Abstract
Purpose.
An increased aqueous level of TGF-β2 has been found in many primary open-angle glaucoma patients. Secreted Protein, Acidic, and Rich in Cysteine (SPARC)-null mice have a lower intraocular pressure. The mechanistic relationship between SPARC and TGF-β2 in trabecular meshwork (TM) is unknown. We hypothesized that TGF-β2 upregulates SPARC expression in TM.
Methods.
Cultured TM cells were incubated with selective inhibitors for p38 MAP kinase (p38), Smad3, p42, JNK, RhoA, PI3K, or TGF-β2 receptor for 2 hours, and then TGF-β2 was added for 24 hours in serum-free media. Quantitative polymerase chain reaction (qPCR) and immunoblot analysis were performed. Immunofluorescent microscopy was used to determine nuclear translocation of signaling proteins. Ad5.hSPARC and Lentiviral shRNA for p38 and Smad3 were constructed, and infected human TM cells.
Results.
SPARC was upregulated by TGF-β2 in the human TM cells (3.8 ± 1.7-fold, n = 6, P = 0.01 for protein and 7.1 ± 3.7-fold, n = 6, P = 0.01 for mRNA), while upregulation of SPARC had no effect on TGF-β2. TGF-β2–induced SPARC expression was suppressed by inhibitors against p38 (−40.3 ± 20.9%, n = 10, P = 0.0001), Smad3 (−56.2 ± 18.9%, n = 10, P = 0.0001), JNK (−49.1 ± 24.6%, n = 10, P = 0.0001), and TGF-β2 receptor (−83.6 ± 14.4%, n = 6, P = 0.003). Phosphorylation and translocation of Smad3, p38, and MAPKAPK2 were detected at 30 minutes and 1 hour, respectively, following TGF-β2 treatment. Phosphorylation of JNK and c-jun was detected before TGF-β2 treatment. SPARC was suppressed 31 ± 13% (n = 5, P < 0.0001) by shRNA-p38 and 41 ± 3% (n = 5, P < 0.0001) by shRNA-Smad3.
Conclusions.
TGF-β2 upregulates SPARC expression in human TM through Smad-dependent (Smad2/3) or -independent (p38) signaling pathways. SPARC may be a downstream regulatory node of TGF-β2–mediated IOP elevation.
Keywords: SPARC, matricellular proteins, TGF-β2, Smad3, p38
TGF-β2 upregulates SPARC via Smad3 or p38 in the human trabecular meshwork.
Introduction
Primary open-angle glaucoma (POAG) is characterized by progressive optic nerve damage and visual loss.1 POAG is a leading cause of irreversible visual impairment and blindness, affecting more than 70 million individuals worldwide.2 An elevated IOP, beyond the structural and/or vascular capabilities of the optic nerve, is a causative risk factor for POAG.3,4 The elevated IOP of POAG is caused by poor aqueous drainage through the principle drain of the eye, the trabecular meshwork (TM).5 The underlying regulatory mechanisms controlling aqueous outflow resistance in the TM are not fully elucidated. However, pathologic changes of extracellular matrix (ECM) within the TM are involved.4,6
TGF-β2 has been demonstrated to be higher, as much as 3-fold, in the aqueous humor of POAG patients compared to age-matched people without glaucoma.7–10 In many disorders, TGF-β2 mediates fibrosis and ECM deposition,11–13 suggesting that TGF-β2 may have a similar role in the TM. In vitro, TGF-β2 increases the secretion of certain structural and regulatory ECM proteins.14–17 In perfused human cadaveric anterior chambers, TGF-β2 increased IOP, and the production of fibronectin and plasminogen activator inhibitor-1 (PAI-1).18,19 The downstream mechanistic pathways leading to these changes are unknown.
Matricellular proteins are nonstructural secreted glycoproteins that regulate ECM turnover and deposition in many human tissues.20 The matricellular protein family includes Secreted Protein, Acidic, and Rich in Cysteine (SPARC), thrombospondins 1 and 2, tenascins C and X, hevin, and osteopontin.21–23 SPARC is one of the most highly transcribed genes in TM and found throughout the TM,24 especially in the juxtacanalicular (JCT) region, where aqueous humor outflow resistance is highest and believed to be the anatomic location of outflow regulation.20 We demonstrated that SPARC-null mice have a 15% to 20% lower IOP compared to their corresponding wild-type mice due to enhanced aqueous drainage,25 indicating that SPARC may have a regulatory role in IOP.
