Abstract
Mesenchymal stromal cells (MSC) are differentiation competent cells and may generate, among others, mature osteoblasts or chondrocytes in vitro and in vivo. Laminin-5 and type I collagen are important components of the extracellular matrix. They are involved in a variety of cellular and extracellular activities including cell attachment and osteogenic differentiation of MSC. MSC were isolated and expanded using media conforming good medical practice (GMP)-regulations for medical products. Cells were characterized according to the defined minimal criteria for multipotent MSC. MTT- and BrdU-assays were performed to evaluate protein-dependent (laminin-5, laminin-1, type I collagen) metabolic activity and proliferation of MSC. MSC-attachment assays were performed using protein-coated culture plates. Osteogenic differentiation of MSC was measured by protein-dependant mineralization and expression of osteogenic marker genes (osteopontin, alkaline phophatase, Runx2) after three, seven and 28 days of differentiation. Marker genes were identified using quantitative reverse-transcription polymerase chain reaction. Expansion of MSC in GMP-conforming media yielded vital cells meeting all minimal criteria for MSC. Attachment assay revealed a favorable binding of MSC to laminin-5 and type I collagen at a protein concentration of 1–5 fmol/µL. Compared to plastic, osteogenic differentiation was significantly increased by laminin-5 after 28 days of culture (P<0.04). No significant differences in gene expression patterns were observed. We conclude that laminin-5 and type I collagen promote attachment, but laminin-1 and laminin-5 promote osteogenic differentiation of MSC. This may influence future clinical applications.
Key words: attachment, mesenchymal stromal cells, osteogenic differentiation, type I collagen, laminin-5.
Introduction
Mesenchymal stem cells (MSC) were originally described by Friedstein et al. in 1966 as clonal, plastic adherent cells with a high proliferation rate and the potential of differentiation into cells of mesodermal origins including osteocytes, chondrocytes and adipocytes in vitro.1 According to the consensus in nomenclature, these cells were termed multipotent mesenchymal stromal cells.2,3 For both cell populations, the acronym MSC may be used.3 The ability of MSC to differentiate into numerous tissues reveals promising potential for tissue engineering, cell therapies and future clinical applications.
Cell differentiation is regulated by soluble factors (e.g. TGF-β, BMPs, insulin-like growth factor, dexamethasone) and the extracellular matrix (ECM) surrounding the cells in which both mechanisms are closely connected. Several ECM-proteins, such as type I collagen, laminin-5, and vitronectin, have been identified as regulators of MSC-adhesion and inducers of osteogenic differentiation in vitro.4–10 Fetal calf serum (FCS) is widely used for cell culture,4–7 but in the context of possible further clinical applications animal serum does not meet the European Good Manufacturing Practice (GMP) regulations for medical products. The risk of immunological reactions, prion transmission and potential metabolic changes in cultured cells must be considered.11–13 Therefore, we use a GMP-compatible protocol employing MSC-expansion and differentiation media in which FCS is replaced by human plasma and platelet extract as described previously.13–15 In this study, we examined the effects of laminin-5, laminin-1 and type I collagen on MSC, their in vitro proliferation, adhesion to these matrix proteins and their osteogenic differentiation.
Materials and Methods
Isolation, expansion and cell culture of human bone marrow mesenchymal stromal cells
Bone marrow samples were obtained from 14 patients (age 45 to 72 years) undergoing total hip arthroplasty (femur shafts) or pelvic osteotomy at the Department of Orthopaedic Surgery at UKT (Tübingen, Germany) or the BGU Trauma Center (Tübingen, Germany), after written consent was given. This study has been approved by the local ethics committee of the University of Tübingen (Germany).
Bone marrow was washed with phosphate-buffered saline (PBS, BioWhittaker) and a lipid layer was removed after centrifugation. MSC were isolated using Ficoll density-gradient centrifugation and mononucleated cells were collected in the interphase (Ficoll-Paque Premium, GE Healthcare, Munich, Germany). The cells were washed with PBS, centrifuged and seeded at a density of 1×106 to 1×107 cells/cm2 in 75 cm2 cell culture falcons (BD Biosciences, Heidelberg, Germany) in expansion medium containing low-glucose Dulbecco's modified Eagle medium (DMEM, Lonza, Basel, Switzerland) supplemented with 5% human fresh frozen plasma (FFP), 5% platelet concentrate (108 platelets/mL medium, Blood Donor Center, University of Tubingen), 2 mM L-glutamine (Lonza, Basel, Switzerland), 1000 IU heparin sodium (Roth, Karlsruhe, Germany), 100 U/mL penicillin G, and 100 µg/mL streptomycin (Gibco, Invitrogen, Karlsruhe, Germany) at 37°C in a humidified atmosphere with 95% air and 5% CO2. After 24 h of incubation, non-adherent cells were removed and adherent cells were detached by trypsin-EDTA, passaged and expanded in fresh cell culture medium. The medium was changed at least twice a week. Cells were detached with Accutase® (PAA, Pasching, Austria) and passaged when reaching 80% confluence. They were seeded at a density of 1.5×105 cell/flask. Cells were used for experiments after three passages.
