Abstract
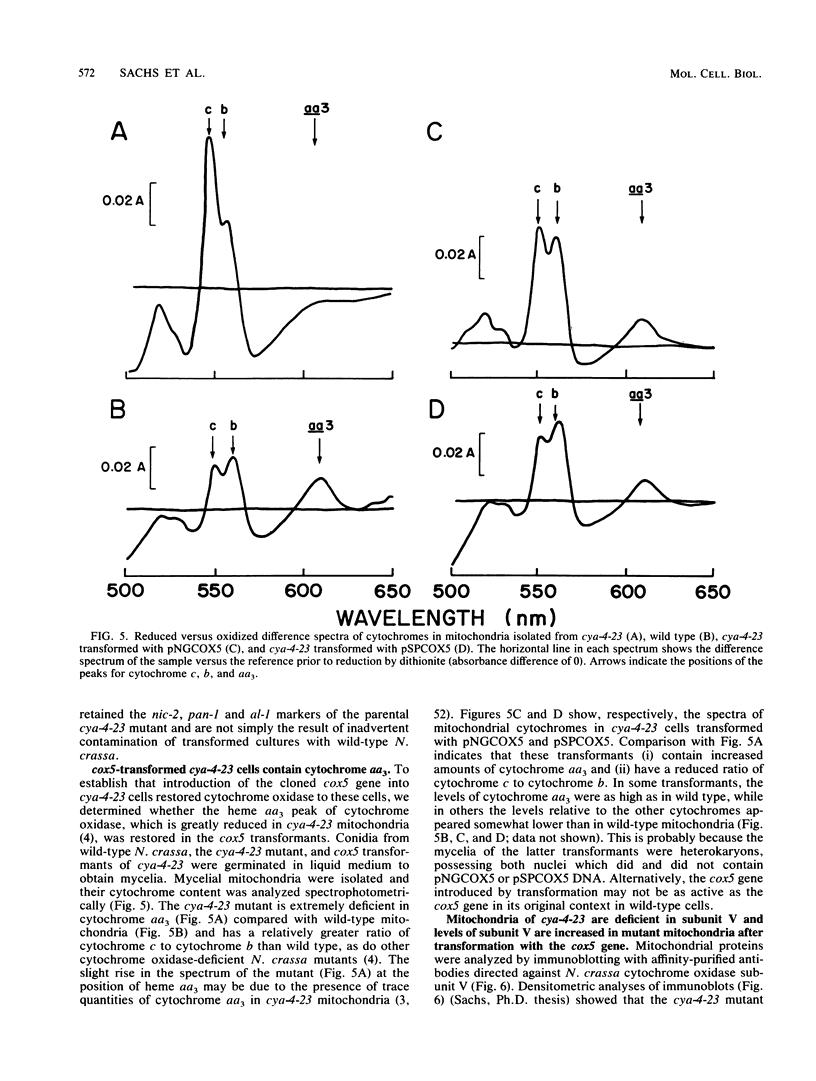
The sequences of cDNA and genomic DNA clones for Neurospora cytochrome oxidase subunit V show that the protein is synthesized as a 171-amino-acid precursor containing a 27-amino-acid N-terminal extension. The subunit V protein sequence is 34% identical to that of Saccharomyces cerevisiae subunit V; these proteins, as well as the corresponding bovine subunit, subunit IV, contain a single hydrophobic domain which most likely spans the inner mitochondrial membrane. The Neurospora crassa subunit V gene (cox5) contains two introns, 398 and 68 nucleotides long, which share the conserved intron boundaries 5'GTRNGT...CAG3' and the internal consensus sequence ACTRACA. Two short sequences, YGCCAG and YCCGTTY, are repeated four times each in the cox5 gene upstream of the mRNA 5' termini. The cox5 mRNA 5' ends are heterogeneous, with the major mRNA 5' end located 144 to 147 nucleotides upstream from the translational start site. The mRNA contains a 3'-untranslated region of 186 to 187 nucleotides. Using restriction-fragment-length polymorphism, we mapped the cox5 gene to linkage group IIR, close to the arg-5 locus. Since one of the mutations causing cytochrome oxidase deficiency in N. crassa, cya-4-23, also maps there, we transformed the cya-4-23 strain with the wild-type cox5 gene. In contrast to cya-4-23 cells, which grow slowly, cox5 transformants grew quickly, contained cytochrome oxidase, and had 8- to 11-fold-higher levels of subunit V in their mitochondria. These data suggest (i) that the cya-4 locus in N. crassa specifies structural information for cytochrome oxidase subunit V and (ii) that, in N. crassa, as in S. cerevisiae, deficiencies in the production of nuclearly encoded cytochrome oxidase subunits result in deficiency in cytochrome oxidase activity. Finally, we show that the lower levels of subunit V in cya-4-23 cells are most likely due to substantially reduced levels of translatable subunit V mRNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Buxton F., Patel V., Giles N. H., Vapnek D. 5'-Untranslated sequences of two structural genes in the qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1955–1959. doi: 10.1073/pnas.79.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman N. J., Lomax M. I., Grossman L. I. Two bovine genes for cytochrome c oxidase subunit IV: a processed pseudogene and an expressed gene. Gene. 1987;55(2-3):219–229. doi: 10.1016/0378-1119(87)90282-4. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Collins R. A. A regulatory system controlling the production of cytochrome aa3 in Neurospora crassa. Mol Gen Genet. 1978 Oct 25;166(1):1–13. doi: 10.1007/BF00379723. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Nargang F. E., Collins R. A., Zagozeski C. A. Nuclear cytochrome-deficient mutants of Neurospora crassa: isolation, characterization, and genetic mapping. Mol Gen Genet. 1977 Jun 24;153(3):247–257. doi: 10.1007/BF00431590. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Cytoplasmic Mutants Selected from Continuously Growing Cultures of NEUROSPORA CRASSA. Genetics. 1969 Mar;61(3):643–659. doi: 10.1093/genetics/61.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Werner S. Cytochrome c oxidase subunits in nuclear and extranuclear cytochrome-aa3-deficient mutants of Neurospora crassa. Eur J Biochem. 