The relationship between TGF-β2 and SPARC in TM is not fully elucidated. In response to TGF-β2 stimulation, SPARC is the most highly produced protein by TM cells.26 We hypothesized that TGF-β2 is an upstream regulator of SPARC. In our study, we examined the effect of TGF-β2 stimulation along with selective inhibition of various TGF-β2 signaling pathways on SPARC expression in normal human TM endothelial cells to determine the regulatory relationship and specific signaling pathways.
Materials and Methods
Tissue Samples, Cell Cultures and Reagents
Human TM was dissected from corneoscleral rims discarded from corneal surgery at the Massachusetts Eye and Ear Infirmary (Boston, MA). We have demonstrated previously the suitability of this tissue for molecular biologic experiments.27 TM tissue was isolated from the anterior segment, segmented, and assigned for the development of primary cell cultures. We conducted our studies in compliance with the tenets of the Declaration of Helsinki.
Primary human TM cells were cultured from cadaveric donor anterior segments aged 9, 35, 42, 45, 47, 62, and 70 years using a previous reported protocol.28 The cultures were maintained in Dulbecco's modified Eagle's media (DMEM; Invitrogen, Carlsbad, CA) containing 20% fetal bovine serum (FBS), 1% L-glutamine (2 mM), and gentamicin (0.1 mg/mL) at 37°C in a 10% CO2 atmosphere. All the cells used were from confluent passage–4 or –5 cultures that had been allowed to differentiate (i.e., incubate for beyond the time point at which confluence is noted) in complete media for 3 days.
Small molecule inhibitors for p38 (SB202190; Sigma-Aldrich; St. Louis, MO), JNK (SP600125; Sigma-Aldrich), p42/44 (PD98059; Sigma-Aldrich), RhoA (Y-27632 dihydrochloride; Sigma-Aldrich), PI3K (LY-294002 hydrochloride; Sigma-Aldrich), Smad3 (SIS3; EMD Chemicals, Gibbstown, NJ), and TGF-β2 receptor (SB431542; EMD Chemicals) were used to block each pathway selectively. Each inhibitor was added into cell cultures at 10 μM in serum-free media for 2 hours before TGF-β2 was added. TGF-β2 (R&D Systems, Minneapolis, MN) was reconstituted in 4 mM HCl solution containing 0.1% human serum albumin according to the manufacturer's instructions. Cultured TM cells were treated with TGF-β2 (2 ng/mL) at 37°C in a time-dependent manner (i.e., 0, 6, 12, and 24 hours). Conditioned media were harvested at each time-point and analyzed by immunoblot analysis. Control cells were treated with 4 mM HCl solution containing 0.1% human serum albumin without TGF-β2 (i.e., vehicle). Topro3 and Alexa Fluor 568 phalloidin (Invitrogen) were used to identify the nucleus and F-Actin, respectively.
Overexpression of SPARC by Adenoviral Infection
TM cell cultures were incubated with 2% FBS media and infected with 50 MOI of Ad5.control or Ad5.hSPARC for 18 hours. Then, the equal volume of 10% FBS media was added and TM cells were cultured further for 24 hours. TM cultures were replaced with serum-free media for 24 hours. The conditioned media were analyzed using immunoblot assays.
RNA Isolation and Quantitative PCR
Total RNA was isolated using Trizol (Invitrogen) according to the manufacturer's protocol. Briefly, cultured TM cells were lysed with Trizol buffer, and chloroform was added. After centrifugation, total nucleic acid was isolated from the aqueous layer using ethanol precipitation. After digesting DNA by DNase I, total RNA was isolated and stored at −80°C for no more than one month before further experiments.
Complementary DNA (cDNA) was synthesized using the M-MLV RT kit (Promega, Madison, WI) according to the manufacturer's instructions with minor modifications. Briefly, 250 ng of total RNA was used as a template in 50 μL of reaction mixture including 250 ng of oligo-dT primer, 1× reaction buffer, 0.2 mM of dATG, dCTG, dGTG, dATG, 20 units of RNase inhibitor, 200 units of M-MLV reverse transcriptase. The reaction mixture was incubated at 42°C for 1 hour and used for quantitative PCR (qPCR).
qPCR was performed to detect relative mRNA levels. Specific RNA messages were amplified in SYBR green I master mix (Applied Biosystems, Inc., Foster City, CA) using AB StepOnePlus (Applied Biosystems, Inc.). Quantification of the genes of interest was calculated by fold increase to β-Actin expression using Step One software v2.0 (Applied Biosystems, Inc.). All qPCRs were performed in triplicates. Primers for human SPARC and β-Actin were designed using the Primer 3 program (available in the public domain at http://frodo.wi.mit.edu) spanning introns with an expected qPCR product of 200 bp (Table 1).
Table 1. .
