dynamic MT plus ends in vitro (Lampert et al., 2010).
The Ska (spindle and kinetochore associated) complex (Hanisch et al., 2006), composed of three subunits (Ska1, Ska2, and Ska3/Rama1), has also been shown to be important for the end-on kinetochore-MT attachment. The kinetochore association of the Ska complex has been shown to be dependent on the Ndc80 complex (Gaitanos et al., 2009), possibly through a direct interaction (Chan et al., 2012; Zhang et al., 2012). Depletion of any of the Ska subunits results in reduced stability of kinetochore-MT attachment (Gaitanos et al., 2009; Raaijmakers et al., 2009; Welburn et al., 2009). The Ska complex-coated beads can bind and move along the MTs and track shortening (depolymerizing) MT plus ends (Welburn et al., 2009). Although the Ska complex has been proposed to be the functional homolog to the Dam1 complex in metazoans (Guimaraes and Deluca, 2009), electron microscopy studies show no evidence for the ring-like structure of the Ska complex in vitro (Jeyaprakash et al., 2012). In contrast, the Ska core complex shows a W-shape dimer of coiled coils with MT-binding domains at both ends (Jeyaprakash et al., 2012), which is a symmetric structure ideally suited for the diffusion properties on MTs (Cooper and Wordeman, 2009).
4.3. Fibril-Connector Model
Both the Hill-sleeve and the bias-diffusion models assume that the kinetochore attachment sites are expected to localize at MT sides, but very close to MT plus ends. An alternative model has been proposed that kinetochores bind to the luminal side of peeling protofilaments through fibril-like attachments (Fig. 6.2C). This fibril-connector model is based on the electron microscopy imaging of mitotic PtK1 cells (McIntosh et al., 2008): the protofilaments appear to be curved at the growing and shortening plus ends of MTs and connected to the inner kinetochores by fibrils, which are not observed on non-kinetochore MTs.
The molecular components of the fibrils remain unidentified. However, several kinetochore-associated proteins are likely to be filamentous. CENP-E, the kinetochore-associated kinesin-like motor protein, has a ~200 nm long and flexible coiled coil resembling the longest fibrils that have been observed with electron microscopy (Kim et al., 2008). Rotary shadowing EM of Ndc80 has revealed that this hetero-tetrameric complex is a ~57 nm-long rod (Wei et al., 2005). By unidirectional shadowing and electron microscopy, XMAP212 appears as an elongate molecule of about 60 nm with some flexibility (Cassimeris et al., 2001). However, all of these three proteins/protein complexes have been shown to bind to MT lattice. Another possible candidate is CENP-F, a ~400 kD protein with a predicted structure consisting of two long coil domains that flank a central flexible core. CENP-F localizes at the outer kinetochore region and extends into the fibrous corona by immune-electron microscopy (Rattner et al., 1993). By MT pellet assay, several in vitro translated CENP-F fragments have been shown to bind to MTs (Feng et al., 2006).
4.4. Tracking the Ends: Contributions of Motors and Microtubule Plus-End Interacting Proteins
The kinetochore-associated plus end motor CENP-E can stabilize the MT capture. Antibodies against CENP-E, but not cytoplasmic dynein, can slow or stop chromosome motion on disassembling MTs in vitro (Lombillo et al., 1995), arguing a role of CENP-E in maintaining the attachments with depolymerizing MTs. In primary mouse fibroblasts without CENP-E, most aligned kinetochores bound only half the normal number of MTs and polar chromosomes have no obvious attached MTs (Putkey et al., 2002). Another mitotic centromere-associated kinesin (MCAK) is an MT disassemblase (Desai et al., 1999; Walczak et al., 1996; Wordeman and Mitchison, 1995). MCAK tracks MT tips by binding to EB1 (Montenegro Gouveia et al., 2010) and regulates spindle MT length to promote kinetochore-MT attachment (Domnitz et al., 2012).
Besides kinetochore associated components, a group of MT plus-end binding proteins have also emerged to be involved in mediating the connections between kinetochores and dynamic MT plus-ends. The members of the EB1 protein family can bind to plus ends of cytoplasmic, spindle, and astral MTs (Berrueta et al., 1998; Mimori-Kiyosue et al., 2000; Morrison et al., 1998) and track MT plus-ends in an in vitro reconstitution system by TIRF microscopy (Bieling et al., 2007). EB1 has been shown to associate only with the trailing kinetochore (in a sister kinetochore pair) where there is a net kinetochore MT growth (polymerization) (Tirnauer et al., 2002). EB1 knockdown mediated by siRNA in cells (Draviam et al., 2006; Green et al., 2005) or immuno-depletion from cycled Xenopus egg extracts (Zhang et al., 2007) produces bi-polar mitotic spindles with misaligned chromosomes and/or kinetochore-MT attachment defects.
