Abstract
Uniform conclusions about therapeutic concepts and survival time of bone and soft tissue sarcoma patients are difficult due to the heterogeneity of histological subtypes as well as the different responses to neoadjuvant therapy. The subject of this retrospective study was the analysis of tumour free survival, risk and prognostic factors of sarcoma patients treated by limb sparing techniques or amputation. We included 118 patients with soft tissue sarcoma of the extremities treated primarily or secondarily at our institution between 1990 and 2008 with a minimum follow-up of 12 months. Data about the tumour free survival time, operative techniques and potential prognostic factors were analysed. The tumour-specific and overall survival were significantly influenced by two factors: the grading and distant metastases present at time of diagnosis. Optimal multimodal therapeutic concepts at a specialized Cancer Center decreased the risk of local recurrence. The importance of optimal preoperative and surgical course concerning the oncological long term outcome was investigated. The decrease in local recurrence as a result of multimodal therapeutic concepts at a specialized Cancer Center was confirmed. To evaluate the individual prognosis of a patient, multiple factors have to be considered. Factors for a poor prognosis are primary metastasis, high-grade tumours and several histological entities (e.g. synovial sarcoma, not other specified).
Key words: soft tissue sarcoma, oncological outcome, prognostic factors.
Introduction
Bone and soft tissue sarcoma occur with a rare incidence of 1–2% of all malignancies. Because of advancements in multimodal therapy concepts, the number of limb sparing operations as well as the overall survival time have increased in recent years.1
Due to the heterogeneity of histological subtypes as well as the different response to neoadjuvant therapy, uniform conclusions about therapeutic concepts and survival time are difficult.
A major determinant of the outcome is R0-resection with adequate margins, as a microscopically positive R1-resection correlates with an increased risk of local recurrence.2–6 In cases with no adequate margins, a re-resection to achieve negative margins should be performed as it directly correlates with improved local control, metastasis-free survival and disease-specific overall survival.7
Some promising outcomes in patients with high-risk sarcomas (large, GII/GIII, narrow margin, high-risk histological subtypes) were achieved by a preoperative combination of radiotherapy and chemotherapy (neoadjuvant) followed by an adequate R0-resection.8,9 This kind of therapy may also lead to a decrease in tumour-volume and less radical surgery could be performed.10
The survival-rate for patients with metastatic disease (stage III according to Enneking/ IV UICC/AJCC) is poor. The most common site of metastases after primary treatment of an extremity sarcoma is the lung and they usually occur within 2–3 years after surgical treatment.11,12 For exclusion of lung metastases, a computed tomography (CT) of the chest should be performed every six months after tumour resection.13
Patients with widely disseminated metastatic disease are best treated with palliative chemotherapy, although their survival rate is poor.11–13
The subject of this retrospective study was the analysis of tumour free survival, risk and prognostic factors of patients treated by limb sparing techniques or amputation.
Materials and Methods
We reviewed the medical records of all patients who presented with the diagnosis of a soft tissue sarcoma at our hospital in the period between 1990 and 2008. Criteria for the inclusion in this study were the diagnosis of a soft tissue sarcoma confirmed by pathology, meaningful clinical information and histological material and achievement of a minimum postoperative follow-up of 12 months. To complete the medical records, we contacted the local family doctor.
We analyzed the data with regard to tumour free survival time, operative techniques and potential prognostic factors (e.g. TNM staging, grading, histological entity, resection status). Because of the long observation period (1990–2008) and because most of the tumours originated in the axial skeleton, the Enneking classification system of orthopaedic oncology was used. It had been proved to be reliable, reproducible and of prognostic importance.14,15
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations. The Kaplan-Meier method was used to estimate tumor-specific and overall survival, with the log-rank test to determine significance. Hazard ratios and 95% confidence intervals for mortality were estimated with the use of Cox proportional-hazards regression models.
All reported P values are two-sided, with a significance level of 0.05 and have not been adjusted for multiple testing. All analyses were performed with the use of SPSS software.