Primer Sequences
|
|
Forward |
Reverse |
Intron, Spanned |
| SPARC, human | 5′-GTGCAGAGGAAACCGAAGAG-3′ | 5′-AAGTGGCAGGAAGAGTCGAA-3′ | 4 and 5 |
| β-Actin, human | 5′-GGCATCCTCACCCTGAAGTA-3′ | 5′-GGGGTGTTGAAGGTCTCAAA-3′ | 3 and 4 |
Immunoblot Analysis
Cultured TM cells were homogenized and lysed in 1× radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% Igegal, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) with protease inhibitors (25 mTIU/mL aprotinin, 0.05 mg/mL phenylmethylsulfonyl fluoride [PMSF], 1 mM EDTA, 1 mM EGTA, and 1 μg/mL leupeptin to a final volume of 1 mL with RIPA buffer). Equal amounts of total protein from cell lysates or conditioned media were mixed with 2× reducing buffer (125 mM of Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.01% bromophenol blue) at a 1:10 ratio and were boiled at 100°C for 3 minutes with reducing reagent (5 mg/mL dithiothreitol [DTT]) or without it (nonreducing condition). The samples were analyzed using 10% polyacrylamide gels (PAGE). SDS-PAGE was performed at 100 V in tank buffer (250 mM Tris, 192 mM glycine, and 0.1% SDS) using the XCell SureLock Mini-Cell system (Invitrogen). The separated proteins were transferred onto a nitrocellulose membrane with 0.45 μm pore size (Invitrogen) in blotting buffer (250 mM Tris, 192 mM glycine, and 10% methanol). The membrane was incubated for 1 hour in 0.5× blocking buffer (Rockland, Gilbertsville, PA) at room temperature. The membrane then was incubated with a primary antibody (Table 2) in 0.5× blocking buffer, overnight at 4°C. The next day, the membrane was washed 3 times with TBS/T (50 mM Tris-HCl, 150 mM NaCl, 0.05% Tween20) for 10 minutes and then incubated with IRDye 800-conjugated donkey antigoat IgG (1:10,000), goat antimouse IgG (1:10,000) or goat antirabbit IgG (1:10,000; Rockland) for 1 hour. The membrane was washed with 1× TBS/T, 3 times at room temperature (RT) for 10 minutes and scanned using the Odyssey Infrared Imaging System (Li-Cor, Lincoln, Nebraska). The band density of proteins was quantified using Odyssey densitometric software (version 1.2). We stripped the membrane using Newblot Nitro Stripping Buffer (Li-cor) and added antibody to detect the dephosphorylated target proteins.
Table 2. .
The Primary Antibodies
|
Primary |
Dilution in 0.5× Blocking Buffer |
Company |
| Goat anti-human SPARC IgG | 1:1000 | R&D Systems Inc. (Minneapolis, MN) |
| Mouse anti-human TGF-β2 IgG | 1:1000 | Abcam (Cambridge, MA) |
| Rabbit anti-human phospho-p38 IgG | 1:1000 | Cell Signaling Technology (Danvers, MA) |
| Rabbit anti-human p38 IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human phospho-MAPKAPK2 IgG (T334) | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human MAPKAPK2 IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human phospho-Smad3 IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human Smad3 IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human phospho-JNK IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human JNK IgG | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human phospho-c-jun | 1:1000 | Cell Signaling Technology |
| Rabbit anti-human c-jun | 1:1000 | Cell Signaling Technology |
| Mouse anti-human β-Actin IgG | 1:1000 | Cell Signaling Technology |
Immunofluorescent Staining
Cultured TM cells in 8-well slides were fixed with 4% paraformaldehyde in PBS (pH 7.4) at 4°C, for 30 minutes. They then were washed in 1× PBS for 10 minutes, twice at RT. The cells were permeabilized with 0.2% Triton-100 in 1× PBS for 5 minutes and washed in 1× PBS. The cells then were blocked in 1× PBS including 3% BSA for 1 hour at RT. Primary antibody was applied to each section at 4°C overnight. Optimal primary antibody concentrations were determined empirically by serial antibody dilution (1:50–1:200). Slides were washed with 3% BSA-1 × PBS for 10 minutes, 3 times. Secondary antibodies (1:100) were applied to detect the target protein. Nucleus was stained with TO-PRO-3 (1:400) or DAPI (Invitrogen), and F-Actin was stained with Alexa Fluor 568 phalloidin (1:40; Invitrogen), respectively. Labeled tissues were analyzed with the Leica TCS SP2 spectral confocal laser scanning microscope (Leica Microsystems, Exton, PA).