Most MT tip-binding proteins have the ability to physically associate with a number of other tip-tracking proteins creating a complex web of interactions to integrate their activities at the MT plus ends (Akhmanova and Steinmetz, 2008). EB1 could play a central role at the interface of kinetochores and MT plus ends, since EB1 has been shown to interact with APC (Berrueta et al., 1999; Honnappa et al., 2005; Su et al., 1995), CLIP170 (Blake-Hodek et al., 2010; Dixit et al., 2009; Goodson et al., 2003), Clasp1 (Mimori-Kiyosue et al., 2005), p150 Glued (Askham et al., 2002; Hayashi et al., 2005), and XMAP215 (Kronja et al., 2009), all of which have been shown to be essential for mitosis (Joglekar et al., 2010; Maiato et al., 2004).
The interaction with growing MT plus ends is an intrinsic property of the tip-tracking protein EB1 (Mimori-Kiyosue et al., 2000; Slep and Vale, 2007), but the mechanism and the role of its stable association with the trailing sister kinetochore are not clear. In interphase cells, the mDia formin proteins have been shown to serve as the scaffold for EB1 and APC at cell cortex to stabilize MTs in promoting cell migration (Wen et al., 2004). The formin mDia3 has been shown to localize at the kinetochores in an MT-independent manner and knockdown of the formin mDia3 by siRNA results in chromosome misalignment phenotypes in mammalian cells, reminiscent of the depletion of the Ndc80 and the Ska1 complexes (Cheng et al., 2011; Yasuda et al., 2004). Using an mDia3 mutant that cannot bind to EB1, it has been shown that the mDia3-EB1 interaction is essential for mDia3’s role in metaphase chromosome alignment (Cheng et al., 2011), indicating that this interaction can be one of the connections between kinetochores and the plus ends of growing MTs (Mao, 2011). Finally, the role of EB1 and APC in stabilizing kinetochore-MT attachment could be regulated by two kinetochore-associated mitotic kinases, Bub1 and BubR1 (Kaplan et al., 2001; Zhang et al., 2007).
5. CHROMOSOME CONGRESSION AND OSCILLATION AT METAPHASE PLATE
5.1. Classic Model of Congression and Oscillation
After capturing spindle MTs from opposite poles and becoming bi-orientated, sister kinetochores undergo a series of regular oscillations toward the spindle equator, a process called “congression”, and continue to oscillate at the metaphase plate (Skibbens et al., 1993). It has been proposed that this kinetochore “directional instability” involves the pulling force at the “leading” kinetochore and the pushing force at the “trailing” one and the tension generated between sister kinetochores could control the switch (Skibbens et al., 1993). Later analysis by video-light microscopy combined with a laser beam to sever the connection between a pair of bi-oriented sister kinetochores have shown that the “leading” kinetochore continued the motion whereas the “trailing” one stopped (Khodjakov and Rieder, 1996). This result has indicated that the major force moving the chromosome pair is generated at the sister kinetochore with depolymerizing MTs. The sister kinetochores alternatively “lead” during oscillation and congression processes rather than a direct movement until the force is balanced at the spindle equator (Hayden et al., 1990).
5.2. Congression Before Bi-orientation without End-on Attachment
By live-cell light microscopy and correlative electron microscopy, cells subjected to a sequence of mitotic inhibitors to allow detailed analysis of congression movement have mono-oriented kinetochores congressing toward the metaphase plate via lateral attachments with existing kinetochore MT fibers (Kapoor et al., 2006). Since CENP-E inhibition produces polar chromosomes, it is argued that CENP-E, the processive plus end-directed kinetochore motor (Kim et al., 2008; Yardimci et al., 2008), is responsible for the transport of the mono-oriented chromosomes. However, the nature of the existence of the polar chromosomes in CENP-E-depleted cells needs to be more carefully examined. CENP-E depletion with siRNA, as well as expressing a non-phosphorylatable BubR1 mutant at the CENP-E-dependent BubR1 auto-phosphorylation site, results in a decrease of Aurora B-mediated Ndc80 phosphorylation at unattached kinetochores (Guo et al., 2012). Furthermore, expressing a phosphomimetic BubR1 mutant at the auto-phosphorylation site substantially reduces the incidence of polar chromosomes in CENP-E-depleted cells (Guo et al., 2012).
5.3. Regulation of Chromosome Congression and Oscillation
Besides MT depolymerization at the leading kinetochore being the main energy source for chromosome oscillation (Khodjakov et al., 1996), how kinetochores coordinate oscillatory movements with the attachment remains largely unknown. Kinetochores generally bind to bundles of many MTs (up to 25–30 bundled MTs in mammalian cells), which contain both polymerizing and depolymerizing MT plus ends (McIntosh et al., 2008). Therefore, the dynamics of these kinetochore-bound MT plus ends must be, at least, partially synchronized for oscillation to occur (Civelekoglu-Scholey et al., 2006; Gardner and Odde, 2006). This is probably achieved by a much slower tubulin turnover rate (Hyman and Mitchison, 1990; Zhai et al., 1995), which could also facilitate the attachments. The molecular mechanism to coordinate the dynamics of kinetochore-bound MT bundles is not clear. One of the attractive candidates is the kinetochore-associated formin mDia3 (Mao, 2011). The mDia formin proteins not only directly interact with the tip-tracking proteins EB1 and APC (Cheng et al., 2011; Wen et al., 2004), but also reduce rates of both polymerization and depolymerization of MTs (Bartolini et al., 2008).
