Abstract
Purpose.
During inflammation of the ocular surface, increased proinflammatory cytokines depress tear protein secretion, suggesting that a decline in lacrimal cell function contributes to dry eye. Lacritin, a glycoprotein secreted from lacrimal acinar cells, may function as an autocrine factor to stimulate tear protein secretion. The purpose of the present experiment was to investigate lacritin-induced protein secretion in normal and cytokine-pretreated (inflammation model) monkey acinar cells.
Methods.
Acinar cells from monkey lacrimal glands were cultured with or without tumor necrosis factor alpha (TNF-α) plus interferon gamma (IFN-γ). Protein secretion was induced by lacritin or carbachol (Cch, positive control). Proteins were detected and identified by immunoblotting and immunocytochemistry. Intracellular Ca2+ was measured with the fluorophore Calcium-4, and cell damage was determined by LDH leakage into the culture medium.
Results.
In cultured monkey acinar cells, lacritin stimulated tear protein secretion in normal cells without elevating intracellular Ca2+. In contrast, Cch elevated intracellular Ca2+ and release of tear proteins. This contrast suggested an alternate, calcium-independent mechanism for lacritin-induced protein secretion. TNF-α plus IFN-γ caused LDH leakage from sensitive human corneal epithelial cells, but even higher doses of TNF-α plus IFN-γ did not cause LDH leakage from monkey acinar cells, suggesting a higher tolerance against these cytokines. In cytokine-treated acinar cells, lacritin stimulated protein secretion as much as that in normal cells. In contrast, Cch-induced elevation of Ca2+ and release of proteins were depressed by cytokines.
Conclusions.
Lacritin might be a useful biotechnology-based treatment agent against ocular surface diseases where endogenous lacritin is inadequate.
Keywords: lacritin, monkey, lacrimal acinar cells, tear protein secretion, cytokine
Lacritin promoted secretion of tear protein from cultured monkey acinar cells. Even when acinar cells were pretreated with cytokines (inflammation model), lacritin stimulated protein secretion as much as from normal cells. In contrast, calbachol-induced secretion was depressed by cytokines.
Introduction
The functions of the tear film include maintaining the moisture of the eye surface, supplying oxygen and nutrients to the avascular cornea, protecting the ocular surface by entrapping and sweeping debris and microorganisms, preventing infection by supplying antibacterial substances and antibodies, and maintaining normal osmolarity and tear film stability.1 Dry eye occurs when there are insufficient tears to provide these functions.
Inflammation of the ocular surface also plays a key role in dry eye syndrome.2 Increased concentrations of proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) have been found in the tears of dry eye patients.3–5 In a murine model of human Sjögren syndrome (MRL/lpr), cytokine expression was upregulated,6,7 and acetylcholine release was impaired following a decrease in protein secretion from the lacrimal gland.8 Proinflammatory cytokines interleukin-1 alpha, beta, or TNF-α also inhibited peroxidase secretion induced by neurotransmitters from mouse lacrimal glands.7
Secretion of tear proteins from lacrimal glands responds to neurotransmitters from parasympathetic and sympathetic nerves. The major neurotransmitters are parasympathetic acetylcholine, vasoactive intestinal peptide, and sympathetic norepinephrine. Each neurotransmitter activates a different signaling pathway.9 For example, in cultured monkey lacrimal acinar cells, we previously reported that acetylcholine analog carbachol (Cch) increased intracellular Ca2+, activated the protein kinase C (PKC) pathway, and promoted secretion of the tear protein lacritin.10
Lacritin protein expression has been detected in primates, and high expression was found in human lacrimal and meibomian glands.11 Recently, lacritin expression was also found in nonprimate horse tears.12 Monkey lacritin was also identified in monkey tears, and expressions of lacritin mRNA and protein were found in monkey lacrimal gland.13 Cholinergic stimulation induced secretion of lacritin from monkey lacrimal acinar cells, suggesting that lacritin is produced in the lacrimal gland and is secreted onto the ocular surface.10 Since a decrease of tear lacritin was reported in patients with blepharitis14 and in contact lens–related dry eye,15 lacritin might play an important role in maintaining tear fluid and the ocular surface. Indeed, lacritin promoted protein secretion from cultured rat acinar cells16 and stimulated proliferation in cultured human corneal cells and salivary ductal cells.17,18 Furthermore, in vivo instillation of recombinant human lacritin stimulated basal tear flow in rabbits.19 However, no data are available on lacritin-induced secretion of tear proteins in the more relevant monkey model. Thus, the purpose of the present study was to determine if lacritin induces protein secretion from monkey lacrimal acinar cells cultured under physiologic and inflammatory conditions.
Materials and Methods
Experimental Animals
Lacrimal glands from rhesus monkeys (Macaca mulatta), ranging in age from 1 to 10 years, were obtained at necropsy from procedures unrelated to the present studies from the Oregon National Primate Research Center (Beaverton, OR). Tear fluids were collected from adult monkeys (Macaca fascicularis; Evebioscience Co., Ltd., Wakayama, Japan). Experimental animals were handled in accordance with the ARVO Statement for the use of Animals in Ophthalmic and Vision Research and the Guiding Principles in the Care and Use of Animals (DHEW Publication, NIH 80-23).
