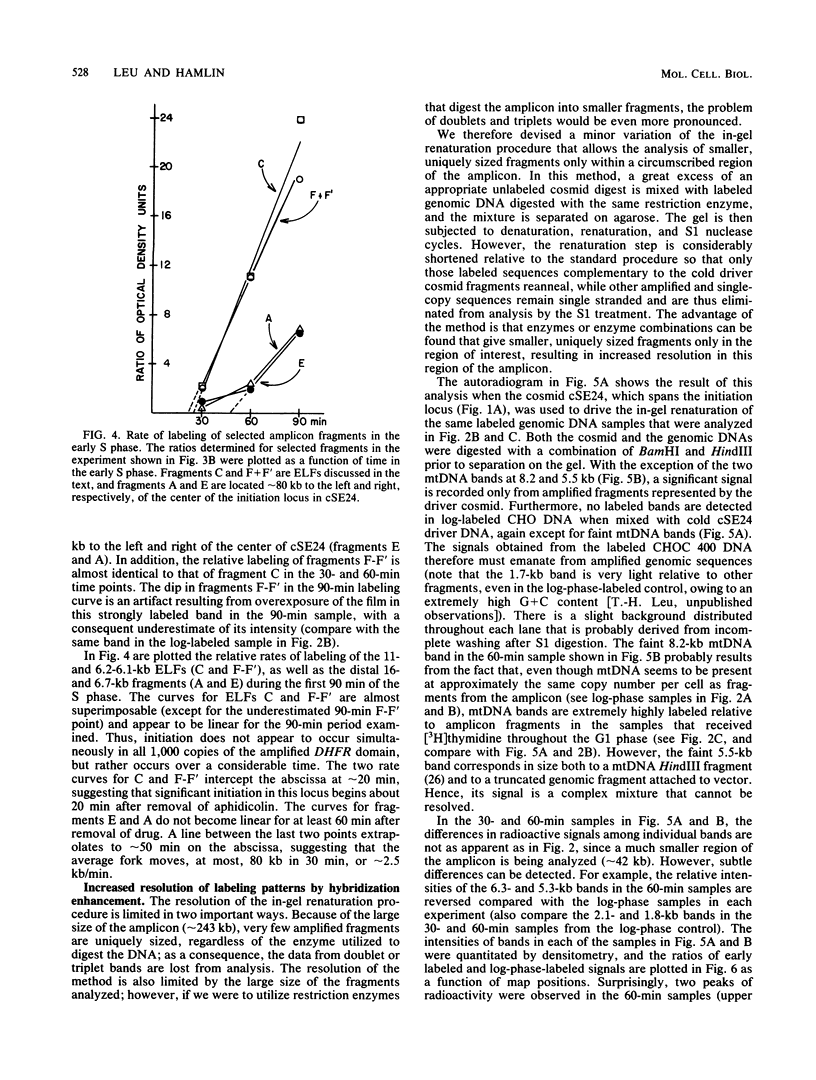
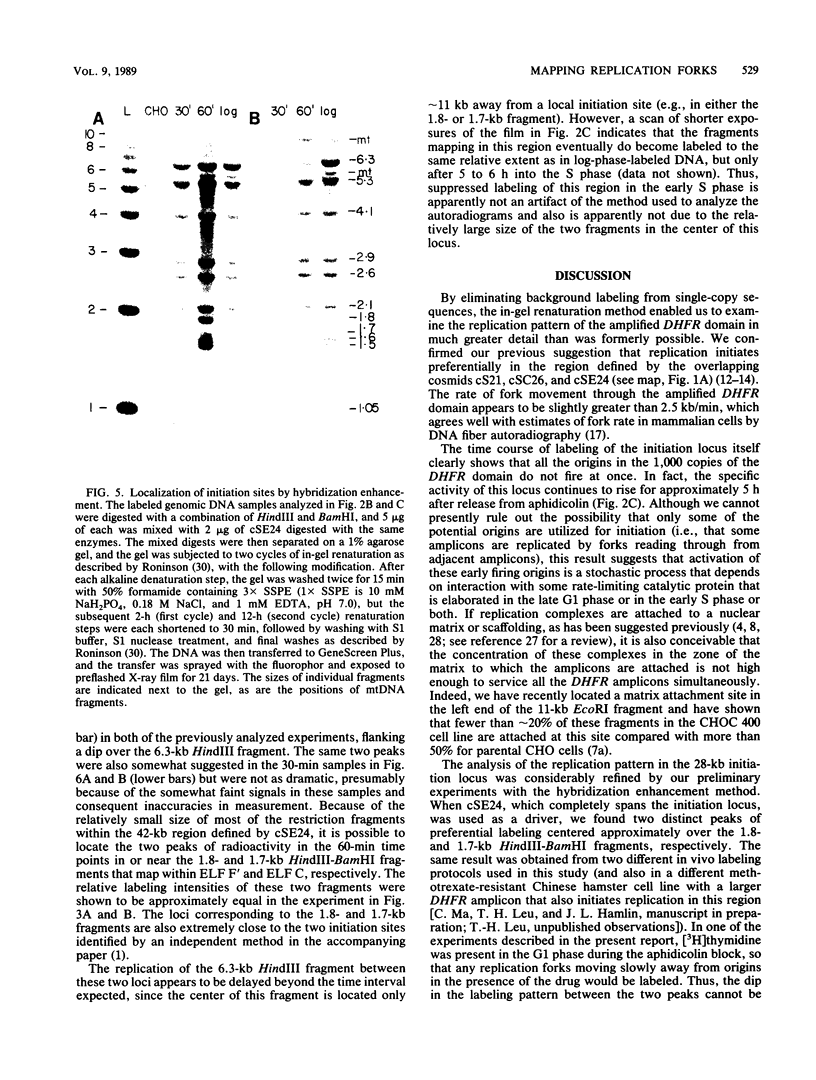
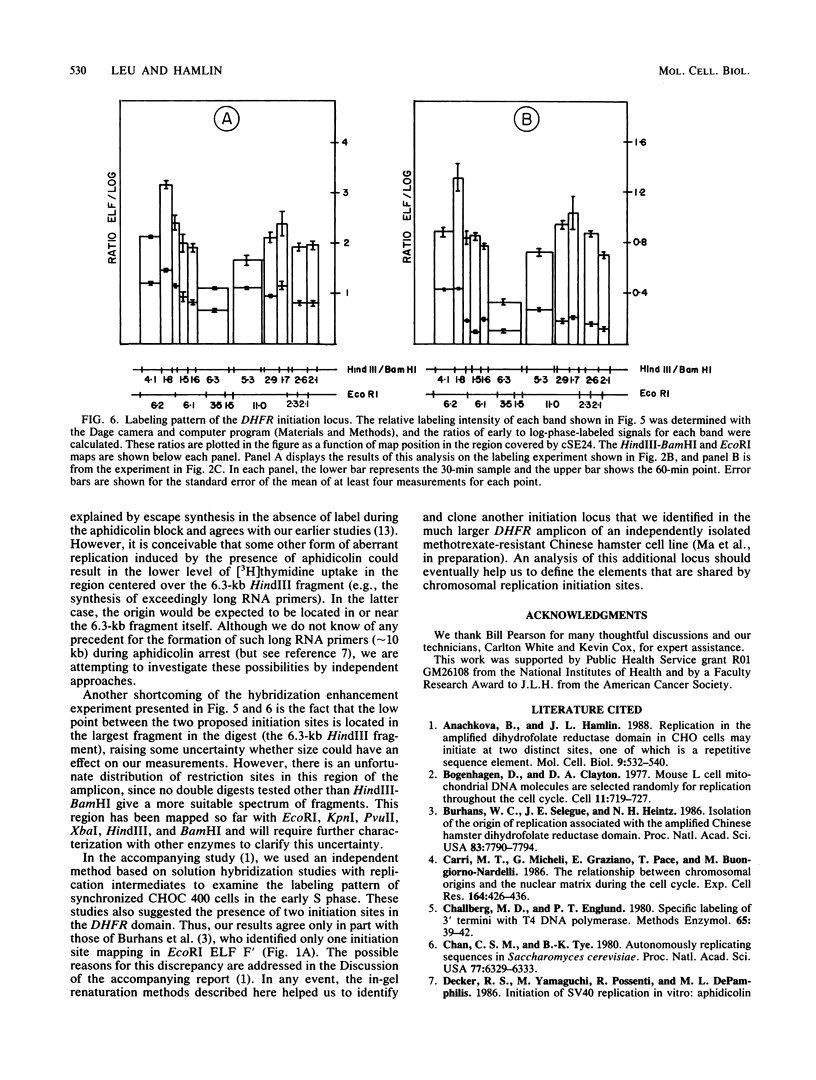
Abstract
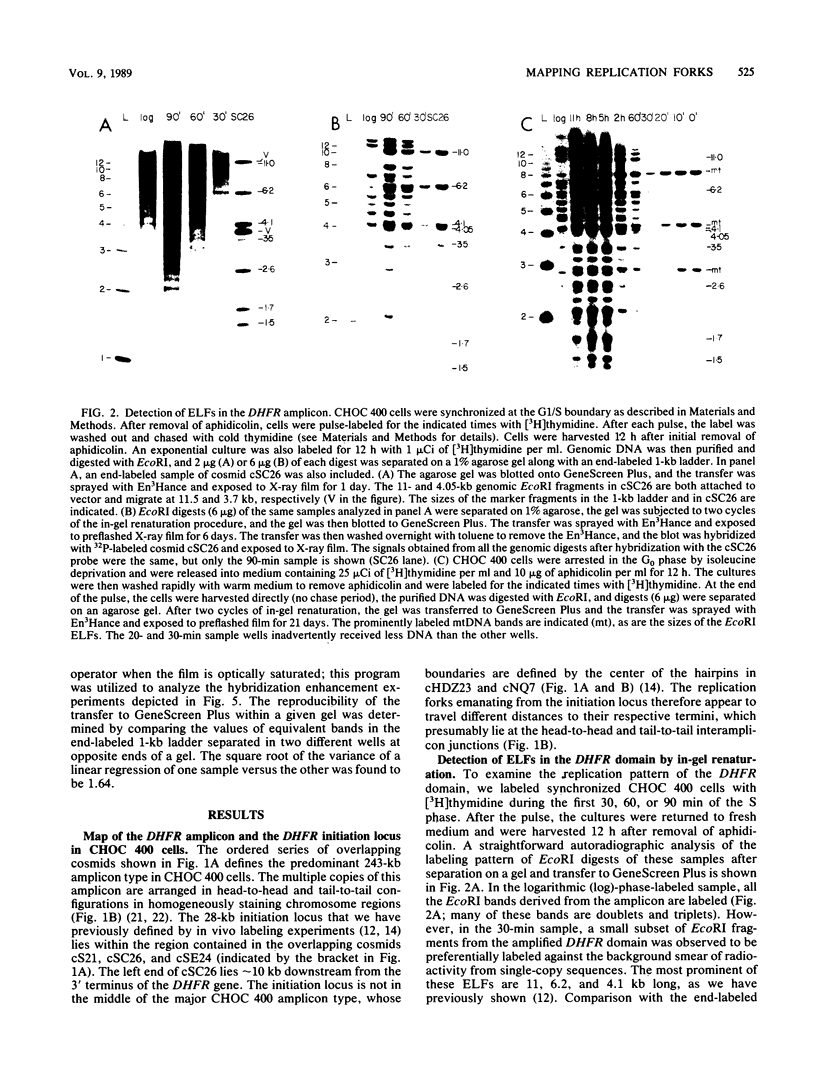
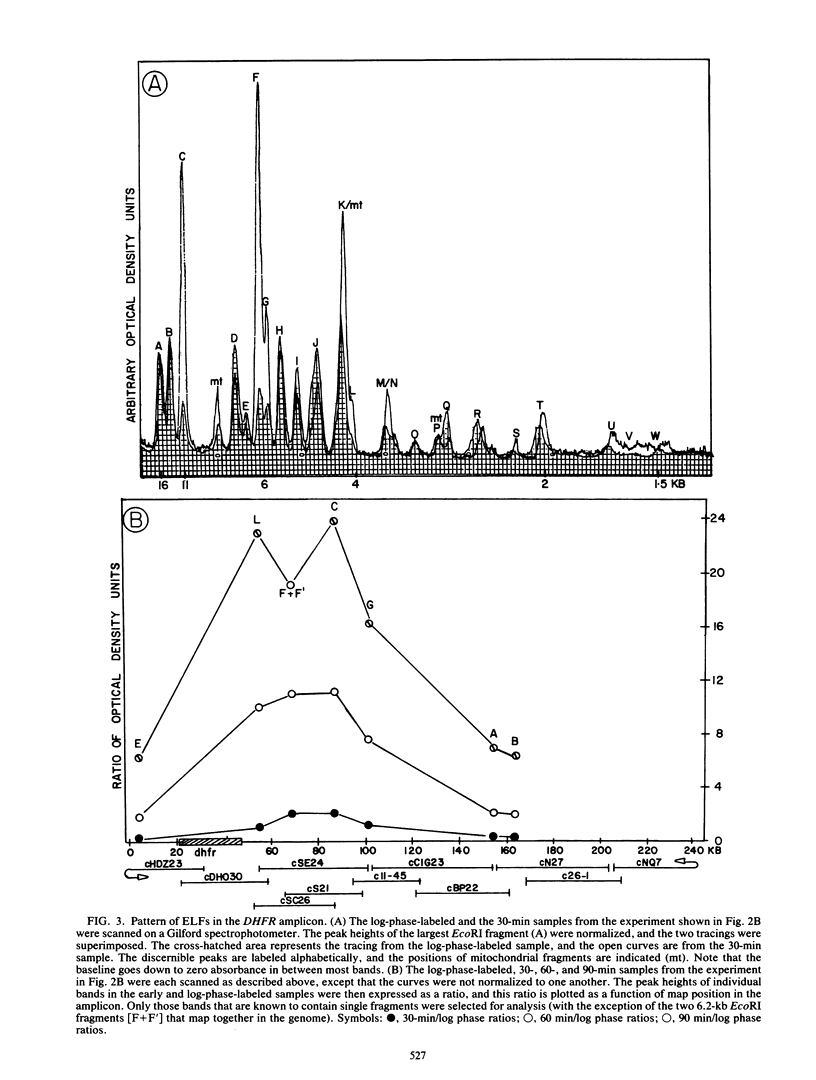
Utilizing an in vivo labeling method on synchronized cultures, we have previously defined a 28-kilobase (kb) replication initiation locus in the amplified dihydrofolate reductase domain of a methotrexate-resistant Chinese hamster ovary cell line (CHOC 400) (N. H. Heintz and J. L. Hamlin, Proc. Natl. Acad. Sci. USA 79:4083-4087, 1982; N. H. Heintz and J. L. Hamlin, Biochemistry 22:3552-3557, 1983; N. H. Heintz, J. D. Milbrandt, K. S. Greisen, and J. L. Hamlin, Nature [London] 302:439-441, 1983). To locate the origin of replication in this 243-kb amplicon with more precision, we used an in-gel renaturation procedure (I. Roninson, Nucleic Acids Res. 11:5413-5431, 1983) to examine the labeling pattern of restriction fragments from the amplicon in the early S phase. This method eliminates background labeling from single-copy sequences and allows quantitation of the relative radioactivity in individual fragments. We used this procedure to follow the movement of replication forks through the amplicons, to roughly localize the initiation locus, and to estimate the rate of fork travel. We also used a slight modification of this method (termed hybridization enhancement) to illuminate the labeling pattern of smaller restriction fragments derived solely from the initiation locus itself, thereby increasing resolution. Our preliminary results suggest that there are actually two distinct initiation sites in the amplicon that are separated by approximately 22 kb.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anachkova B., Hamlin J. L. Replication in the amplified dihydrofolate reductase domain in CHO cells may initiate at two distinct sites, one of which is a repetitive sequence element. Mol Cell Biol. 1989 Feb;9(2):532–540. doi: 10.1128/mcb.9.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977 Aug;11(4):719–727. doi: 10.1016/0092-8674(77)90286-0. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Isolation of the origin of replication associated with the amplified Chinese hamster dihydrofolate reductase domain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7790–7794. doi: 10.1073/pnas.83.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrì M. T., Micheli G., Graziano E., Pace T., Buongiorno-Nardelli M. The relationship between chromosomal origins of replication and the nuclear matrix during the cell cycle. Exp Cell Res. 1986 Jun;164(2):426–436. doi: 10.1016/0014-4827(86)90041-8. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Specific labeling of 3' termini with T4 DNA polymerase. Methods Enzymol. 1980;65(1):39–43. doi: 10.1016/s0076-6879(80)65008-3. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6329–6333. doi: 10.1073/pnas.77.11.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Hamlin J. L. Matrix attachment regions are positioned near replication initiation sites, genes, and an interamplicon junction in the amplified dihydrofolate reductase domain of Chinese hamster ovary cells. Mol Cell Biol. 1988 Dec;8(12):5398–5409. doi: 10.1128/mcb.8.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Wenink P. W., Poddighe J. Permanent attachment of replication origins to the nuclear matrix in BHK-cells. Nucleic Acids Res. 1986 Apr 25;14(8):3241–3249. doi: 10.1093/nar/14.8.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frappier L., Zannis-Hadjopoulos M. Autonomous replication of plasmids bearing monkey DNA origin-enriched sequences. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6668–6672. doi: 10.1073/pnas.84.19.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hatton K. S., Dhar V., Brown E. H., Iqbal M. A., Stuart S., Didamo V. T., Schildkraut C. L. Replication program of active and inactive multigene families in mammalian cells. Mol Cell Biol. 1988 May;8(5):2149–2158. doi: 10.1128/mcb.8.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. In vivo effects of cytosine arabinoside on deoxyribonucleic acid replication in Chinese hamster ovary cells. 