Characterization of mesenchymal stromal cells
The MSC were characterized by the defined minimal criteria for multipotent mesenchymal stromal cells, such as adherence to plastic, expression or lack of expression of defined cell-surface antigens analyzed by flow cytometry (CD11b−, CD14−, CD34−, CD45−, CD73+, CD90+, and CD105+) and the ability for in vitro differentiation into osteoblasts, adipocyts and chondroblasts.16
Attachment assays
For cell attachment assays, laminin-1 (Sigma), laminin-5 (Abcam, Cambridge, MA, USA) and type I collagen (BD Bioscience) were solved in PBS in concentrations of 1 fmol/µL, 10 fmol/µL, 100 fmol/µL or 500 fmol/µL. Tissue culture plates were coated with 1 µL aliquots of the protein solutions (about 1 µm2 of plate area). BSA (bovine serum albumin) served as control. These spots were air-dried and the plates were blocked with 200 µL 1% BSA/PBS (Sigma) for 1 h and washed with PBS prior to the assay. Then 200 µL of MSC suspension in PBS were loaded on the spot for attachment for 15 min, washed with PBS, and adherent cells were microscopically photographed (Zeiss Anxiovert 13 S, Jena, Nikon camera).
MTT cell proliferation assay
For measurement of protein-dependant differences in metabolic activity of MSC, we used the MTT assay to quantify mitochondrial enzymatic activity; 3000 MSC per well were seeded in a 96-well plate (coated with laminin-5, laminin-1, type I collagen or plastic as control) for 24 h and the activity of mitochondrial reductase was quantified as described by the supplier by measuring the absorbance of the produced agent at λ=450 nm and λ=620 nm after 2.5 h of incubation using the ELISA reader (EL800, Bio Tek, Bad Friedrichshall, Germany).
BrdU cell proliferation assay
For further measurement of a possible time-dependent proliferation of MSC we used the BrdU assay quantifying DNA synthesis after three, seven, 16 and 24 h of incubation (BrdU-Kit I (Roche). Three thousand MSC per well were seeded in a 96-well plate (coated with laminin-5, laminin-1, type I collagen or plastic as control), 10 µmol 5-bromo-2'-deoxy-uridine (BrdU) were added. Analysis of incorporated BrdU was performed with anti-BrdU antibodies which were then linked with anti-mouse IgG fluorescein. Counterstaining was performed with DAPI and visualized using a fluorescence microscope (DM IRBE, Leica).
Osteogenic differentiation and von Kossa stain
To explore osteogenic differentiation of the cells, MSC of 10 different patients were seeded at 5×104 cells/2 mL/well (coated with 5 mL of 1 µg/mL laminin-5, laminin-1, type I collagen or plastic as control) in 6-well plates. MSC in starvation medium and in non-coated plates/flasks served as controls. After four days of MSC expansion, growth medium was replaced by osteogenic induction medium consisting of high glucose DMEM, FCS 10%, 0.1 µM dexamethason, 10 nM β-glycerolphosphate, 0.17 mM L-ascorbic acid, 100 U/mL penicillin G, 100 µg/mL streptomycin and fungicides. Progress of osteogenic differentiation was analyzed at various time points after three, seven and 28 days of differentiation. The cultures were stained according to the von Kossa staining protocol to quantify mineralization. The plates were scanned and images were analyzed for staining density (Image J, NIH, USA). The level of confluent blackness was given as a number between 0 and 200, with 0 being black and 200 being white. For visualization, reciprocal value was calculated and normalized to the control.