Lentiviral Constructs for shRNA
Several lentiviral constructs of shRNA targeting Smad3 or p38 were purchased from Open Biosystems (Huntsville, AL; available in the public domain at www.openbiosystems.com). To screen out the most effective shRNA sequence, 1.0 μg of each construct was transfected into HT1080 cell lines. Then, target protein was analyzed in immunoblot. The selected construct was transfected into a 297T cell line as dictated by the manufacturer's protocol. Briefly, the day before transfection, the HEK293T cells were plated at a density of 5.5 × 106 cells per 100 mm of plate. On the day of transfection, DNA/Arrest-In complexes were made by mixing 9 μg of construct plasmid DNA, 28.5 μg of packaging mix, and 187.5 μg of Arrest-In transfection reagent in 2 mL of Opti-MEM (Invitrogen). After incubating for 20 minutes at RT, 3 mL of Opti-MEM were added to the transfection complexes. Then, the transfection complex was added to the cells. The cells were incubated at 37°C, 5% CO2 for 6 hours. Afterwards, 5 mL of DMEM (10% FBS, 1× penicillin-streptomycin [10,000 units and 10,000 mg /mL, respectively; pen/strep] and 1× L-glutamate) were added. The cells then were incubated further at 37°C, 5% CO2 chamber for 48 hours. Lentivirus carrying a random sequence (i.e., “scrambled” or “nonsilencing”) shRNA was used as a control.29 After 48 hours of incubation, lentivirus-containing supernatant was harvested and collected into a 15 mL sterile tube, then used for further experiments.
Cultured TM cells in 6 well-plates were transfected with 4 μg/mL of polybrene by adding 1 mL of shRNA- or nonsilent lentivirus. The cells then were incubated overnight at 37°C, 5% CO2. Fresh DMEM (10 mL; 20% FBS, 1× pen/strep [10,000 units and 10,000 mg/mL, respectively] and 1× L-glutamate) was replaced and the infected cells were cultured further for 3 days.
Statistics
Statistical analyses were performed using SigmaStat version 1.0 (Jandel Corp., San Rafael, CA). P values were calculated by Student's t-test and P < 0.05 was considered statistically significant when testing two groups. When testing our multiple inhibitors, we had seven experimental groups; applying the Bonferroni correction gives us an alpha value of P < 0.0071. All data were presented as the mean ± SD. Sample size (n) referred to the number of primary cell lines each derived from a distinct cadaveric donor.
Results
In cultured TM cells, TGF-β2 upregulated SPARC mRNA and protein in conditioned media as shown by quantitative PCR (7.1 ± 3.7-fold, n = 6, P = 0.01) and immunoblot analysis (3.8 ± 1.7-fold, n = 6, P = 0.01, Fig. 1).
Figure 1. .
Analysis of SPARC levels following TGF-β2 treatment (2 ng/mL) in cultured TM cells (n = 6). SPARC mRNA was analyzed and normalized with β-Actin. SPARC levels in conditioned media were analyzed by immunoblotting. n, sample size.
An increased protein level was seen as early as 6 hours and increased linearly over 24 hours (Fig. 2).
Figure 2. .

Time course of SPARC levels following TGF-β2 treatment (2 ng/mL). Conditioned media (30 μL) was analyzed using 10% SDS-PAGE (nonreducing condition).
To investigate a potential reciprocal relationship between SPARC and TGF-β2, we examined the level of TGF-β2 following SPARC overexpression in cultured TM cells and found that TGF-β2 was not affected by increased SPARC expression (Fig. 3).
Figure 3. .

TGF-β2 expression following SPARC overexpression (50 MOI) in cultured TM cells (n = 3). TGF-β2 levels in conditioned media were analyzed by immunoblotting. Conditioned media (30 μL) was analyzed using 10% SDS-PAGE (nonreducing condition).
To investigate which TGF-β2 signaling pathways were involved, we selected several small molecule inhibitors to p38, Smad3, p42/44, RhoA, PI3K, JNK, and the TGF-β2 receptor. Compared to TGF-β2–only treatment, TGF-β2–mediated SPARC expression was suppressed translationally by p38 (−40.3 ± 20.9%, n = 10, P = 0.0002), Smad3 (−56.2 ± 18.8%, n = 10, P = 0.000001), JNK (−49.1 ± 24.6%, n = 10, P = 0.0001), and TGF-β2 receptor (−76.2 ± 18.0%, n = 10, P = 0.0000003) inhibitors, but not by the p42, RhoA, or PI3K inhibitors (Fig. 4). In cultured TM cells, SPARC was upregulated via Smad3, p38, and JNK.
Figure 4.