1979 Jul;98(1):9–18. doi: 10.1111/j.1432-1033.1979.tb13154.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Cerletti N., Schatz G. Cytochrome c oxidase from bakers' yeast. Photolabeling of subunits exposed to the lipid bilayer. J Biol Chem. 1979 Aug 25;254(16):7746–7751. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cumsky M. G., Trueblood C. E., Ko C., Poyton R. O. Structural analysis of two genes encoding divergent forms of yeast cytochrome c oxidase subunit V. Mol Cell Biol. 1987 Oct;7(10):3511–3519. doi: 10.1128/mcb.7.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan W., Bibus C. R., Schatz G. The cytoplasmically-made subunit IV is necessary for assembly of cytochrome c oxidase in yeast. EMBO J. 1985 Jan;4(1):179–184. doi: 10.1002/j.1460-2075.1985.tb02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R. Translational control of transcription in eukaryotes. Cell. 1986 Apr 25;45(2):155–156. doi: 10.1016/0092-8674(86)90378-8. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Snyder M., Chang L. M., Davis R. W., Campbell J. L. Isolation of the gene encoding yeast DNA polymerase I. Cell. 1985 Nov;43(1):369–377. doi: 10.1016/0092-8674(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Kadenbach B. Regulation of respiration and ATP synthesis in higher organisms: hypothesis. J Bioenerg Biomembr. 1986 Feb;18(1):39–54. doi: 10.1007/BF00743611. [DOI] [PubMed] [Google Scholar]
- Kinnaird J. H., Fincham J. R. The complete nucleotide sequence of the Neurospora crassa am (NADP-specific glutamate dehydrogenase) gene. Gene. 1983 Dec;26(2-3):253–260. doi: 10.1016/0378-1119(83)90195-6. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Homison G., Tzagoloff A. Nuclear mutants of Saccharomyces cerevisiae with altered subunits 4, 5, and 6 of cytochrome oxidase. J Biol Chem. 1985 May 25;260(10):5871–5874. [PubMed] [Google Scholar]
- Kuhn-Nentwig L., Kadenbach B. Orientation of rat liver cytochrome c oxidase subunits investigated with subunit-specific antisera. Eur J Biochem. 1985 Nov 15;153(1):101–104. doi: 10.1111/j.1432-1033.1985.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Yanofsky C. Cloning and characterization of the multifunctional his-3 gene of Neurospora crassa. Gene. 1985;39(2-3):129–140. doi: 10.1016/0378-1119(85)90306-3. [DOI] [PubMed] [Google Scholar]
- Lustig A., Padmanaban G., Rabinowitz M. Regulation of the nuclear-coded peptides of yeast cytochrome c oxidase. Biochemistry. 1982 Jan 19;21(2):309–316. doi: 10.1021/bi00531a017. [DOI] [PubMed] [Google Scholar]
- Malatesta F., Darley-Usmar V., de Jong C., Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Arrangement of subunit IV in beef heart cytochrome c oxidase probed by chemical labeling and protease digestion experiments. Biochemistry. 1983 Sep 13;22(19):4405–4411. doi: 10.1021/bi00288a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McEwen J. E., Ko C., Kloeckner-Gruissem B., Poyton R. O. Nuclear functions required for cytochrome c oxidase biogenesis in Saccharomyces cerevisiae. Characterization of mutants in 34 complementation groups. J Biol Chem. 1986 Sep 5;261(25):11872–11879. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Stevens J. N., Selker E. U., Morzycka-Wroblewska E. Identification and chromosomal distribution of 5S rRNA genes in Neurospora crassa. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2067–2071. doi: 10.1073/pnas.82.