There are several other proteins and protein complexes that have also been implicated in affecting chromosome oscillatory movements. Inhibition of cytoplasmic dynein function at the kinetochore (Varma et al., 2008) or depletion of Kif18A (Stumpff et al., 2008) produces an increase in the magnitude of kinetochore oscillations along the major spindle axis. The MT depolymerase Kif18A and the minus-end motor cytoplasmic dynein could mechanically influence MT depolymerization during kinetochore oscillations. Furthermore, the Kif18A accumulates in a gradient manner on the kinetochore-attached MTs dependent on its motor activity, and thus, possibly regulating the switch of the oscillatory direction (Stumpff et al., 2008). On the other hand, depletion of the CENP-A nucleosome-associated and CENP-A distal (NAC/CAD) complexes results in suppressed kinetochore oscillations (Amaro et al., 2010). The loss of NAC/CAD complexes could affect the recruitment of other outer kinetochore components that are important for regulating MT dynamics at the kinetochores (Cheeseman et al., 2008). Alternatively, one of the components of the CENP-A NAC/CAD complexes, CENP-Q, has been shown to make direct physical interactions with MTs in vitro (Amaro et al., 2010); however, whether CENP-Q can directly influence MT dynamics remains untested.
The polar ejection force at chromosome arms as a result of the interaction between chromokinesins and spindle MTs could balance poleward kinetochore forces, and thus, influence chromosome congression and oscillation. Drosophila Nod, the first identified chromokinesin, is required for proper alignment and segregation of meiotic chromosomes (Afshar et al., 1995; Zhang et al., 1990). Immunodepletion of the Xenopus homolog, Kid, from egg extracts results in congression defects and metaphase chromosome misalignment (Antonio et al., 2000; Funabiki and Murray, 2000). Antibody-induced inhibition of Kid in human cells blocks oscillations, but not congression (Levesque and Compton, 2001).
6. KINETOCHORE-MICROTUBULE ATTACHMENT ERROR CORRECTION
6.1. Attachment Error Correction Mechanisms Centered with Aurora B
The geometry of a pair of sister kinetochores favors proper bi-oriented kinetochore-MT attachment, termed amphitelic, in which one sister kinetochore captures MT from one spindle pole and the other one is attached to the opposite pole (Loncarek et al., 2007). However, improper attachments, such as syntelic attachments (both sister kinetochores attach to the same pole) and merotelic attachments (a single kinetochore captures MTs from both spindle poles), frequently occur in early prometaphase, producing polar chromosomes in metaphase (Hauf et al., 2003). Current studies clearly demonstrate that Aurora B is a central component actively involved in the error correction process (Lampson and Cheeseman, 2011; Walczak and Heald, 2008). Aurora B is a family member of serine/threonine protein kinases (Kimura et al., 1997) and has the preferred phosphorylation consensus sequence as [RK]x[TS][ILV] (Cheeseman et al., 2002). In budding yeast, Ipl1, the yeast homolog of Aurora B, facilitates bi-orientation by promoting turnover of kinetochore MTs until tension is generated when the sister kinetochores are attached to opposite spindle poles (Tanaka et al., 2002). In vertebrates, inhibiting Aurora B kinase activity with small molecules or depleting Aurora B with siRNA results in an increase of numerous mono-oriented chromosomes with syntelic attachment (Ditchfield et al., 2003; Hauf et al., 2003). Aurora B is enriched at merotelic attachment sites (Knowlton et al., 2006) and promotes the turnover of kinetochore MTs to reduce segregation errors (Cimini et al., 2006).
MCAK is the first substrate of the Aurora B kinase that has been argued to be involved in attachment error correction. MCAK is enriched at merotelic attachments (Knowlton et al., 2006). Depletion of the centromeric MCAK with a centromere dominant-negative protein in mammalian cultured cells results in kinetochore-MT attachment defects, including merotelic and syntelic attachments (Kline-Smith et al., 2004). These results would make MCAK an attractive candidate to depolymerize improperly attached MTs upon Aurora B activation were it not that Aurora B phosphorylation of MCAK actually inhibits its MT disassembly activity (Andrews et al., 2004; Lan et al., 2004; Ohi et al., 2004).