Purification of Native Lacritin From Monkey Tears
Adult monkeys were secured in a monkey chair, and 50 μL saline (Otsuka Pharmaceutical, Tokyo, Japan) was instilled in the nasal side of one eye by a micropipette. After blinking, diluted tear fluids were collected from the temporal side of the palpebral fissure.
Diluted tear fluids (20 mL) were mixed with 80 mL 50 mM sodium phosphate solution (pH 7.0) and applied onto a cationic exchange column (HiTrap SP Sepharose; GE Healthcare Life Sciences, Buckinghamshire, UK) and eluted with 50 mM sodium phosphate solution containing 10 mM NaCl (pH 7.0). Flow-through fractions containing lacritin were collected and concentrated using disposable ultrafiltration spin columns (Vivaspin 20; Sartorius Stedim Biotech S.A., Aubagne Cedex, France). The concentrated sample was then purified on a gel filtration column (Superdex 75 HiLoad 16/60; GE Healthcare Life Sciences) with phosphate-buffered saline (PBS, pH 7.4). The gel filtration fractions comprising the lacritin peak were pooled and subjected to SDS-PAGE and immunoblotting to document the purity of our lacritin preparation, using 15% Tris-Tricine gels (c-PAGEL; ATTO Corp., Tokyo, Japan). Gels were stained with commercial stain (Coomassie Brilliant Blue R-250; Nacalai Tesque, Inc., Kyoto, Japan). Immunoblotting was performed by a conventional method20 with primary antibody against the N-terminus of Macaca mulatta lacritin polypeptide (Ser-Ser-Asp-Ser-Thr-Asp-Ala-Asp-Pro-Ala-Gln-Glu-Ala-Gly-Thr-Ser-Lys-Pro-Asn-Glu-Cys) and peroxidase-conjugated goat antirabbit secondary antibody (Thermo Fisher Scientific Inc., Rockford, IL).
Culture of Monkey Lacrimal Acinar Cells and Collection of Secreted Tear Proteins
Lacrimal acinar cells were isolated as described previously,13 and were plated at 1 × 105 cells/cm2 onto rat collagen I (0.01 mg/cm2; BD Biosciences, San Jose, CA) coated plates with Dulbecco's modified Eagles's medium/Ham's F-12 Nutrient Mixture (Life Technologies, Carlsbad, CA) containing 10 ng dexamethazone/mL (Sigma-Aldrich, St. Louis, MO), 50 ng EGF/mL (Life Technologies), 10 μg glutathione/mL (Sigma-Aldrich), 1× insulin-transferrin-sodium selenite media supplement (Sigma-Aldrich), 25 μg l-ascorbic acid/mL (Sigma-Aldrich), 1 mM putrescine (Sigma-Aldrich), and 50 μg gentamicin/mL (Life Technologies). After culture for approximately 16 hours at 37°C to allow adhesion and formation of epithelial-like morphology, the acinar cells were rinsed and pretreated with supplement-free medium for 30 minutes. Acinar cells were stimulated for 10 minutes with monkey lacritin or carbachol (Cch; Sigma-Aldrich) at the concentrations indicated in the figures. The cultured medium was then collected, and detached cells were removed by centrifugation. The collected media were concentrated and subjected to immunoblotting. To determine calcium requirements for protein secretion, calcium-free medium (EpiLife Medium; Life Technologies) was used during pretreatment and stimulation in replace of medium containing calcium.
Since the amount of tear proteins secreted into the culture medium was less than 0.1% of total tear proteins within the cultured acinar cells, and variability of cell numbers had a negligible influence on results, protein secretion was expressed as the amount in the medium not as a ratio to cellular number.
Establishment of Inflammatory Conditions in Lacrimal Acinar Cells
To mimic inflammatory conditions, monkey lacrimal acinar cells were cultured with TNF-α (R&D Systems, Minneapolis, MN) and IFN-γ (R&D Systems) for an additional 1, 2, or 3 days. To compare with damage due to inflammation on the ocular surface, human corneal epithelial cells (HCE-T; a gift from Kaoru Sasaki, Ideta Eye Hospital, Kumamoto, Japan) were also treated with TNF-α and/or IFN-γ at indicated concentrations, after plating at 3 × 104 cells/cm2 and preculturing for approximately 16 hours.
After incubation, the medium was collected, and leakage of LDH was measured as a marker for cell damage (LDH Cytotoxicity Detection Kit; Takara Bio Inc., Shiga, Japan). The cells were harvested into buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, protease inhibitor (complete Mini-EDTA free; Roche Diagnostics Corp., Indianapolis, IN), and phosphatase inhibitor cocktail I and II (EMD Chemicals Inc., Philadelphia, PA), and then lysed by sonication. Supernates were obtained by centrifugation for 10 minutes at 16,000g, and protein concentrations were determined with the BCA protein assay (Thermo Fisher Scientific Inc.) using bovine serum albumin (BSA) as a standard.