Analysis of osteogenic marker genes
RNA was isolated from cells after osteogenic differentiation for three, seven or 14 days (RNesay-Mini Kit, Qiagen, Hilden, Germany). Cells were detached and the pellets disrupted in RLT buffer (1% β-mercaptoethanol) and RNA was isolated following the manufacturer's protocol. Reverse transcription of the RNA into cDNA was achieved with 1 µg RNA using a cDNA synthesis kit (Clontech, Mountain View CA, USA). Gene-specific cDNA was amplified by quantitative PCR (LightCycler 1.5, Roche), each according to the manufactorer's protocol. Primer pairs for alkaline phosphatase (MWG, Ebersberg, Germany), osteopontin, osteocalcin (LCSearch, Heidelberg, Germany, sequences not published) and runt-related transcription factor 2 (Runx-2, MWG) were used. The used primer sequences were:
AP:
up 5′- ATT ACC TGG ACA TCG GCA AC -3′
down 5′- TTG GGC ACC ACA TCA TAG AA -3′
Runx-2:
up 5′- TCT GGC CTT CCA CTC TCA GT -3′
down 5′- GAC TGG CGG GGT GTA AGT AA -3′
In each PCR, GAPDH served as housekeeping reference gene as described.17 To quantify the RNA synthesis the number of RNA copies was divided by the number of GAPDH copies. All gene analyzes were performed on three cultures of MSC and repeated twice.
Statistical analysis
Representative data were presented as mean values. Statistical analyses were performed using ANOVA. P<0.05 was considered significant.
Results
Expansion of mesenchymal stromal cells
MSC were expanded to the second or third passage. The cells presented as fibroblast-like adherent and proliferating cells (Figure 1). All populations (n=14) were characterized by flow cytometry revealing expression of CD73, CD90 and CD105 and the absence or minimal expression of CD14, CD34 and CD45 (data not shown). The proliferation capacity of MSC in GMP-conforming media was high with an average time of 10.6 days till expansion into the next passage (passage doubling time).
Figure 1.
Mesenchymal stromal cells cultured in animal serum-free media in passage 2 after three (A) and nine (B) days with increasing confluence. After 10–11 days, cells were transferred to the next passage.
Attachment assay
Adhesion was measured by incubation of MSC in protein-coated tissue culture plates using cultures of MSC of 9 patients. Coated bovine serum albumin served as blocking agent and as negative control. The assay was performed with protein amounts of 1, 5, 100 and 500 fmol/µL per spot. A protein amount of 5 fmol/µL was the lower limit for attachment to type I collagen and laminin-5, although some MSC attached to spots of 1 fmol/µL type I collagen. Visualization of adherent MSC revealed type I collagen to be most effective, followed by laminin-5 and laminin-1 (Figure 2).
Figure 2.

Representative compilation of attachment of mesenchymal stromal cells on plates coated with proteins (solution of 1 µL) of different concentrations. BSA (bovine serum albumin) served as control. Mesenchymal stromal cells preferentially bound to type I collagen and laminin-5 at a minimal concentration of 1–5 fmol/µL.
MTT and BrdU assays
MTT assay was performed using cultures of MSC of 8 patients. Cells of all cultures were vital without showing significant differences between the proteins used for coating. Growth on cell culture plastic (control) revealed the same cell viability index and metabolic activity (average extinction rate 0.39–0.43; data not shown) compared to the protein-coated samples.
BrdU assay was performed using cultures of 9 patients. MSC were expanded on protein-coated 96-well plates and BrdU-incorporation was measured after three, seven, 16 und 24 h. On average, 73% of MSC of laminin-5 coated wells proliferated after 24 h, compared to type I collagen with 70% followed by plastic (67%) and laminin-1 (63%). Differences did not reach statistical significance. After three, seven and 16 h, differences between proteins and plastic were negligible: for example, average proliferation of MSC on laminin-5 coated plates was 25% (3 h), 42% (7 h) and 51% (16 h).
Osteogenic differentiation, von Kossa stain, and analysis of osteogenic marker genes
MSC were expanded on protein-coated plates (for von Kossa stain) or in protein-coated cell culture flasks (for transcript analysis of marker genes) with osteogenic differentiation medium for three, seven, 14 and 28 days. Osteogenic differentiation of the MSC was analyzed using von Kossa stain. Plates were evaluated macroscopically and also scanned to explore the degree of mineralization indicated by black silver precipitates. Compared to plastic after 28 days of culture, laminin-5 coated plates contained significant more calcium-phosphate granula (P<0.04), followed by laminin-1 (P<0.09) and type I collagen (Figure 3). No spontaneous osteogenic differentiation of MSC in starvation medium was observed.
Figure 3.