Aurora B also phosphorylates a group of MT-associated proteins, including the Dam1 complex (Cheeseman et al., 2002), the KMN (KNL1-Mis12-Ndc80) network (Cheeseman et al., 2006; DeLuca et al., 2006; Welburn et al., 2010), and the formin mDia3 (Cheng et al., 2011). The Aurora B-mediated phosphorylation reduces the MT-binding activity of these proteins (Cheeseman et al., 2006; Cheng et al., 2011; Welburn et al., 2010), which could destabilize improperly attached kinetochore MTs. Furthermore, the Aurora B phosphorylation can also inhibit the cooperation between the Ndc80 complex and either the Dam1 complex (Lampert et al., 2010) or the Ska complex (Chan et al., 2012) to control kinetochore-MT attachments.
6.2. Spatial Separation Model
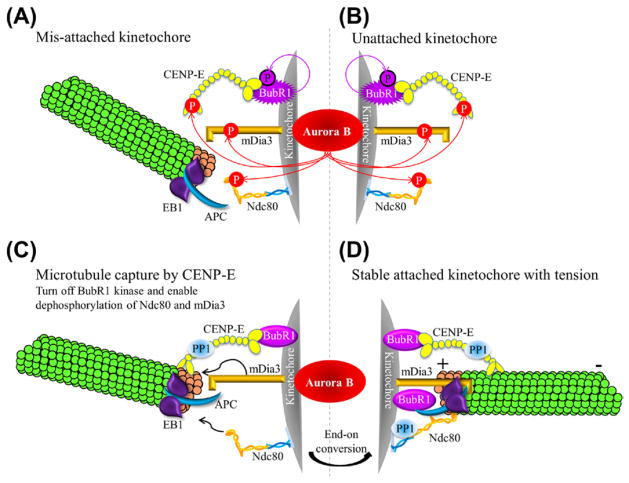
One of the important unresolved questions for the error correction mechanism is how to differentiate between proper and improper attachments. A “spatial separation” model has suggested whether the attachment is stabilized or not depends on the physical distance between the Aurora B kinase and its kinetochore-associated substrates (Lampson and Cheeseman, 2011). Bi-oriented proper attachments exerting tension across the sister kinetochores (Akiyoshi et al., 2010; Nicklas, 1997) can separate Aurora B, which localizes at the inner centromere, from its outer kinetochore substrates (Keating et al., 2009; Liu et al., 2009). The dephosphorylated form of these Aurora B substrates is able to bind to MTs and stabilize the correct attachments (Cheeseman et al., 2006; Cheng et al., 2011; DeLuca et al., 2006; Welburn et al., 2009). Conversely, with improper attachments in which there is little or no tension, the Aurora B kinase is physically close to and phosphorylates its substrates, resulting in reduced MT binding affinity, which leads to MT destabilization (Fig. 6.3A).
Figure 6.3. A model for establishing proper stable kinetochore-microtubule attachment.
(A and B) Mis-attached (A) or unattached (B) kinetochores have Aurora B-mediated phosphorylation of the KMN network (represented by Ndc80 complex in the cartoon), CENP-E, and the formin mDia3, which causes destabilization of improperly attached kinetochore microtubules. (C) Upon spindle microtubule capture, CENP-E can turn off the kinase activity and auto-phosphorylation of BubR1 and recruit PP1. Both activities are essential to reduce Aurora B-mediated phosphorylation on kinetochore-associated substrates. These coordinated events enable the Ndc80 and mDia3 to bind to microtubules. (D) After converting into end-on attachment to produce tension, the inter kinetochore stretch separates the inner centromeric Aurora B from outer kinetochore substrates, resulting in stable kinetochore-microtubule attachment. (For color version of this figure, the reader is referred to the online version of this book.)
This “spatial separation” model assumes that tension (inter-kinetochore stretch) can physically separate the inner centromere-associated Aurora B from its substrates localized at the outer kinetochore. However, a population of active Aurora B kinase has been shown to be enriched at the outer kinetochore in both HeLa and PtK1 cells throughout mitosis (DeLuca et al., 2011). Furthermore, in order to produce tension, the phosphorylated form of these outer kinetochore components has to be reversed for the initial spindle MT capture in the vicinity of Aurora B kinase (Fig. 6.3B).
6.3. “Sensor”–Dependent Regulation of Aurora B-mediated Phosphorylation
An alternative model to “spatial separation” is the regulation of intrinsic kinase activity of Aurora B in response to spindle MT capture and/or subsequent tension generation. Aurora B is a component of the CPC, which also includes INCENP, Borealin/Dasra, and Survivin (Jeyaprakash et al., 2007). Within the complex, INCENP stimulates Aurora B kinase activity (Bishop and Schumacher, 2002; Honda et al., 2003; Sessa et al., 2005) and directly binds to MTs (Adams et al., 2001; Wheatley et al., 2001). Survivin can also stimulate Aurora B kinase activity (Chen et al., 2003). In budding yeast, Bir1 and Sli15, the homologs of Survivin and INCENP, act as tension sensors by linking centromere to MTs and activate Ipl1 (Aurora B) in attachment error correction (Sandall et al., 2006). TD-60, an inner centromere protein, has also been shown to be essential for Aurora B activation along with MTs (Rosasco-Nitcher et al., 2008).