Suppression by small molecule inhibitors of TGF-β2 induced SPARC expression (n = 3). Conditioned media (30 μL) was analyzed using 10% SDS-PAGE (nonreducing condition). Lane 1: no inhibitor (in) and no TGF-β2. Lane 2: TGF-β2 only. Lane 3: p42/44 inhibitor (PD98059) and TGF-β2. Lane 4: p38 inhibitor (SB202190) and TGF-β2. Lane 5: Smad3 inhibitor (SIS3). Lane 6: JNK inhibitor (SP600125). Lane 7: PI3K inhibitor (LY-294,002 hydrochloride). Lane 8: RhoA inhibitor (Y-27,632 dihydrochloride). Lane 9: TGF-β2 receptor inhibitor (SB431542) and TGF-β2. *P < 0.01 (n = 10). Compared to TGF-β2 only, the inhibitors to p38, smad3, JNK, and the TGF-β2 receptor blunted the induction of SPARC.
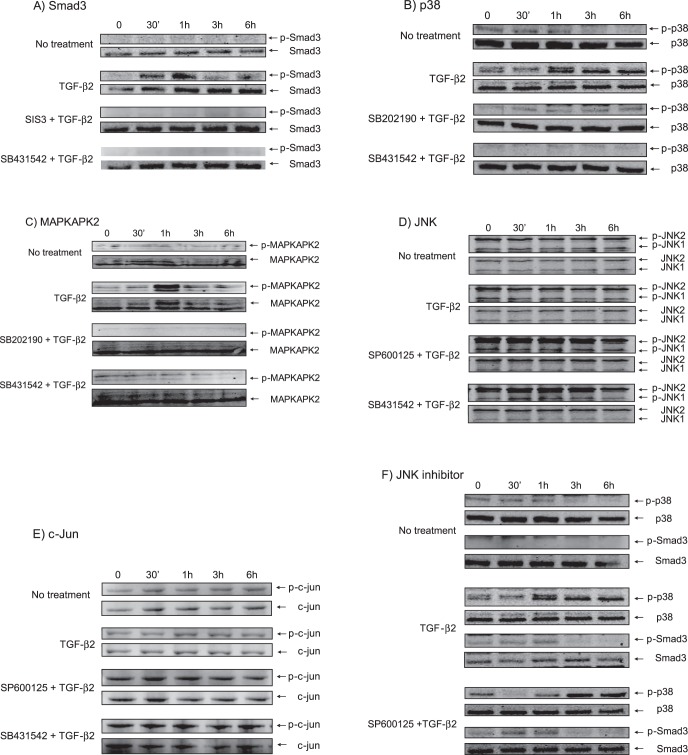
To investigate further the involvement of Smad3, p38, and JNK, we examined their phosphorylation. We found that Smad3 was phosphorylated within 30 minutes following TGF-β2 treatment (Fig. 5A). The phosphorylation of Smad3 peaked at 1 hour, and then decayed rapidly and returned to baseline at 3 and 6 hours. Phosphorylation of p38 was seen first at 1 hour and was stable for 6 hours (Fig. 5B). Phosphorylation of MAPKAPK2, which is one of the direct substrates of activated p38,30 peaked at 1 hour, and then decayed rapidly and returned to baseline at 3 and 6 hours (Fig. 5C), although phosphorylation of p38 was stable for 6 hours and phosphorylation of JNK was detected before TGF-β2 treatment. Thus, the JNK inhibitor did not affect the phosphor-JNK level (Fig. 5D). We also found that c-Jun was phosphorylated before TGF-β2 treatment indicating that JNK already was activated before the treatment (Fig. 5E). The JNK inhibitor did not affect either Smad3 or p-38 signaling pathways (Fig. 5F). The TGF-β2 receptor inhibitor completely blocked the effect of TGF-β2 on SPARC and served as a positive control.
Figure 5. .
Phosphorylation of signaling molecules in cultured TM cells after TGF-β2 treatment (2 ng/mL). Cell lysates (30 μg) was analyzed using 10% SDS-PAGE (reducing condition). Dephosphorylated target proteins were used as a loading control. (A) Phosphorylation of Smad3 was seen at 30 minutes on TGF-β2 treatment and reached a peak at 1 hour. (B) Phosphorylation of p38 began 1 hour after TGF-β2 treatment and was detected up to 6 hours. (C) Phosphorylation of MAPKAPK2, the substrate of activated p38, reached a peak at 1 hour after TGF-β2 treatment. (D) Phosphorylation of JNK already was detected before TGF-β2 treatment. (E) Phosphorylation of c-jun already was detected before TGF-β2 treatment. (F) JNK inhibitor (SP600125) did not affect phosphorylation of Smad3 and p38. SIS3, Smad3 inhibitor; SB202190, p38 inhibitor; SP600125, JNK inhibitor; SB431542, TGF-β2 receptor inhibitor.