Immunoblotting
Denatured protein samples from cells and media were separated by 4–12% Bis-Tris gels (NuPAGE; Life Technologies) with 2(N-morpholino)ethane sulfonic acid buffer (Life Technologies). The proteins were then electrotransferred from the gels to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked with 0.5% skim milk in Tris-buffered saline with 0.05% Tween 20 (TTBS) for 30 minutes at room temperature, and incubated overnight at 4°C with primary antibodies for lacritin (dilution: 1:1000),13 lipocalin (1:100; Santa Cruz Biotechnology, Inc. Santa Cruz, CA), lactoferrin (1:5000; Sigma-Aldrich), phosphorylated STAT1 (1:1000; Cell Signaling Technology, Inc. Danvers, MA), STAT1 (1:1000; Cell Signaling Technology), IκB (1:1000; Santa Cruz Biotechnology, Inc.), or β-actin (1:1000; Sigma-Aldrich) in 1% BSA in TTBS. The membranes were then rinsed in TTBS and incubated for 1 hour at room temperature with HRP-conjugated goat antirabbit secondary antibody (1:10,000–5000; Santa Cruz Biotechnology, Inc.), HRP-conjugated donkey antigoat secondary antibody (1:5000; Santa Cruz Biotechnology, Inc.), or HRP-conjugated bovine antimouse secondary antibody (1:5000; Santa Cruz Biotechnology, Inc.). Immunopositive proteins were detected by chemiluminescence (ECL plus or ECL prime; GE Healthcare Life Sciences). Images were captured with a (FluorChem FC2 imager; Alpha Innotech Corp., San Leandro, CA).
Immunocytochemistry
Cultured acinar cells were fixed with −20°C 100% methanol, incubated for 3 minutes at −20°C, and rinsed with Dulbecco's phosphate-buffered saline (DPBS; Life Technologies). Cells were blocked and permeabilized for 15 minutes in DPBS containing 1% BSA and 0.1% Triton X-100 at room temperature. Samples were incubated overnight at 4°C with primary antibodies for lacritin (dilutions: 1:200), lipocalin (1:200), or lactoferrin (1:200). After the cells were rinsed in DPBS with 0.05% Tween 20 (TPBS), they were incubated for 1 hour at room temperature with Alexa Fluor 488-labeled antirabbit IgG or antigoat IgG (1:1000; Life Technologies) and DAPI (1:1000; Life Technologies). Stained cells were rinsed with DPBS and then photographed with an inverted microscope (Axiovert 200, equipped with an AxioCam MRc5; Carl Zeiss Vision, GmbH, Hallbergmoos, Germany). Images were compiled in image-analysis software (ImageJ Launcher 1.4.3.67, GIMP 2.6.11; ImageJ software, provided in the public domain, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsbweb.nih.gov/ij/index.html).
Measurement of Intracellular Calcium
Cultured acinar cells were incubated with calcium binding fluorophore Calcium-4 (Molecular Devices, Sunnyvale, CA) in loading buffer (Hank's balanced salt solution with 20 mM HEPES, pH 7.4) for 1 hour at 37°C. Fluorescent measurements were performed on a microplate reader (Flexstation 3; Molecular Devices) every 1.5 seconds for 3 minutes. Cch and/or lacritin were added following 20 seconds of initial background fluorescent measurements. Results are expressed as the background-adjusted fluorescence intensity versus time.
Statistical Analysis
Data were analyzed by Dunnett's test or Student's t-test with statistical software (JMP 8.0.1 or 10.0.0; SAS Institute Japan Ltd., Tokyo, Japan). P < 0.05 was considered statistically significant.
Results
Purification of Lacritin From Monkey Tears
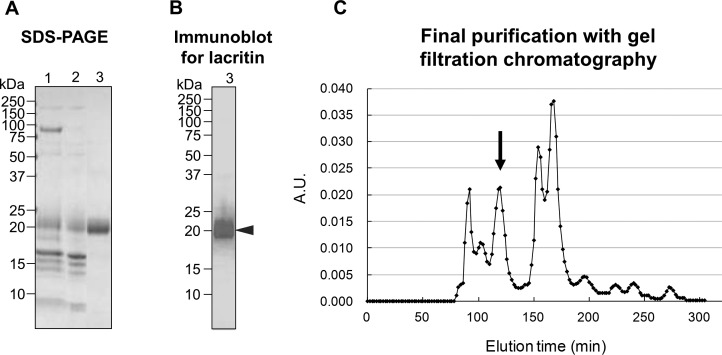
Raw monkey tears contained lacritin at the expected size of 21 kDa (Fig. 1B, arrowhead), along with other major protein bands at 87 and <20 kDa (Fig. 1A, lane 1). During the first stage of purification, lacritin (isoelectric point [pI] = 4.9) did not bind to a cationic exchange column and was present in the flow-through fraction. However, the column removed the 87-kDa band (Fig. 1A, lane 2), identified by immunoblotting as lactoferrin (data not shown). Gel filtration chromatography eluted a purified lacritin peak between 110 and 130 minutes (Fig. 1C, arrow) and removed the contaminating bands below 20 kDa (Fig. 1A, lane 3). The pooled fractions contained lacritin without other obvious contaminating proteins with the final purity calculated to be more than 98%.