Another candidate that can regulate Aurora B-mediated phosphorylation in attachment error correction is the kinetochore-associated mitotic kinase BubR1, along with its regulator, the kinetochore-associated motor CENP-E. Kinetochores do not form stable attachment with spindle MTs in BubR1-depleted human cells (Lampson and Kapoor, 2005) and Xenopus egg extracts (Zhang et al., 2007). This attachment defect is in part attributed to an increase in Aurora B kinase activity judging by the increased levels of Aurora B-mediated CENP-A (Lampson and Kapoor, 2005) and Ndc80 phosphorylation (Guo et al., 2012). The kinase activity of BubR1, as well as its auto-phosphorylation, is stimulated upon its interaction with CENP-E (Guo et al., 2012; Mao et al., 2003; Weaver et al., 2003). Upon spindle MT capture by CENP-E, the BubR1 kinase activity is silenced in a ternary complex of BubR1-CENP-E-MTs (Mao et al., 2005) and BubR1 becomes non-phosphorylated at the auto-phosphorylation site (Guo et al., 2012). The non-phosphorylated form of BubR1, which normally resides on attached kinetochores, is able to reduce the levels of Aurora B-mediated Ndc80 phosphorylation at kinetochores (Guo et al., 2012). Therefore, the capture of spindle MTs by kinetochore-associated motor CENP-E results in a decrease of BubR1 kinase activity and auto-phosphorylation and, therefore, reduced levels of Aurora B-mediated phosphorylation at kinetochores. Along with a local increase of KNL- and CENP-E-bound PP1 (Kim et al., 2010; Liu et al., 2010; Rosenberg et al., 2011), this first essential step will reverse the Aurora B-dependent inactivation state of the core MT-binding proteins, such as the KMN network, to initiate kinetochore-MT attachment (Fig. 6.3C). The subsequent tension generated between the sister kinetochores pulls these kinetochore-associated substrates further away from Aurora B at the inner centromere to enhance the stability of kinetochore-MT connections (Fig. 6.3D).
6.4. Kinetochore-Associated Protein Phosphatase Activity
Activity of kinases is usually restricted by protein phosphatases. PP1 is the likely phosphatase for opposing the Aurora B kinase at kinetochores. Studies with a PP1 mutant (glc7-10) of Saccharomyces cerevisiae have revealed that the phosphatase activity is important for the MT binding activity of the kinetochore in vitro and in vivo (Sassoon et al., 1999). The budding yeast PP1 is recruited to the kinetochore by the Fin1 protein (Akiyoshi et al., 2009). In human cells, time-lapse imaging reveals that the fluorescently-labeled PP1γ protein localizes to kinetochores and exchanges rapidly with the diffuse cytoplasmic pool (Trinkle-Mulcahy et al., 2003). Two kinetochore-associated proteins, KNL1 (Liu et al., 2010) and CENP-E (Kim et al., 2010), have been shown to directly interact with PP1 through a conserved docking motif. KNL1-mediated kinetochore recruitment of PP1 opposes Aurora B kinase activity and is important for the formation of cold-stable kinetochore-associated MT fibers (Liu et al., 2010). Injecting an antibody, which inhibits PP1-mediated dephosphorylation of CENP-E, in human cells produces polar chromosomes that cannot form stable MT attachment (Kim et al., 2010).
7. CONCLUSIONS
It is now clear that the process to ensure each pair of sister chromatids ending up at the metaphase plate prior to chromosome segregation is much more complex than initially imagined. Significant progress has been made in identifying active components in stabilizing kinetochore-MT attachment and in powering chromosome movement to ensure accurate chromosome segregation. There are many kinetochore-associated kinases; however, mechanistically the role and the regulation for majority of them have yet to be identified. More efforts are needed in the coming years to understand how forces generated at the interface between kinetochores and MTs can control mitotic progression and mitotic checkpoint signals.
Acknowledgments
We thank all members of the Mao laboratory for stimulating discussion. The work in the Mao laboratory is supported by a grant from the National Institutes of Health (GM089768) and a Research Scholar grant from the American Cancer Society (RSG-09-027-01-CCG) to Y.M. Y.M. is a recipient of Irma T. Hirschl/Monique Weill-Caulier Trusts Research Award.