Results
General data
One-hundred eighteen patients were included with a soft tissue sarcoma treated at our institution during the observation period and had a minimum follow-up of 12 months. 60 were female and 58 were male. The average age of patients at the time of diagnosis was 54.8±17 years. The average time between symptom onset and diagnosis was 10.4±17.9 months, the time between diagnosis and surgery was 32.1±29.3 days. In 58 patients (49.2%) the sarcoma occurred in the thigh, in the upper arm in 14 patients (11.9%) and the lower leg with 12 patients (10.2%). Localisations of the different kind of tumours are shown in Figure 1.
Figure 1.
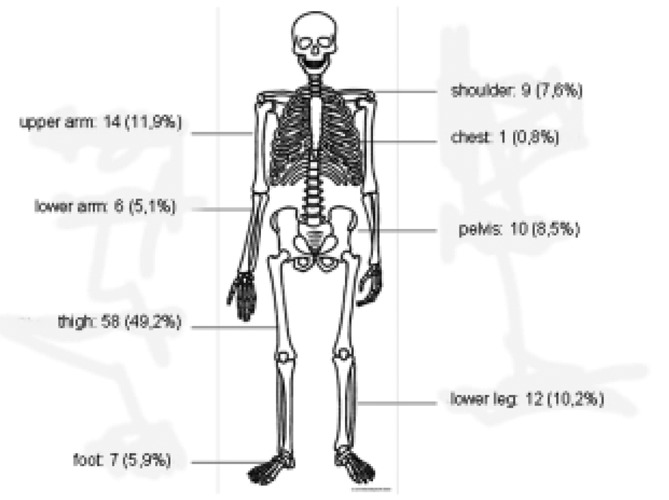
Locations of the analysed soft tissue sarcomas.
The most common imaging technique for the assessment of the primary tumour at diagnosis was the magnetic resonance imaging (MRI). It was performed in 87.2% of all patients. To determine the degree of spread of the tumour, preferred imaging procedures were CT of the chest (71.6%) and abdomen (37.6%), X-ray of the chest (57.8%) and bone scintigraphy (65.1%).
Liposarcoma and NOS (not other specified) were the most common histological entities, occurring in 49.15% of patients (Figure 2).
Figure 2.
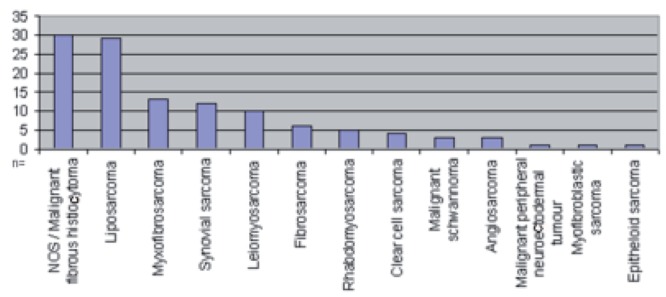
Histological entities (n=118).
A stage IIb according to Enneking was observed in 54% of the cases. The grading was >GI in 78% of the tumours.
Stage T2b was most frequently diagnosed (69.0%). The regional lymph nodes were affected in only 3.5% of all study patients at the time of initial diagnosis. 6.8% of patients had distant metastases at first presentation.
The mean follow-up after surgery was 58 months.
At the end of the observation period, the patient's status was re-evaluated. At this time no clinical or radiological evidence of disease was seen in 50.85% of patients. Every fourth patient died as a result of the sarcoma and its consequences. No data could be collected in every tenth case. Six percent of patients died of another disease unrelated to the tumour. Almost seven percent were alive at the end of the observation period with evidence of the tumour (Figure 3).
Figure 3.
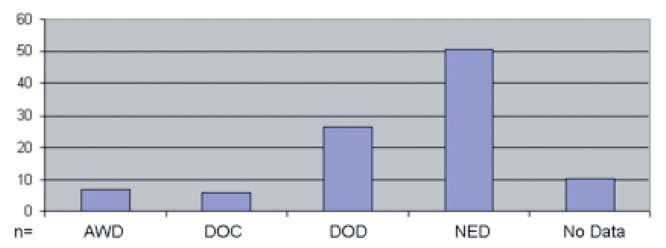
Status of disease at the end of the observation period (n=118). AWD, alive with disease; DOC, death from other causes; DOD, death from disease; NED, no evidence of disease.