As Smad3 and p38 were phosphorylated in cultured TM cells, we investigated the translocation of these molecules into the nucleus following TGF-β2 treatment. Phosphorylated-Smad3 (p-Smad3, Fig. 6A), phosphorylated-p38 (p-p38, Fig. 6B), and phosphorylated-MAPKAPK2 (Fig. 6C) each were translocated into nucleus, suggesting that this translocation is required for SPARC upregulation by TGF-β2. However, phosphorylated-JNK (Fig. 6D) and phosphorylated c-jun (Fig. 6E), the substrate of JNK, were translocated into nucleus before TGF-β2 treatment, also suggesting that JNK already was activated before TGF-β2 treatment.
Figure 6. .
Nuclear translocation of signaling molecules (green) in cultured TM cells following TGF-β2 treatment (2 ng/mL, 1 hour incubation for p-Smad3, 2 hours for p-p38, 2 hours for p-MAPKAPK2, 30 minutes for p-JNK and p-c-jun). (A) Nuclear translocation of p-Smad3. (B) Nuclear translocation of p-p38. (C) Nuclear translocation of p-MAPKAKPK2, the substrate of activated p38. (D) Nuclear location of p-JNK. (E) Nuclear location of p-c-jun, the substrate of JNK. Primary antibodies for p-Smad3, p-p38, p-MAPKAPK2, p-JNK, and p-c-jun (green), TO-PRO-3 (blue) for nucleus and Alexa Fluor 568 phalloidin (red) for F-Actin were used.
To investigate if the SPARC suppression was due specifically to p38 or Smad3 and not from a nonspecific effect of the small molecule inhibitors, lentiviruses including shRNA targeting p38 or Smad3 were constructed (Fig. 7). In cultured TM cells, shRNA against p38 and Smad3 suppressed these proteins by 54 ± 6% (n = 5, P < 0.0001) and 65 ± 6% (n = 5, P < 0.0001), respectively. Concurrently, TGFβ2-mediated SPARC upregulation was suppressed 31 ± 13% (n = 5, P < 0.0001) by shRNA-p38 and 41 ± 3% (n = 5, P < 0.0001) by shRNA-Smad3.
Figure 7. .
SPARC suppression by shRNA targeting p38 or Smad3. SPARC downregulation following p38 or Smad3 suppression in cell lysates and conditioned media was analyzed by immunoblot analysis, and the band intensity was measured to quantify relative expression. ***P < 0.0001 (n = 5).
Discussion
Our data indicated that SPARC mRNA and protein levels are regulated by TGF-β2. Specifically, JNK signaling provides some baseline level of stimulation, while smad2/3 and p38 signaling pathways allow for an additional graded response. The SPARC protein begins to appear as early as 6 hours and increases gradually over 24 hours. Our corroborating findings from shRNA and small molecule inhibitors, as well as the demonstration of nuclear translocation of phosphorylated proteins strongly argue for the involvement of these specific pathways. Interestingly, we discovered that phosphorylation, and translocation of the phosphorylated proteins of smad-3 and p38 occur at distinct times and in a specific sequence.
The cause of compromised aqueous drainage through the trabecular meshwork in POAG is not understood fully. However, patients with POAG have a higher level of aqueous TGF-β2 compared to age-matched unaffected people.7–10 We did not observe any difference in the responsiveness to TGF-β2 among the cell cultures based on age. TGF-β2 induces the expression of a variety of ECMs in cultured TM cells and increases the IOP of anterior segment organ perfusion studies.19 These observations suggest TGF-β2 as an important part of the pathogenesis of POAG. The mechanistic pathways responsible for TGF-β2's effects in trabecular meshwork are unknown. We found that SPARC null mice have a lower IOP due to enhanced aqueous drainage.25 SPARC is highly expressed in the juxtacanalicular TM and is one of the mostly highly upregulated genes in response to mechanical stretch of TM cells, implicating SPARC as an important protein for IOP regulation.24,31 However, we found that upregulation of SPARC has no effect on TGF-β2 in cultured TM cells. Thus, SPARC appears to be a downstream regulatory node mediating the effects of TGF-β2, which previously was unrecognized.