References
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and Aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar K, Barton NR, Hawley RS, Goldstein LS. DNA binding and meiotic chromosomal localization of the Drosophila nod kinesin-like protein. Cell. 1995;81:129–138. doi: 10.1016/0092-8674(95)90377-1. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Akiyoshi B, Nelson CR, Ranish JA, Biggins S. Quantitative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev. 2009;23:2887–2899. doi: 10.1101/gad.1865909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576–579. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–U868. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro AC, Samora CP, Holtackers R, Wang E, Kingston IJ, Alonso M, Lampson M, McAinsh AD, Meraldi P. Molecular control of kinetochore-microtubule dynamics and chromosome oscillations. Nat Cell Biol. 2010;12:319–329. doi: 10.1038/ncb2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, Vernos I. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate [see comments] Cell. 2000;102:425–435. doi: 10.1016/s0092-8674(00)00048-9. [DOI] [PubMed] [Google Scholar]
- Askham JM, Vaughan KT, Goodson HV, Morrison EE. Evidence that an interaction between EB1 and p. 150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell. 2002;13:3627–3645. doi: 10.1091/mbc.E02-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R, Scaerou F, Mische S, Wojcik E, Lefebvre C, Gomes R, Hays T, Karess R. In vivo dynamics of the rough deal checkpoint protein during Drosophila mitosis. Curr Biol. 2004;14:56–61. doi: 10.1016/j.cub.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Berrueta L, Kraeft SK, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, Pellman D, Bierer BE. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 2007;450:1100–1105. doi: 10.1038/nature06386. [DOI] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake-Hodek KA, Cassimeris L, Huffaker TC. Regulation of microtubule dynamics by Bim1 and Bik1, the budding yeast members of the EB1 and CLIP-170 families of plus-end tracking proteins. Mol Biol Cell. 2010;21:2013–2023. doi: 10.1091/mbc.E10-02-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack LS, Applen SE, Berman J. The requirement for the Dam1 complex is dependent upon the number of kinetochore proteins and microtubules. Curr Biol. 2011;21:889–896. doi: 10.1016/j.cub.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Gard D, Tran PT, Erickson HP. XMAP215 is a long thin molecule that does not increase microtubule stiffness. J Cell Sci. 2001;114:3025–3033. doi: 10.1242/jcs.114.16.3025. [DOI] [PubMed] [Google Scholar]
- Caudron M, Bunt G, Bastiaens P, Karsenti E. Spatial coordination of spindle assembly by chromosome-mediated signaling gradients. Science. 2005;309:1373–1376. doi: 10.1126/science.1115964. [DOI] [PubMed] [Google Scholar]
- Chan YW, Jeyaprakash AA, Nigg EA, Santamaria A. Aurora B controls kinetochore-microtubule attachments by inhibiting Ska complex-KMN network interaction. J Cell Biol. 2012;196:563–571. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Hori T, Fukagawa T, Desai A. KNL1 and the CENP-H/I/K complex coordinately direct kinetochore assembly in vertebrates. Mol Biol Cell. 2008;19:587–594. doi: 10.1091/mbc.E07-10-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jin S, Tahir SK, Zhang H, Liu X, Sarthy AV, McGonigal TP, Liu Z, Rosenberg SH, Ng SC. Survivin enhances Aurora-B kinase activity and localizes Aurora-B in human cells. J Biol Chem. 2003;278:486–490. doi: 10.1074/jbc.M211119200. [DOI] [PubMed] [Google Scholar]
- Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, Mao Y. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell. 2011;20:342–352. doi: 10.1016/j.devcel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Civelekoglu-Scholey G, Sharp DJ, Mogilner A, Scholey JM. Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis. Biophysical J. 2006;90:3966–3982. doi: 10.1529/biophysj.105.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Wordeman L. The diffusive interaction of microtubule binding proteins. Curr Opin Cell Biol. 2009;21:68–73. doi: 10.1016/j.ceb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124:622–634. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur EL. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc Natl Acad Sci USA. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domnitz SB, Wagenbach M, Decarreau J, Wordeman L. MCAK activity at microtubule tips regulates spindle microtubule length to promote robust kinetochore attachment. J Cell Biol. 2012;197:231–237. doi: 10.1083/jcb.201108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, McCleland ML, Satinover DL, Stukenberg PT. Measuring the stoichiometry and physical interactions between components elucidates the architecture of the vertebrate kinetochore. Mol Biol Cell. 2005;16:4882–4892. doi: 10.1091/mbc.E05-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Huang H, Yen TJ. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 2006;115:320–329. doi: 10.1007/s00412-006-0049-5. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement [see comments] Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf 3. EMBO J. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MK, Odde DJ. Modeling of chromosome motility during mitosis. Curr Opin Cell Biol. 2006;18:639–647. doi: 10.1016/j.ceb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Gassmann R, Essex A, Hu JS, Maddox PS, Motegi F, Sugimoto A, O’Rourke SM, Bowerman B, McLeod I, Yates JR, 3rd, Oegema K, Cheeseman IM, Desai A. A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 2008;22:2385–2399. doi: 10.1101/gad.1687508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson HV, Skube SB, Stalder R, Valetti C, Kreis TE, Morrison EE, Schroer TA. CLIP-170 interacts with dynactin complex and the APC-binding protein EB1 by different mechanisms. Cell Motil Cytoskelet. 2003;55:156–173. doi: 10.1002/cm.10114. [DOI] [PubMed] [Google Scholar]
- Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177:1005–1015. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes GJ, Deluca JG. Connecting with Ska, a key complex at the kinetochore-microtubule interface. EMBO J. 2009;28:1375–1377. doi: 10.1038/emboj.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kim C, Ahmad S, Zhang J, Mao Y. CENP-E-dependent BubR1 auto-phosphorylation enhances chromosome alignment and the mitotic checkpoint. J Cell Biol. 2012;198:205–217. doi: 10.1083/jcb.201202152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A, Sillje HH, Nigg EA. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 2006;25:5504–5515. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Wilde A, Mal TK, Ikura M. Structural basis for the activation of microtubule assembly by the EB1 and p. 150Glued complex. Mol Cell. 2005;19:449–460. doi: 10.1016/j.molcel.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hill TL. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnappa S, John CM, Kostrewa D, Winkler FK, Steinmetz MO. Structural insights into the EB1-APC interaction. EMBO J. 2005;24:261–269. doi: 10.1038/sj.emboj.7600529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Modulation of microtubule stability by kinetochores in vitro. J Cell Biol. 1990;110:1607–1616. doi: 10.1083/jcb.110.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Santamaria A, Jayachandran U, Chan YW, Benda C, Nigg EA, Conti E. Structural and functional organization of the ska complex, a key component of the kinetochore-microtubule interface. Mol Cell. 2012;46:274–286. doi: 10.1016/j.molcel.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Joglekar AP, Bloom KS, Salmon ED. Mechanisms of force generation by end-on kinetochore-microtubule attachments. Curr Opin Cell Biol. 2010;22:57–67. doi: 10.1016/j.ceb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bouck D, Finley K, Liu X, Wan Y, Berman J, He X, Salmon ED, Bloom KS. Molecular architecture of the kinetochore-microtubule attachment site is conserved between point and regional centromeres. J Cell Biol. 2008;181:587–594. doi: 10.1083/jcb.200803027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating P, Rachidi N, Tanaka TU, Stark MJ. Ipl1-dependent phosphorylation of Dam1 is reduced by tension applied on kinetochores. J Cell Sci. 2009;122:4375–4382. doi: 10.1242/jcs.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Bajer AS, Rieder CL. The force for poleward chromosome motion in Haemanthus cells acts along the length of the chromosome during metaphase but only at the kinetochore during anaphase. J Cell Biol. 1996;132:1093–1104. doi: 10.1083/jcb.132.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Heuser JE, Waterman CM, Cleveland DW. CENP-E combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. J Cell Biol. 2008;181:411–419. doi: 10.1083/jcb.200802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142:444–455. doi: 10.1016/j.cell.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, Okano Y. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J Biol Chem. 1997;272:13766–13771. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- King JM, Hays TS, Nicklas RB. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol. 2000;151:739–748. doi: 10.1083/jcb.151.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MW, Mitchison T. Microtubule dynamics. Nature. 1986;324:621. doi: 10.1038/324621a0. [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Kronja I, Kruljac-Letunic A, Caudron-Herger M, Bieling P, Karsenti E. XMAP215-EB1 interaction is required for proper spindle assembly and chromosome segregation in Xenopus egg extract. Mol Biol Cell. 2009;20:2684–2696. doi: 10.1091/mbc.E08-10-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F, Hornung P, Westermann S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol. 2010;189:641–649. doi: 10.1083/jcb.200912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Levesque AA, Compton DA. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J Cell Biol. 2001;154:1135–1146. doi: 10.1083/jcb.200106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Wirtz D, Zheng Y. A mechanism of coupling RCC1 mobility to RanGTP production on the chromatin in vivo. J Cell Biol. 2003;160:635–644. doi: 10.1083/jcb.200211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombillo VA, Nislow C, Yen TJ, Gelfand VI, McIntosh JR. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Kisurina-Evgenieva O, Vinogradova T, Hergert P, La Terra S, Kapoor TM, Khodjakov A. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature. 2007;450:745–749. doi: 10.1038/nature06344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- Mao Y. FORMIN a link between kinetochores and microtubule ends. Trends Cell Biol. 2011;21:625–629. doi: 10.1016/j.tcb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Abrieu A, Cleveland DW. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 2003;114:87–98. doi: 10.1016/s0092-8674(03)00475-6. [DOI] [PubMed] [Google Scholar]
- Mao Y, Desai A, Cleveland DW. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J Cell Biol. 2005;170:873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Varma D, Vallee R. Emerging functions of force-producing kinetochore motors. Cell Cycle. 2010;9:715–719. doi: 10.4161/cc.9.4.10763. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell. 2001;12:2776–2789. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Grishchuk EL, Morphew MK, Efremov AK, Zhudenkov K, Volkov VA, Cheeseman IM, Desai A, Mastronarde DN, Ataullakhanov FI. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell. 2008;135:322–333. doi: 10.1016/j.cell.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim KS, Hawley RS. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–153. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12:138–143. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- Montenegro Gouveia S, Leslie K, Kapitein LC, Buey RM, Grigoriev I, Wagenbach M, Smal I, Meijering E, Hoogenraad CC, Wordeman L, Steinmetz MO, Akhmanova A. In vitro reconstitution of the functional interplay between MCAK and EB3 at microtubule plus ends. Curr Biol. 2010;20:1717–1722. doi: 10.1016/j.cub.