Biopsy
One third of patients (34.7%) were not assigned to our department until external pre-treatment. Patients treated primarily at our institution received a diagnostic biopsy in 98.2% (81.7% open biopsy). Patients who underwent primary surgery at an external hospital underwent a biopsy prior to the resection in only 8.1% of the cases. No significant differences concerning the grading or the tumour status according to Enneking in the subgroups of patients (external operation vs. surgery at a cancer center) were seen.
Status of resection and local relapse
Despite initial R0-resection in 97% of the cases (82.4% of patients treated at our institution, 18.4% of patients treated externally), local relapse within the observation period was seen in 21.7% of the patients. The risk of local recurrence was significantly less in the group of patients treated at our institution from the beginning: 9.1% vs 17.2% in the first year, 12.5% vs 32.5% after 3 years and 21.2% vs 45.7% after 5 years (P=0.013) (Figure 4).
Figure 4.
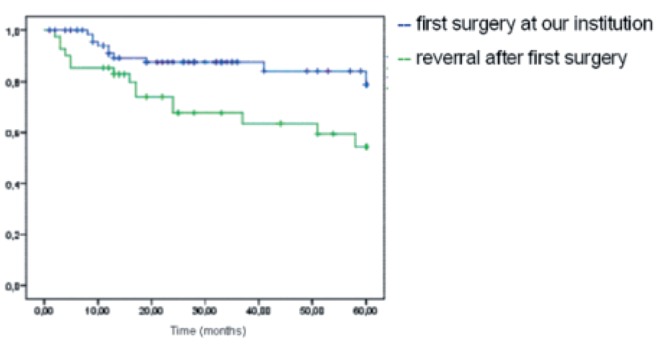
Relapse-free time depending on the institution at which first surgery was performed.
The risk of local recurrence increased with the T stage (P=0.056) and significantly with the tumour stage according to Enneking: stage I 8.7%; 8.7%; 17%; stage II 9.2%; 18.7%; 29.1%; stage III 25%; 50%; 55% one/three/five years after surgery (P=0.04).
A higher risk of local recurrence was also seen in patients without adjuvant therapy (P=0.089) and patients without initial R0 resection (despite local re-resection) (P=0.179).
Distant metastases and overall survival
During the observation period, 45 of 114 patients (39.5%) developed distant metastases. The lung was the mainly affected organ (34.7%).
Analysis of survival, depending on the grading, showed a statistically significant difference: one year after diagnosis all patients with low grade sarcoma (G1) were still alive (90.5% after three, 82.3% after five years). In the high grade sarcoma group (G>1) 95.3% of study patients were still alive one year after surgery, (83% after three, 48.8% after five years) (α=0.035). The median survival for high grade sarcomas was 60 months (Figure 5).
Figure 5.
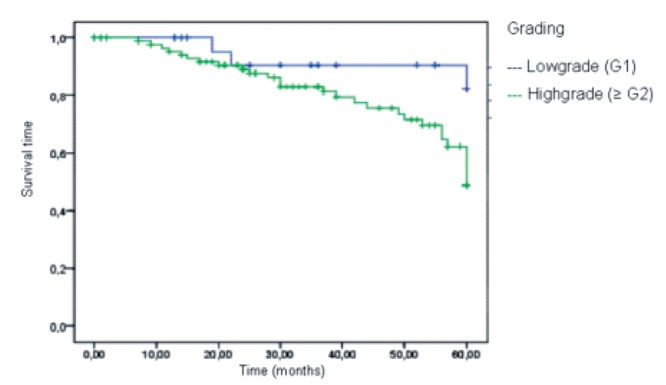
Tumour specific survival depending on the grading.
The median survival diminished significantly with primary metastases at first presentation (median survival 20 months).
Regarding the tumour-specific survival depending on the tumour stage according to Enneking, a 5-year survival of 86.1%, 53.7% and 33.3% in stage I, II, and III was seen (P<0.05). Concerning the T stage, 71.8% and 55.2% of patients after T1 or T2 sarcomas survived after 5 years (P=0.172).
Multivariant analysis
Patients pre-treated at an external hospital had, compared with those who underwent primary surgery at our institution, twice the risk of local tumour recurrence (P=0.003). Similarly, the risk for patients who received no adjuvant therapy (radio- or chemotherapy) was doubled (P=0.082). Another factor influencing the risk of relapse was the tumour size. The risk of local relapse between T2- and T1-sarcomas was 4.5-fold higher (P=0.046). Regarding the stage according to Enneking (III vs II and II vs I) a three-fold increased risk of local recurrence was associated with the higher tumour stage (P=0.029) (Table 1).