Figure 1.
Steps in purification of lacritin from monkey tears, showing SDS-PAGE (∼2 μg/lane) (A), immunoblotting for lacritin (∼0.2 μg/lane) (B), and final gel filtration chromatography with lacritin fractions eluting at 110 to 130 minutes ([C], arrow). Lane 1, diluted raw monkey tear fluid; lane 2, the flow through fraction from initial cationic exchange chromatography; lane 3, pooled lacritin fractions of gel filtration chromatography.
Lacritin-Induced Secretion of Tear Proteins From Cultured Monkey Acinar Cells
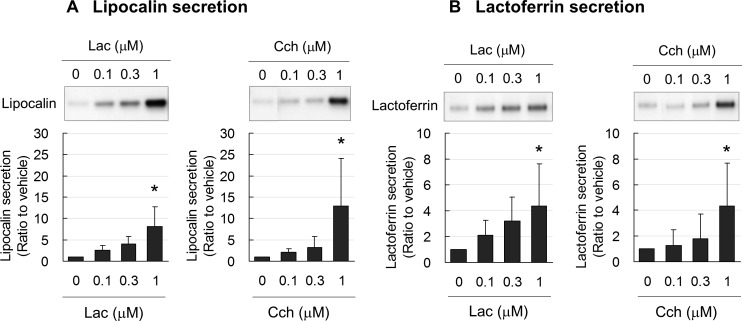
Lacritin induced secretion of the tear proteins lipocalin (Fig. 2A) and lactoferrin (Fig. 2B) in a dose-dependent manner. The effect of lacritin at 1 μM was significant and nearly equal to that of the well-known tear protein stimulator, acetylcholine analog Cch. Thus, 1 μM of lacritin and Cch were used in the following experiments.
Figure 2.
Representative immunoblots and band density measurements for secretion of lipocalin (A) and lactoferrin (B) from cultured monkey lacrimal acinar cells, showing significant, dose-dependent increases caused by lacritin and carbachol. Data are the means ± SD (n = 4). *P < 0.05 relative to nontreated controls (0 μM) (Dunnett's test). Lac, lacritin; Cch, carbachol.
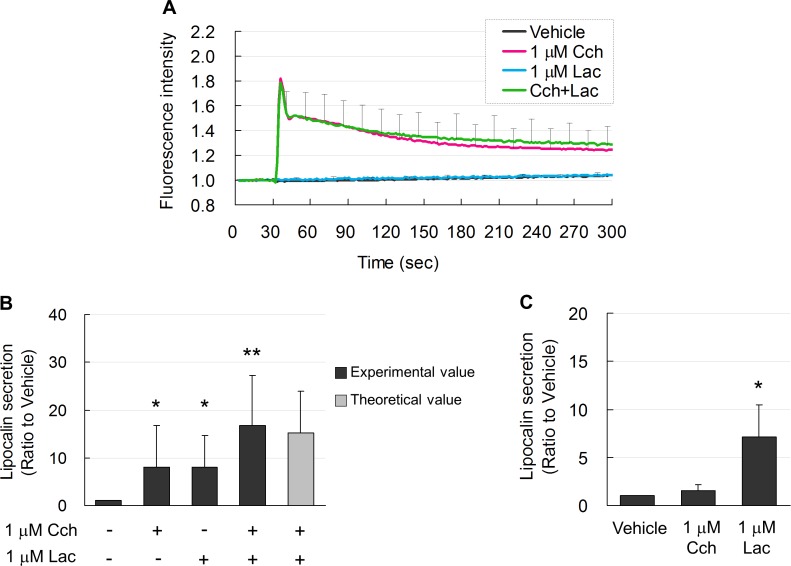
Cch at 1 μM transiently induced an increase in intracellular Ca2+ (Fig. 3A), which gradually decreased with time. In contrast, 1 μM lacritin did not change intracellular Ca2+ levels, although both Cch and lacritin equally induced secretion of lipocalin (Fig. 3B). An additive effect on lipocalin secretion was observed when Cch was combined with lacritin (Fig. 3B). In a previous study,21 lower concentrations of Cch plus another agonist showed a synergistic secretory effect in rabbit lacrimal gland cells. In our model of monkey lacrimal acinar cells, 0.1 μM each of Cch plus lacritin did not show any synergistic effect (data not shown). However, note that addition of lacritin to Cch did not increase intracellular Ca2+ levels observed with Cch alone (Fig. 3A), suggesting that Ca2+ did not directly regulate lacritin-induced protein secretion. This was also confirmed in experiment using calcium-free medium. Lacritin (1 μM) induced lipocalin secretion from monkey lacrimal acinar cells to a similar level as with medium containing calcium (Figs. 3B, 3C). Cch (1 μM) did not increase lipocalin secretion in the calcium-free medium (Fig. 3C). These results suggested that lacritin did not induce entrance of extracellular Ca2+. Calcium was not required for lacritin induction of protein secretion by monkey lacrimal acinar cells.