1. Resolution of differential effects on mitochondrial and nuclear deoxyribonucleic acid synthesis. Biochemistry. 1983 Jul 19;22(15):3552–3557. doi: 10.1021/bi00284a003. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- Holst A., Müller F., Zastrow G., Zentgraf H., Schwender S., Dinkl E., Grummt F. Murine genomic DNA sequences replicating autonomously in mouse L cells. Cell. 1988 Feb 12;52(3):355–365. doi: 10.1016/s0092-8674(88)80028-x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- James C. D., Leffak M. Polarity of DNA replication through the avian alpha-globin locus. Mol Cell Biol. 1986 Apr;6(4):976–984. doi: 10.1128/mcb.6.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Jelinek W. R. Replication of a plasmid bearing a human Alu-family repeat in monkey COS-7 cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4660–4664. doi: 10.1073/pnas.83.13.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney J. E., Hamlin J. L. Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol. 1987 Feb;7(2):569–577. doi: 10.1128/mcb.7.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Looney J. E., Leu T. H., Hamlin J. L. Organization and genesis of dihydrofolate reductase amplicons in the genome of a methotrexate-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1988 Jun;8(6):2316–2327. doi: 10.1128/mcb.8.6.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Azizkhan J. C., Greisen K. S., Hamlin J. L. Organization of a Chinese hamster ovary dihydrofolate reductase gene identified by phenotypic rescue. Mol Cell Biol. 1983 Jul;3(7):1266–1273. doi: 10.1128/mcb.3.7.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Zavala M., Hamlin J. L. Similar 150-kilobase DNA sequences are amplified in independently derived methotrexate-resistant Chinese hamster cells. Mol Cell Biol. 1985 Apr;5(4):619–627. doi: 10.1128/mcb.5.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Restriction map of Chinese hamster mitochondrial DNA containing replication coordinates: comparison with Syrian hamster mitochondrial genome. Gene. 1983 Mar;21(3):249–255. doi: 10.1016/0378-1119(83)90008-2. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Kekelidze M. G., Lukanidin E. M., Scherrer K., Georgiev G. P. Replication origins are attached to the nuclear skeleton. Nucleic Acids Res. 1986 Oct 24;14(20):8189–8207. doi: 10.1093/nar/14.20.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B. Detection and mapping of homologous, repeated and amplified DNA sequences by DNA renaturation in agarose gels. Nucleic Acids Res. 1983 Aug 25;11(16):5413–5431. doi: 10.1093/nar/11.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]