In preliminary experiments, we found that SPARC upregulation increases IOP in cadaveric human anterior segments (Oh DJ, et al. IOVS 2011;52:ARVO E-Abstract 4621). In these perfused segments, there is an increased amount of expression of certain ECM proteins, especially collagen IV, within the JCT region. These changes appear to be a direct result of SPARC rather than a feedback loop with TGF-β2. The SPARC gene resides within the GLC1M locus for juvenile open-angle glaucoma, but coding sequences, copy number variations, and splice sites in SPARC do not appear to cause a glaucoma that is inherited in a Mendelian fashion.32 At this time, the majority of POAG is not the result of mutations inherited in a Mendelian pattern, but rather the result of numerous contributory genes—similar to many complex diseases, such as diabetes and hypertension. It remains to be seen if SPARC itself has a role in the pathogenesis of glaucoma. If it does, it may be as a downstream effector of TGF-β2. However, SPARC does appear to be important to the normal regulation of IOP. In either event, the elucidation of these specific signaling pathways in this report may be critical to developing directed therapies rather than the gross blocking of TGF-β2, which has many normal functions and the disruption of which may cause numerous unwanted side effects.
TGF-β2 triggers several signaling pathways.33–37 The Smad3-dependent pathway has been known as canonical34,35 and there are Smad3-independent pathways, such as the p38 pathway.34 Inhibition of either Smad3 or p38 did not suppress SPARC expression completely. Thus, these two signaling pathways are redundant for TGF-β2 and may provide a functional reserve in TM. In our time-course experiments, we found that Smad3 and p38 were sequentially phosphorylated following TGF-β2 treatment. Smad3 was phosphorylated quickly within 30 minutes (early response), and its phosphorylation peaked at 1 hour. Interestingly, the phosphorylation of Smad3 declined rapidly after 1 hour. However, the phosphorylation of p38 started at 1 hour (late response) and lasted for 6 hours, while phosphorylation of MAPKAPK2, the substrate of activated p38, peaked at 1 hour and then declined thereafter. The physiologic/pathophysiologic significance of the sequential timing of these two pathways in TM is yet unclear.
We found that the small molecule inhibitor of JNK, SP600125, blunted the TGF-β2 induction of SPARC without affecting the phosphorylation of Smad3 or p38. However, phosphorylation of JNK was detected before TGF-β2 treatment. This could indicate a basal level of stimulation provided by JNK stimulation. Alternatively, JNK may not be involved in SPARC regulation, and the effect on SPARC may have been a nonspecific effect of small molecular inhibitor; we were unable to confirm the effects of the small molecular inhibitor with silencing RNA.
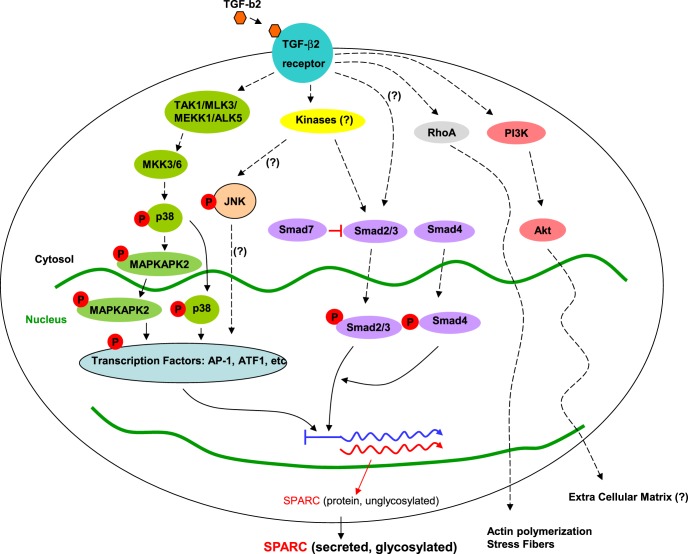
In TM cells, TGF-β2 upregulated SPARC via Smad3 or p38 (Fig. 8). SPARC appears to be a previously unrecognized regulatory node for TGF-β2 mediated ECM and IOP changes. Further study of the downstream pathways of SPARC in the regulation of IOP and its potential contributions to the pathogenesis of glaucoma are needed.
Figure 8. .
Schematic representation of TGF-β2 signaling pathways involved with SPARC regulation in TM cells. Phosphorylation of Smad3 began quickly and disappeared by 1 hour, followed by phosphorylation of p38 and MAPKAPK2 after 1 hour. p42, PI3K, and RhoA do not appear to be involved.
Acknowledgments
Supported by American Glaucoma Society Mid-Career Award, Massachusetts Lions Eye Research Fund, National Eye Institute, EY 019654-01 (DJR) and EY14104 (MEEI Vision-Core Grant).