Figure 3.
(A) Elevated free intracellular Ca2+ (fluorescence intensity) levels in acinar cells after induction by Cch (red line). Intracellular Ca2+ levels during Lac induction (blue line) were not increased and were similar to vehicle-treated controls (black line). Combined treatment with Cch and Lac (Cch + Lac) did not further increase Ca2+ (green line). (B) Lipocalin secretion induced by Cch and Lac was similar. Lac plus Cch enhanced lipocalin secretion. Light gray bar shows the theoretical additive effect of Cch- and Lac-induction. Data are means ± SD (n = 6). *P < 0.05 relative to vehicle-treated controls and **P < 0.05 relative to Cch- or Lac-treated alone (Student's t-test). (C) Ca2-free medium abolished lipocalin secretion induced by Cch. Lacritin-induced lipocalin secretion in Ca2-free medium was the same as that in medium containing Ca2. Data are means ± SD (n = 3). *P < 0.05 relative to vehicle (Dunnett test).
Establishment of an Inflammation Model in Cultured Acinar Cells
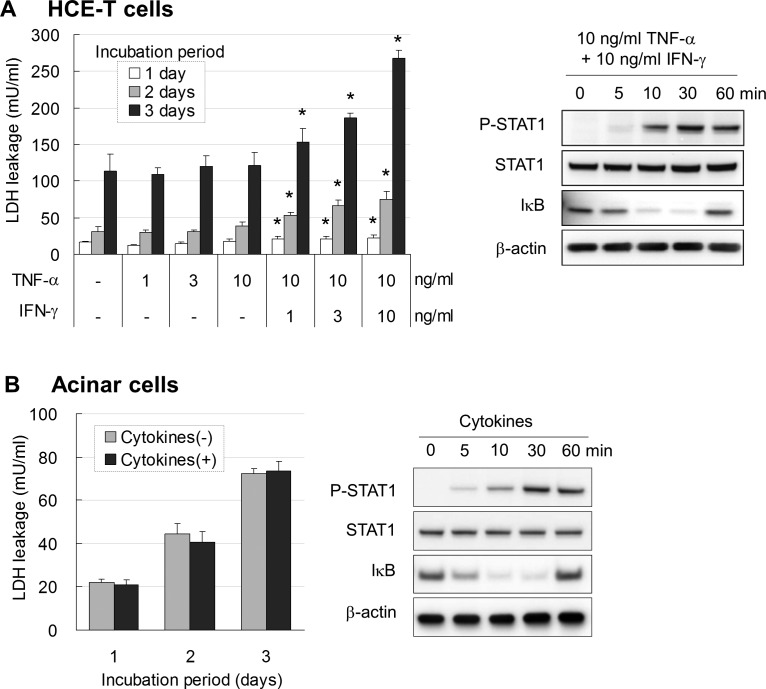
By itself, 10 ng inflammatory cytokine TNF-α/mL did not increase LDH leakage from cultured HCE-T cells (Fig. 4A). However, addition of the proinflammatory cytokine IFN-γ to TNF-α treatment significantly increased leakage of LDH in a dose-dependent manner on days 1, 2, and 3 after stimulation. Inflammatory mediator STAT1 was phosphorylated 10 minutes after stimulation by 10 ng each TNF-α and IFN-γ/mL, and P-STAT1 remained increased at 60 minutes (Fig. 4A, right). Loss of IκB was observed 10 minutes after stimulation by TNF-α plus IFN-γ and then returned to initial level by 60 minutes. Phosphorylation of STAT1 and degradation of the transcription-related inflammatory factor IκB confirmed activation of downstream signal transduction by IFN-γ and TNF-α, respectively.
Figure 4.
(A) LDH leakage from HCE-T cells was not affected by TNF-α at levels up to 10 ng/mL. Addition of IFN-γ to TNF-α significantly increased LDH leakage on days 1, 2, and 3. Data are the means ± SD (n = 5). *P < 0.05 relative to nontreated group at each cultured period (Dunnett's test). Right: Immunoblots showing that combining TNF-α with IFN-γ at 10 ng/mL each caused phosphorylation of STAT1 and loss of IkB. (B) LDH leakage from monkey lacrimal acinar cells was not increased at any cultured period even when treated with high concentrations (10 ng/mL) of TNF-α and INF-γ. Data are the means ± SD (n = 3). Right: Immunoblot showing that treatment with 10 ng/mL each TNF-α and IFN-γ caused phosphorylation of STAT1 and loss of IkB.