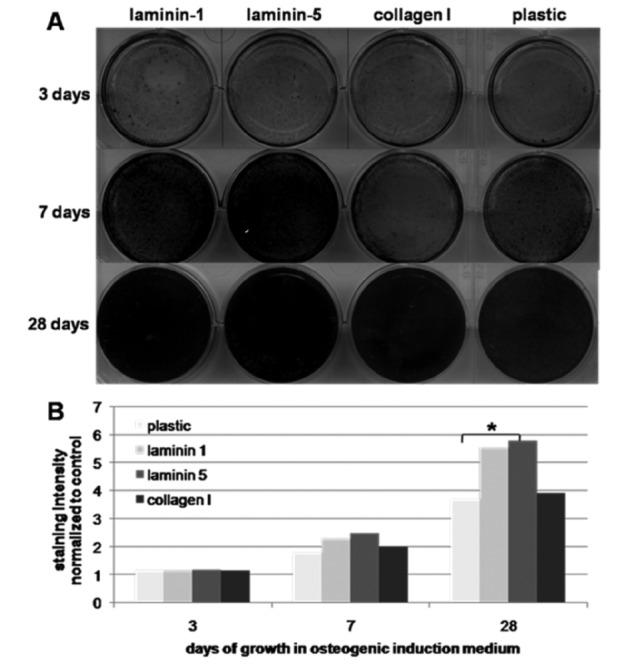
Von Kossa stain (A) and measurement of blackness of silver precipitates (B) after growth of mesenchymal stromal cells on plates coated with different proteins and osteogenic induction medium after three, seven and 28 days. Laminin-5 induced the greatest production of calcium granula with significant differences compared to plastic after 28 days (P<0.04).
To analyze osteogenic differentiation capacity, osteogenic marker genes alkaline phosphatase, osteopontin, osteocalcin and Runx-2 were investigated on 3 cultures. All four genes were expressed whereas analysis of gene expression patterns did not reveal significant differences between MSC cultured in coated flasks and the uncoated control after three, seven and 14 days (data not shown).
Discussion
Cells expanded for clinical applications have to meet the quality standards set by the health authorities (e.g. EMA, FDA). We, therefore, isolated and expanded MSC with animal serum-free medium (FFPP: fresh frozen plasma platelet) meeting current GMP-regulations.13 Due to different donors and preparation techniques, the amounts and the composition of growth factors varies within the plasma employed here.18 For that reason, serum samples from 15–25 individuals tested for growth factor content and sera from suitable donors were then pooled and stored at −70°C.13 Comparably, MSC vary considerably depending on health and age of MSC donors, and depend on culture conditions, number of passages, and other variables during in vitro expansion.
MSC expanded in FFPP medium showed a high proliferation capacity and fulfilled the minimal criteria for MSC with expression of CD 90, CD 73 and CD 105 and lack of CD 14, CD 34 and CD 4, as described in previous studies.13,14,19,20
By MTT and BrdU assays, MSC high proliferation rates were confirmed, but without detectable influences by the coating proteins employed on the metabolic activity. This could be due to the short time of exposure to the proteins (only 24 h). Furthermore, FFPP media activated higher proliferation of MSC compared to standard media.15 Therefore, minor changes in MSC proliferation facilitated by laminins or collagen possibly remained below detection levels. Alternatively, the cell cycle and mitochondrial metabolism was not directly influenced by ECM-proteins investigated in our study. In contrast, Lindner et al. found a higher proliferation rate of MSC in the presence of ECM proteins when they studied a longer proliferation period compared to our study.21
Klees et al. revealed, that laminin-5 induces adhesion, osteogenic gene expression and differentiation of human MSC through an ERK-dependent pathway.5,7,22–24 In their experiments, MSC were expanded in media containing FCS. Similar results are described for type I collagen.4,25 By using FFPP medium for culturing MSC, our study supports the previous findings of increased binding of MSC to the ECM-proteins type I collagen and laminin-5. In that context, we have to consider that we used a specific amount of proteins without noting their exact amount of binding sites, especially when comparing different proteins. Concerning laminin-5, the 3 LG3-domain was described to induce adhesion and osteogenic differentiation of MSC.7 Isolated use of such domains (peptide fragments) would possibly increase binding strength to MSC and could be part of further studies.
Evaluation of von Kossa stain was performed by scanning the plates and measuring the blackness of the silver precipitates. Evaluating the amount of produced calcium granula is described as an alternative.7
Regarding osteogenic differentiation, our study revealed a significant advantage of laminin-5 compared to type I collagen-coated or uncoated plastic growth after 28 days of culture in osteogenic differentiation medium, which is consistent with previous studies.5,22 Laminin-1 also promoted osteogenesis. But this did not reach statistical significance (P<0.09).