Disclosure: M.H. Kang, None; D.-J. Oh, None; J.-H. Kang, None; D.J. Rhee, None
References
- 1. Weinreb RN. Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol. 2007; 42: 396–398 [PubMed] [Google Scholar]
- 2. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720 [DOI] [PubMed] [Google Scholar]
- 4. Rohen JW. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983; 90: 758–765 [DOI] [PubMed] [Google Scholar]
- 5. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801 [DOI] [PubMed] [Google Scholar]
- 6. Lutjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989; 48: 139–147 [DOI] [PubMed] [Google Scholar]
- 7. Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001; 239: 109–113 [DOI] [PubMed] [Google Scholar]
- 8. Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002; 46: 249–253 [DOI] [PubMed] [Google Scholar]
- 9. Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001; 239: 199–207 [DOI] [PubMed] [Google Scholar]
- 10. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994; 59: 723–727 [DOI] [PubMed] [Google Scholar]
- 11. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huggins JT, Sahn SA. Causes and management of pleural fibrosis. Respirology. 2004; 9: 441–447 [DOI] [PubMed] [Google Scholar]
- 13. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006; 69: 213–217 [DOI] [PubMed] [Google Scholar]
- 14. Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48: 715–726 [DOI] [PubMed] [Google Scholar]
- 15. Li J, Tripathi BJ, Tripathi RC. Modulation of pre-mRNA splicing and protein production of fibronectin by TGF-beta2 in porcine trabecular cells. Invest Ophthalmol Vis Sci. 2000; 41: 3437–3443 [PubMed] [Google Scholar]
- 16. Zhao X, Ramsey KE, Stephan DA, Gene Russell P. and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 2004; 45: 4023–4034 [DOI] [PubMed] [Google Scholar]
- 17. Zhao X, Russell P. Versican splice variants in human trabecular meshwork and ciliary muscle. Mol Vis. 2005; 11: 603–608 [PubMed] [Google Scholar]
- 18. Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 153–158 [DOI] [PubMed] [Google Scholar]
- 19. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006; 47: 226–234 [DOI] [PubMed] [Google Scholar]
- 20. Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009; 88: 694–703 [DOI] [PubMed] [Google Scholar]
- 21. Hughes RC, Taylor A, Sage H, Hogan BL. Distinct patterns of glycosylation of colligin, a collagen-binding glycoprotein, and SPARC (osteonectin), a secreted Ca2+-binding glycoprotein. Evidence for the localisation of colligin in the endoplasmic reticulum. Eur J Biochem. 1987; 163: 57–65 [DOI] [PubMed] [Google Scholar]
- 22. Yost JC, Bell A, Seale R, Sage EH. Purification of biologically active SPARC expressed in Saccharomyces cerevisiae. Arch Biochem Biophys. 1994; 314: 50–63 [DOI] [PubMed] [Google Scholar]
- 23. Brekken RA, Sage EH. SPARC. a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001; 19: 816–827 [DOI] [PubMed] [Google Scholar]
- 24. Rhee DJ, Fariss RN, Brekken R, Sage EH, Russell P. The matricellular protein SPARC is expressed in human trabecular meshwork. Exp Eye Res. 2003; 77: 601–607 [DOI] [PubMed] [Google Scholar]
- 25. Haddadin RI, Oh DJ, Kang MH, et al. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009; 50: 3771–3777 [DOI] [PubMed] [Google Scholar]
- 26. Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Quantitative proteomics: TGFbeta(2) signaling in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011; 52: 8287–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003; 77: 749–756 [DOI] [PubMed] [Google Scholar]
- 28. Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000; 20: 347–350 [PubMed] [Google Scholar]
- 29. Walsh CT, Radeff-Huang J, Matteo R, et al. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J. 2008; 22: 4011–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelley MJ, Rose A, Song K, Lystrup B, Samples JW, Acott TS. p38 MAP kinase pathway and stromelysin regulation in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48: 3126–3137 [DOI] [PubMed] [Google Scholar]
- 31. Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001; 42: 1505–1513 [PubMed] [Google Scholar]
- 32. Chen LJ, Tam PO, Tham CC, et al. Evaluation of SPARC as a candidate gene of juvenile-onset primary open-angle glaucoma by mutation and copy number analyses. Mol Vis. 2010; 16: 2016–2025 [PMC free article] [PubMed] [Google Scholar]
- 33. Yao K, Ye PP, Tan J, Tang XJ. Shen Tu XC. Involvement of PI3K/Akt pathway in TGF-beta2-mediated epithelial mesenchymal transition in human lens epithelial cells. Ophthalmic Res. 2008; 40: 69–76 [DOI] [PubMed] [Google Scholar]
- 34. Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011; 11: 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dunker N, Krieglstein K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000; 267: 6982–6988 [DOI] [PubMed] [Google Scholar]
- 36. Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007; 84: 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Itoh Y, Kimoto K, Imaizumi M, Nakatsuka K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007; 84: 464–472 [DOI] [PubMed] [Google Scholar]