In contrast to HCE-T cells, monkey lacrimal acinar cells showed no increase of LDH leakage at any culture period when treated with 10 ng each TNF-α and IFN-γ/mL (Fig. 4B). However, downstream signals were activated similarly as in HCE-T cells (Fig. 4B, right).
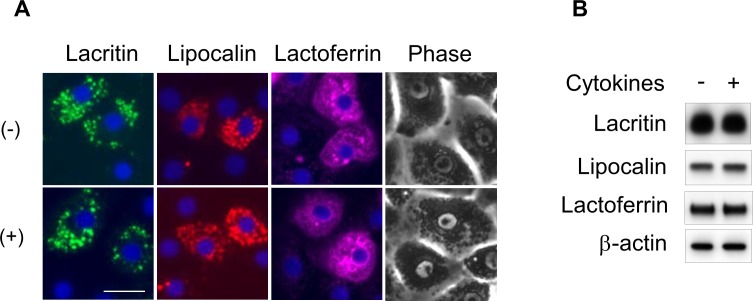
Cultured acinar cells showed an epithelial-like morphology with granules in the cytoplasm (Fig. 5A, top). This was not changed by treatment with 10 ng each TNF-α and IFN-γ/mL for 1 day (Fig. 5A, bottom). The localization and amounts of major acinar cell proteins secreted into tear, lacritin, lipocalin, and lactoferrin were not changed by treatment with cytokines (Figs. 5A, 5B). Thus, monkey lacrimal acinar cells apparently maintained normal morphology even after treatment with cytokines.
Figure 5.
(A) Immunocytochemistry and phase-contrast microscopy in monkey lacrimal acinar cells cultured for 1 day with 10 ng/mL each TNF-α and IFN-γ (+), showing similar localization of lacritin (green), lipocalin (red), and lactoferrin (magenta) as observed in nontreated control cells (−). Nuclei stained (blue) with DAPI. Scale bar: 20 μm. (B) Immunoblots showing equal amounts of lacritin, lipocalin, and lactoferrin in acinar cells with (+) and without (−) treatment with cytokines. β-Actin was the loading control.
Lacritin, but Not Cch, Induced Protein Secretion From Acinar Cells Treated With Cytokines
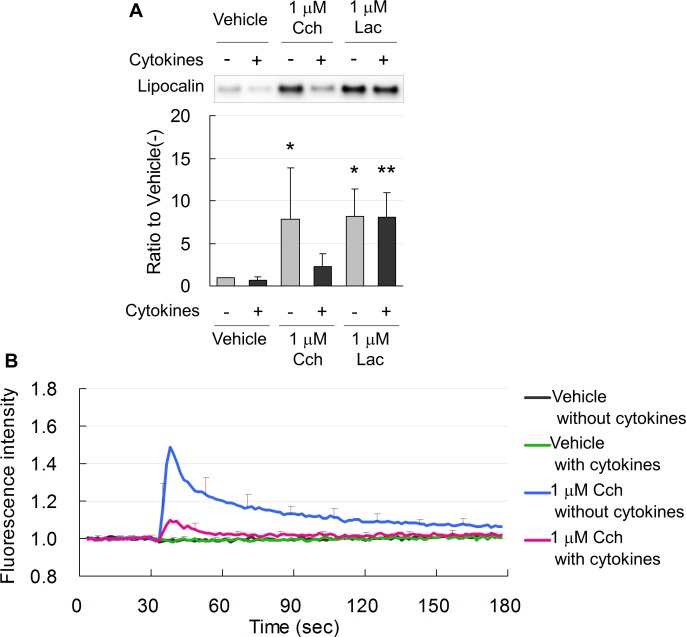
Lacritin and Cch equally increased secretion of lipocalin from nontreated, normal acinar cells (Fig. 6A). In acinar cells treated with 10 ng each TNF-α and INF-γ/mL, lacritin also induced secretion of lipocalin as much as in normal cells. In contrast, treatment with cytokines diminished Cch-induced secretion of lipocalin (Fig. 6A).
Figure 6.
(A) Representative immunoblot and band density measurements, showing that Lac and Cch equally induced lipocalin secretion from normal acinar cells not treated with cytokines (−). In acinar cells treated with 10 ng/mL each TNF-α and IFN-γ for 1 day (+), lacritin, but not Cch, induced secretion of lipocalin. Data are means ± SD (n = 6). *P < 0.05 relative to normal cells treated with vehicle and **P < 0.05 relative to cytokine-treated cells treated with vehicle. (B) Intracellular Ca2+ was increased by Cch in normal acinar cells (blue line) and was suppressed in cells treated for 1 day with 10 ng/mL each TNF-α and IFN-γ (red line).
Elevation of intracellular Ca2+ by Cch was suppressed in cells treated for 1 day with cytokines (Fig. 6B). This was well associated with diminished secretion of lipocalin (Fig. 6A).