We found only slight differences in RNA expressions of osteogenic marker genes in MSC after a few days of osteogenic induction on protein-coated versus non-coated dishes, which correlate with a previous study.6 One explanation would be the small number of observed probes. In addition, extraction of mRNA from mineralizing probes is technically challenging. Therefore, progress in osteogenic differentiation makes it increasingly difficult to extract suitable mRNA for cDNA synthesis and quantitative PCR analyses. Furthermore, MSC from bone marrow are already primed for bone repair. In contrast to, for example, MSC from placenta, they express some genes associated with osteogenesis spontaneously in vitro. RNA analyses are very sensitive and we revealed osteogenic differentiation stain even in non-coated plates after 3 days.
In summary, we report that human bone marrow derived MSC proliferate well in animal serum-free GMP-conforming media, attach to type I collagen, and with lower affinity to laminin-5 and laminin-1. Osteogenic differentiation of MSC is not promoted by type I collagen, but by attachment to laminin-1 and more so by laminin-5. We conclude that coating orthopedic implants with type I collagen may not facilitate regeneration of bony defects, despite its high affinity to MSC. Our data confirm that laminin-5 or laminin-5-derived peptides serve better in bone repair, as this material promotes both firm attachment and osteogenic differentiation of MSC.
Acknowledgments:
we would thank Tanja Abruzzese and Elisabeth Kienzle for their excellent technical support. This study was supported in part by Aesculap and by grants from the Landesstiftung Baden-Württemberg and from the BMBF to WKA.
References
- 1.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 2.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 3.Owen M. Marrow stromal stem cells. J Cell Sci. 1988;10(Suppl 1):63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 4.Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klees RF, Salasznyk RM, Kingsley K, et al. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol Biol Cell. 2005;16:881–90. doi: 10.1091/mbc.E04-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cool SM, Nurcombe V. Substrate induction of osteogenesis from marrow-derived mesenchymal precursors. Stem Cells Develop. 2005;14:632–42. doi: 10.1089/scd.2005.14.632. [DOI] [PubMed] [Google Scholar]
- 7.Klees RF, Salasznyk RM, Ward DF, et al. Dissection of the osteogenic effects of laminin-332 utilizing specific LG domains: LG3 induces osteogenic differentiation, but not mineralization. Exp Cell Res. 2008;314:763–73. doi: 10.1016/j.yexcr.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews S, Bhonde R, Gupta PK, Totey S. Extracellular matrix protein mediated regulation of bone marrow derived human mesenchymal stem cells. Differentiation. 2012 doi: 10.1016/j.diff.2012.05.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Mostafa NZ, Fitzsimmons R, Major PW, et al. Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor, and bone morphogenetic protein-2. Connect Tissue Res. 2012;53:117–31. doi: 10.3109/03008207.2011.611601. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Goss B, Shi W, et al. Laminin, VEGF, and bone matrix protein expression in uroepithelial bone induction — a canine model. Connect Tissue Res. 2006;47:102–9. doi: 10.1080/03008200600646360. [DOI] [PubMed] [Google Scholar]
- 11.Tuschong L, Soenen SL, Blaese RM, et al. Immune response to fetal calf serum by two adenosine deaminase-defi cient patients after T cell gene therapy. Hum Gene Ther. 2002;13:1605–10. doi: 10.1089/10430340260201699. [DOI] [PubMed] [Google Scholar]
- 12.Mannello F, Tonti GA. Concise review. No breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serumfree, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603–9. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 13.Muller I, Kordowich S, Holzwarth C, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8:437–44. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 14.Felka T, Schäfer R, De Zwart P, Aicher WK. Animal serum-free expansion and differentiation of human mesenchymal stromal cells. Cytotherapy. 2010;12:143–53. doi: 10.3109/14653240903470647. [DOI] [PubMed] [Google Scholar]
- 15.Felka T, Schäfer R, Schewe B, et al. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17:1368–76. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen R, Morrison T, Herrmann M, Wittwer C. Quantitative PCR by continuous fluorescence monitoring of a double strand DNA specific binding dye. Biochemica. 1998;2:8–11. [Google Scholar]
- 18.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 19.Kocaoemer A, Kern S, Kluter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25:1270–8. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 20.Arpornmaeklong P, Kochel M, Depprich R, et al. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33:60–70. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 21.Lindner U, Kramer J, Behrends J, et al. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 22.Klees RF, Salasznyk RM, Vandenberg S, et al. Laminin-5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. 2007;262:106–14. doi: 10.1016/j.matbio.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salasznyk RM, Klees RF, Boskey A, Plopper GE. Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5. J Cell Biochem. 2007;100:499–514. doi: 10.1002/jcb.21074. [DOI] [PubMed] [Google Scholar]
- 24.Lam MT, Longaker MT. Comparision of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1499. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem (Tokyo) 2001;129:133–8. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]