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Germann U. A., Lerch K. Isolation and structural organization of the Neurospora crassa copper metallothionein gene. EMBO J. 1985 Oct;4(10):2665–2668. doi: 10.1002/j.1460-2075.1985.tb03985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsoulis G., Hilger F., Fink G. R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986 Jul 18;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OGUR M., ST. JOHN R., NAGAI S. Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science. 1957 May 10;125(3254):928–929. doi: 10.1126/science.125.3254.928. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge B. J. Molecular characterization of the qa-4 gene of Neurospora crassa. Gene. 1984 Dec;32(3):275–287. doi: 10.1016/0378-1119(84)90003-9. [DOI] [PubMed] [Google Scholar]
- Sachs M. S., David M., Werner S., RajBhandary U. L. Nuclear genes for cytochrome c oxidase subunits of Neurospora crassa. Isolation and characterization of cDNA clones for subunits IV, V, VI, and possibly VII. J Biol Chem. 1986 Jan 15;261(2):869–873. [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Selker E. U., Cambareri E. B., Jensen B. C., Haack K. R. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell. 1987 Dec 4;51(5):741–752. doi: 10.1016/0092-8674(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W. Genetics and biosynthesis of cytochrome c. Annu Rev Genet. 1971;5:257–296. doi: 10.1146/annurev.ge.05.120171.001353. [DOI] [PubMed] [Google Scholar]
- Stade S., Brambl R. Cytochrome c oxidase in cytochrome c oxidase-deficient mutant strains of Neurospora crassa. J Biol Chem. 1981 Oct 25;256(20):10235–10238. [PubMed] [Google Scholar]
- Stuart R. A., Neupert W., Tropschug M. Deficiency in mRNA splicing in a cytochrome c mutant of neurospora crassa: importance of carboxy terminus for import of apocytochrome c into mitochondria. EMBO J. 1987 Jul;6(7):2131–2137. doi: 10.1002/j.1460-2075.1987.tb02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G., Mengel T., Cordingley M., Kadenbach B. Molecular cloning and further characterization of cDNAs for rat nuclear-encoded cytochrome c oxidase subunits VIc and VIII. Eur J Biochem. 1987 Oct 1;168(1):233–237. doi: 10.1111/j.1432-1033.1987.tb13410.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueblood C. E., Poyton R. O. Differential effectiveness of yeast cytochrome c oxidase subunit genes results from differences in expression not function. Mol Cell Biol. 1987 Oct;7(10):3520–3526. doi: 10.1128/mcb.7.10.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S. Preparation of polypeptide subunits of cytochrome oxidase from Neurospora crassa. Eur J Biochem. 1977 Sep 15;79(1):103–110. doi: 10.1111/j.1432-1033.1977.tb11788.x. [DOI] [PubMed] [Google Scholar]
- Woudt L. P., Pastink A., Kempers-Veenstra A. E., Jansen A. E., Mager W. H., Planta R. J. The genes coding for histone H3 and H4 in Neurospora crassa are unique and contain intervening sequences. Nucleic Acids Res. 1983 Aug 25;11(16):5347–5360. doi: 10.1093/nar/11.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Nakagawa M., Herbert J., Lomax M. I., Grossman L. I., Sherbany A. A., Miranda A. F., DiMauro S., Schon E. A. Isolation of a cDNA clone encoding subunit IV of human cytochrome c oxidase. Gene. 1987;55(2-3):205–217. doi: 10.1016/0378-1119(87)90281-2. [DOI] [PubMed] [Google Scholar]