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Musacchio A. Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci. 2011;366:3595–3604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- O’Connell CB, Loncarek J, Kalab P, Khodjakov A. Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J Cell Biol. 2009;187:43–51. doi: 10.1083/jcb.200903076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Nakamura M, Nishitani H, Nishimoto T. Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science. 1999;284:1356–1358. doi: 10.1126/science.284.5418.1356. [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, Wordeman L, Davis TN, Asbury CL. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey FR, Cramer T, Morphew MK, Silk AD, Johnson RS, McIntosh JR, Cleveland DW. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell. 2002;3:351–365. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH. RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J Cell Sci. 2009;122:2436–2445. doi: 10.1242/jcs.051912. [DOI] [PubMed] [Google Scholar]
- Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ. CENP-F is a. ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskelet. 1993;26:214–226. doi: 10.1002/cm.970260305. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- Rosenberg JS, Cross FR, Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol CB. 2011;21:942–947. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez I, Renwick SJ, Crawley K, Karig I, Buck V, Meadows JC, Franco-Sanchez A, Fleig U, Toda T, Millar JB. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 2005;24:2931–2943. doi: 10.1038/sj.emboj.7600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall S, Severin F, McLeod IX, Yates JR, 3rd, Oegema K, Hyman A, Desai A. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–1191. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon I, Severin FF, Andrews PD, Taba MR, Kaplan KB, Ashford AJ, Stark MJ, Sorger PK, Hyman AA. Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes Dev. 1999;13:545–555. doi: 10.1101/gad.13.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit AC, Stoppin V, Chevrier V, Job D, Lambert AM. Cell cycle dependent distribution of a centrosomal antigen at the perinuclear MTOC or at the kinetochores of higher plant cells. Chromosoma. 1994;103:343–351. doi: 10.1007/BF00417882. [DOI] [PubMed] [Google Scholar]
- Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell. 2007;27:976–991. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L. The kine-sin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell. 2008;14:252–262. doi: 10.1016/j.devcel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tirnauer JS, Canman JC, Salmon ED, Mitchison TJ. EB1 targets to kinetochores with attached, polymerizing microtubules. Mol Biol Cell. 2002;13:4308–4316. doi: 10.1091/mbc.E02-04-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Andrews PD, Wickramasinghe S, Sleeman J, Prescott A, Lam YW, Lyon C, Swedlow JR, Lamond AI. Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol Biol Cell. 2003;14:107–117. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma D, Monzo P, Stehman SA, Vallee RB. Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment. J Cell Biol. 2008;182:1045–1054. doi: 10.1083/jcb.200710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Wan X, O’Quinn RP, Pierce HL, Joglekar AP, Gall WE, DeLuca JG, Carroll CW, Liu ST, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Long S, Ciferri C, Westermann S, Drubin D, Barnes G, Nogales E. Architecture and flexibility of the yeast Ndc80 kinetochore complex. J Mol Biol. 2008;383:894–903. doi: 10.1016/j.jmb.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Ramey VH, Westermann S, Leschziner AE, Welburn JP, Nakajima Y, Drubin DG, Barnes G, Nogales E. Architecture of the Dam1 kinetochore ring complex and implications for microtubule-driven assembly and force-coupling mechanisms. Nat Struct Mol Biol. 2007;14:721–726. doi: 10.1038/nsmb1274. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Bonday ZQ, Putkey FR, Kops GJ, Silk AD, Cleveland DW. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J Cell Biol. 2003;162:551–563. doi: 10.1083/jcb.200303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei RR, Sorger PK, Harrison SC. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA. 2005 doi: 10.1073/pnas.0501168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16:374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G. Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- Whyte J, Bader JR, Tauhata SB, Raycroft M, Hornick J, Pfister KK, Lane WS, Chan GK, Hinchcliffe EH, Vaughan PS, Vaughan KT. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J Cell Biol. 2008;183:819–834. doi: 10.1083/jcb.200804114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Zheng Y. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science. 1999;284:1359–1362. doi: 10.1126/science.284.5418.1359. [DOI] [PubMed] [Google Scholar]
- Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardimci H, van Duffelen M, Mao Y, Rosenfeld SS, Selvin PR. The mitotic kinesin CENP-E is a processive transport motor. Proc Natl Acad Sci USA. 2008;105:6016–6021. doi: 10.1073/pnas.0711314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–771. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Kelstrup CD, Hu XW, Hansen MJ, Singleton MR, Olsen JV, Nilsson J. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J Cell Sci. 2012 doi: 10.1242/jcs.104208. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ahmad S, Mao Y. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J Cell Biol. 2007;178:773–784. doi: 10.1083/jcb.200702138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Knowles BA, Goldstein LS, Hawley RS. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell. 1990;62:1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]