Table 1. Prognostic factors for local relapse.
| Hazard ratio | Confidence interval: lower level | Confidence interval: upper level | P-value | |
|---|---|---|---|---|
| Enneking | 2.992 | 1.119 | 7.997 | P=0.029 |
| Stage: I a/b | ||||
| II a/b; III a/b | ||||
| Size: T1, T2 | 4.495 | 1.029 | 19.627 | P=0.046 |
| Surgery | 1.955 | 1.256 | 3.043 | P=0.003 |
| Our institution vs external | ||||
| Adjuvant therapy | 2.073 | 0.912 | 4.712 | P=0.082 |
The tumour-specific and overall survival were significantly influenced by two factors: the grading and present distant metastases at time of diagnosis (Table 2).
Table 2. Prognostic factors for tumour specific survival.
| Hazard ratio | Confidence interval:lower level | Confidence interval:upper level | P-value | |
|---|---|---|---|---|
| Grading | 3.415 | 1.035 | 11.268 | P=0.044 |
| Low grade (G1) | ||||
| High grade (G>2) | ||||
| Distant metastases | 6.107 | 2.341 | 15.929 | P<0.0001 |
| At time of diagnosis | ||||
Discussion and Conclusions
This study points out the influence of preop-erative and surgical course with regard to the oncological outcome of patients with soft tissue sarcoma. Optimal multimodal therapeutic concepts at a specialized Cancer Center decrease the risk of local recurrence (Table 1). In the case of potential malignancy of the tumour and given risk factors (subfascial localisation, size over 5 cm in diameter, inhomogeneity in imaging procedures), a biopsy is required preopera-tively to offer the patients this treatment.
The present study demonstrated the benefit of a biopsy and the importance of an adequate R0-resection. We were able to show that biopsies were less frequently performed in patients transferred after unexpected R1 resections to our institution. This fact might explain the better outcome of patients with a biopsy in regard to local recurrence, as most patients with a high-grade sarcoma were treated with a preoperative combination of radiotherapy and/or chemotherapy to reduce tumour size or improve resectability. These findings support the results of different studies which also found a positive effect of a preoperative combination of radiotherapy and chemotherapy followed by an adequate R0-resection.8,9,16,17
We were able to demonstrate different factors which influence the tumour-specific survival such as clinical staging and grading.
Patients with metastatic disease at initial diagnosis had a poor prognosis with a five year survival of about 33% which is also consistent with the published data of previous studies.11,12,18
According to the literature, important prognostic factors for overall survival, in addition to the histopathological grading, include the size of the primary tumour. The larger the tumour (T stage), the worse the respective survival rates.19–21 Positive surgical resection margins and certain histological subtypes not only increase the rate of local recurrence, but also affect overall survival.20,22–24 Another prognostic factor for overall survival is the location of the tumour. Superficial soft tissue sarcomas have a significantly better prognosis than subfascial located tumours.20,25 We often see a significant delay in the time between symptom onset and diagnosis for deep located lesions. Because of the significantly higher incidence of benign compared to malignant neoplasms, the cause of such a lesion is often trivialized.26
An association between the development of local recurrence and a significantly poorer survival was shown in several studies.27,28
We note several limitations of the present study. We used the clinical staging described by Enneking. Today the classification system by the UICC is much more common. The UICC/AJCC was established in 2010 in its final version. During the observation period the Enneking staging was more commonly used However, the Enneking classification is still reliable, reproducible and of prognostic importance and is used by many orthopaedic surgeons, as it includes the local extent of the tumour, which may be a prognostic factor.15
There are positive aspects in both classification systems and we agree with the statement of Jawad et al.15 that a combination of both classification systems might be of some benefit.
Finally, the clinical use of prognostic factors has been very helpful. However, the use of prognostic factors in individual cases is very difficult because of the biological behaviour and the several histological subtypes of soft tissue sarcomas. All patients with a possible STS should be assigned to a centre which is familiar with these kinds of tumours to optimize their outcome.