Discussion
The major findings of the present study were that (1) native lacritin purified from monkey tears promoted secretion of tear proteins from cultured monkey lacrimal acinar cells via a calcium-independent pathway (Fig. 2). This mechanism differed from Cch-induced/calcium-dependent protein secretion. (2) Even when acinar cells were treated with proinflammatory cytokines TNF-α and IFN-γ, lacritin-induced protein secretion was maintained, as observed in nontreated normal cells. In contrast, Cch-induced secretion of tear proteins was depressed in cytokine-treated acinar cells.
Lacritin-Induced, Calcium-Independent Secretion of Tear Proteins
In contrast to lacritin, the mechanism for secretion by Cch has been well studied, and occurs by a calcium-dependent pathway.10,22,23 In agreement with these previous findings, Cch increased intracellular Ca2+ in our cultured monkey lacrimal acinar cells (Fig. 3A). On the other hand, increased intracellular Ca2+ was not detected in lacritin-treated cells, although protein secretion was stimulated. Additional lacritin added to the Cch treatment showed an additional effect on protein secretion without further increasing Ca2+ (Fig. 3B). These results were different from those of a previous report indicating that lacritin increased intracellular Ca2+ and mitosis in human salivary ductal cells.18 Therefore, we performed Ca2+ measurements using the calciumsensitive fluorophore Calcium 5 (Molecular Devices) with a microscopic imaging technique (SP5 AOBS spectral confocal system; Leica Microsystems, Wetzlar, Germany), similar to the previous report,18 resulting in a significant increase in calcium by Cch, but not by lacritin (data not shown). The previous report also showed that activation of PKCα occurred upstream of elevated Ca2+ and that an inhibitor of PKCα inhibited increased Ca2+. In our monkey lacrimal acinar cells, a PKCα inhibitor did not inhibit lacritin-induced protein secretion (data not shown), although Cch-induced secretion was inhibited as previously reported.10 In addition, lacritin-induced protein secretion and even monkey lacrimal acinar cells were stimulated in Ca2+-free medium (Fig. 3C), suggesting that lacritin may not require Ca2+ influx from extracellular. All these suggest a different mechanism for lacritin-induced protein secretion in monkey lacrimal acinar cells. Minor or very transient increases in Ca2+ might not have been detected in our monkey cells, but Ca2+ signaling was not a major mechanism for lacritin as it is for Cch-induced secretion. Similarly, Ca2+-independent secretion of insulin was observed in pancreatic islet cells cultured with EGTA.24 Further studies are needed to clarify the exact mechanism for lacritin-induced protein secretion.
Lacritin Induced Tear Protein Secretion From Cytokine-Treated Acinar Cells
Dry eye patients show upregulation of inflammatory markers such as HLA-DR, which is reduced by topical treatment with cyclosporin A.25 Proinflammatory cytokines TNF-α and IFN-γ are also increased in the tears of dry eye patients.3–5 In the present study, pretreatment with inflammatory cytokines TNF-α and IFN-γ did not cause obvious changes in the morphology of monkey lacrimal acinar cells (Fig. 5); however, phosphorylation of STAT1 and degradation of IκB occurring in other inflamed cells26,27 were also observed in our lacrimal acinar cells (Fig. 4). Cch-induced secretion of tear proteins was depressed in cytokine-treated cells (Fig. 6A), where mobilization of Ca2+ was decreased (Fig. 6B), suggesting abnormal regulation of Ca2+. In contrast, lacritin induced secretion of proteins from acinar cells under inflammatory conditions as much as in normal cells. Since mobilization of Ca2+ was not required for lacritin to induce secretion of tear proteins (Fig. 3), lacritin was an effective protein inducer in acinar cells under inflammatory conditions as well as in normal cells.
Neurotransmitters released from nerve endings are also major factors for stimulating protein secretion from the lacrimal gland.9 Under inflammatory conditions, release of these neurotransmitters and protein secretion are inhibited,7 leading to dry eye.28–30 In blepharitis, lacritin precursor protein levels were also decreased.14 Under our experimental inflammatory conditions, exogenous lacritin treatment still induced protein secretion, although in actual dry eye patients decreased levels of lacritin may not be high enough to compensate for decreased neurotransmitter-induced tear protein secretion. Thus, treatment with exogenous lacritin may be useful in ocular surface diseases with inadequate levels of endogenous lacritin and/or impaired neurotransmitter release, where Cch is not effective.
Acknowledgments
The authors thank Takeshi Nakajima at Senju Laboratory of Ocular Sciences, Kobe, Japan, for lacritin purification and Anda Cornea at the Imaging and Morphology Support Core, Oregon Health and Science University, for technical assistance.
Supported in part by National Center for Research Resources (NCRR)/National Institutes of Health Grant P51 RR000163 to the Oregon National Primate Research Center, and by NCRR Grant S10 RR024585 to the Imaging and Morphology Support Core, Oregon Health and Science University. The potential conflict of interest for Thomas R. Shearer, Mitsuyoshi Azuma, and Atsuko Fujii was reviewed, and a management plan approved by the OHSU Conflict of Interest in Research Committee was implemented.