References
- 1.Mendenhall WM, Indelicato DJ, Scarborough MT, et al. The management of adult soft tissue sarcomas. Am J Clin Oncol. 2009;32:436–42. doi: 10.1097/COC.0b013e318173a54f. [DOI] [PubMed] [Google Scholar]
- 2.Heslin MJ, Woodruff J, Brennan MF. Prognostic significance of a positive microscopic margin in high-risk extremity soft tissue sarcoma: implications for management. J Clin Oncol. 1996;14:473–8. doi: 10.1200/JCO.1996.14.2.473. [DOI] [PubMed] [Google Scholar]
- 3.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–25. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 4.McKee, Lui DF, Brooks JJ, et al. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85:68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]
- 5.Stojadinovic A, Leung DH, Allen P, et al. Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol. 2002;20:2719–25. doi: 10.1200/JCO.2002.07.154. [DOI] [PubMed] [Google Scholar]
- 6.Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and resection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer. 2003;97:2544–53. doi: 10.1002/cncr.11367. [DOI] [PubMed] [Google Scholar]
- 7.Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:110–7. doi: 10.1245/ASO.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 8.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiother-apy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–27. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 9.Cormier JN, Huang X, Xing Y, et al. Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol. 2004;22:4567–74. doi: 10.1200/JCO.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 10.Pisters PW, Patel SR, Prieto VG, et al. Phase I trial of preopertive doxorubicin-based concurrent chemoradiation and surgical resection for localiced extremity and body wall soft tissue sarcomas. J Clin Oncol. 2004;22:3375–80. doi: 10.1200/JCO.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Putnam JB, Jr, Roth JA, Wesley MN, et al. Analysis of prognostic factors in patients undergoing resection of pulmonary metastases from soft tissue sarcomas. J Thorac Cardiovasc Surg. 1984;87:260–8. [PubMed] [Google Scholar]
- 12.Jablons D, Steinberg SM, Roth JA, et al. Metastasectomy for soft tissue sarcoma. Further evidence for efficacy and prognostic indicators. J Thorac Cardiovasc Surg. 1989;97:695–705. [PubMed] [Google Scholar]
- 13.Kane JM. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol. 2004;16:328–32. doi: 10.1097/01.cco.0000127879.62254.d3. [DOI] [PubMed] [Google Scholar]
- 14.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of muscu-loskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20. [PubMed] [Google Scholar]
- 15.Jawad UM, Scully SP. Classifications in brief: Enneking classification: benign and malignant tumors of the musculoskeletal system. Clin Orthop Relat Res. 2010;468:2000–2. doi: 10.1007/s11999-010-1315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. [Accessed: May 2011];Soft Tissue Sarcoma. 2011 Vol 1 Available from: www.nccn.org. [Google Scholar]
- 17.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens a European Organization for research and treatment of cancer soft tissue and bone sarcoma group study. J Clin Oncol. 1999;17:150–7. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatement of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peabody TD, Monson D, Montag A, et al. A comparison of the prognoses for deep and subcutaneous sarcomas of the extremities. J Bone Joint Surg Am. 1994;76:167–73. doi: 10.2106/00004623-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1.041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 21.Stojadinovic A, Leung DH, Allen P, et al. Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol. 2002;20):4344–52. doi: 10.1200/JCO.2002.07.154. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JJ, Leugg D, Casper ES, et al. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–4. doi: 10.1001/archsurg.134.2.190. [DOI] [PubMed] [Google Scholar]
- 23.Canter RJ, Beal S, Borys D, et al. Interaction of histologic subtype and histo-logic grade in predicting survival for soft-tissue sarcomas. J Am Coll Surg. 2010;210:191–8. doi: 10.1016/j.jamcollsurg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Canter RJ. Why do patients with low-grade soft tissue sarcoma die? Ann Surg Oncol. 2008;15:3550–60. doi: 10.1245/s10434-008-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujimoto M, Aozasa K, Ueda T, et al. Multivariate analysis for histologic prognostic factors in soft tissue sarcomas. Cancer. 1988;62:994–8. doi: 10.1002/1097-0142(19880901)62:5<994::aid-cncr2820620526>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence W, Jr, Donegan WL, Natarjan N, et al. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349–59. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–26. doi: 10.1097/01.SLA.0000048448.56448.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trovik CS, Bauer HC, Alvegard TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–6. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]