Disclosure: A. Fujii, Senju Pharmaceutical Corporation Limited (E); A. Morimoto-Tochigi, Senju Pharmaceutical Corporation Limited (E); R.D. Walkup, Senju Pharmaceutical Corporation Limited (E); T.R. Shearer, Senju Pharmaceutical Corporation Limited (C); M. Azuma, Senju Pharmaceutical Corporation Limited (E)
References
- 1. Tiffany JM. The normal tear film. Dev Ophthalmol. 2008; 41: 1–20 [DOI] [PubMed] [Google Scholar]
- 2. Definition and Classification Subcommittee of the International Dry Eye Workshop 2007 The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 3. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999; 19: 201–211 [DOI] [PubMed] [Google Scholar]
- 4. Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009; 28: 1023–1027 [DOI] [PubMed] [Google Scholar]
- 5. Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011; 52: 7725–7730 [DOI] [PubMed] [Google Scholar]
- 6. Jabs DA, Gérard HC, Wei Y, et al. Inflammatory mediators in autoimmune lacrimal gland disease in MRL/Mpj mice. Invest Ophthalmol Vis Sci. 2004; 45: 2293–2298 [DOI] [PubMed] [Google Scholar]
- 7. Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002; 43: 1429–1436 [PMC free article] [PubMed] [Google Scholar]
- 8. Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2001; 42: 925–932 [PMC free article] [PubMed] [Google Scholar]
- 9. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009; 28: 155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morimoto-Tochigi A, Walkup RD, Nakajima E, Shearer TR, Azuma M. Mechanism for carbachol-induced secretion of lacritin in cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2010; 51: 4395–4406 [DOI] [PubMed] [Google Scholar]
- 11. Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006; 90: 372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laurie DE, Splan RK, Green K, Still KM, McKown RL, Laurie GW. Detection of prosecretory mitogen lacritin in nonprimate tears primarily as a C-terminal-like fragment. Invest Ophthalmol Vis Sci. 2012; 53: 6130–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M. Establishment of an appropriate animal model for lacritin studies: cloning and characterization of lacritin in monkey eyes. Exp Eye Res. 2007; 85: 651–658 [DOI] [PubMed] [Google Scholar]
- 14. Koo B, Lee D, Ha H, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res. 2005; 4: 719–724 [DOI] [PubMed] [Google Scholar]
- 15. Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009; 28: 1109–1117 [DOI] [PubMed] [Google Scholar]
- 16. Sanghi S, Kumar R, Lumsden A, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001; 310: 127–139 [DOI] [PubMed] [Google Scholar]
- 17. Ma P, Beck SL, Raab RW, et al. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J Cell Biol. 2006; 174: 1097–1106 Erratum in: J Cell Biol. 2011;192:365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Wang N, Xie J, et al. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J Cell Biol. 2006; 174: 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samudre S, Lattanzio FA Jr, Lossen V, et al. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci. 2011; 52: 6265–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680–685 [DOI] [PubMed] [Google Scholar]
- 21. Edman MC, Andersson SV, Delbro D, Gierow JP. Functional expression of the adenosine A1 receptor in rabbit lacrimal gland. Exp Eye Res. 2008; 86: 110–117 [DOI] [PubMed] [Google Scholar]
- 22. Zoukhri D, Hodges RR, Dicker DM, Dartt DA. Role of protein kinase C in cholinergic stimulation of lacrimal gland protein secretion. FEBS Lett. 1994; 351: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keryer G, Rossignol B. Effect of carbachol on 45Ca uptake and protein secretion in rat lacrimal gland. Am J Physiol. 1976; 230: 99–104 [DOI] [PubMed] [Google Scholar]
- 24. Komatsu M, Schermerhorn T, Aizawa T, Sharp GW. Glucose stimulation of insulin release in the absence of extracellular Ca2+ and in the absence of any increase in intracellular Ca2+ in rat pancreatic islets. Proc Natl Acad Sci U S A. 1995; 92: 10728–10732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brignole F, Pisella PJ, Goldschild M. De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000; 41: 1356–1363 [PubMed] [Google Scholar]
- 26. Bandyopadhyay SK, de la Motte CA, Kessler SP, Hascall VC, Hill DR, Strong SA. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am J Pathol. 2008; 173: 1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Visekruna A, Joeris T, Seidel D, et al. Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. J Clin Invest. 2006; 116: 3195–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohashi Y, Ishida R, Kojima T, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003; 136: 291–299 [DOI] [PubMed] [Google Scholar]
- 29. Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009; 8: 4889–4905 [DOI] [PubMed] [Google Scholar]
- 30. Versura P, Nanni P, Bavelloni A, et al. Tear proteomics in evaporative dry eye disease. Eye. 2010; 24: 1396–1402 [DOI] [PubMed] [Google Scholar]