Abstract
Aims
Patients with mild heart failure (HF) who are clinically compensated may have normal left ventricular (LV) stroke volume (SV). Despite this, altered intra-ventricular flow patterns have been recognized in these subjects. We hypothesized that, compared with normal LVs, flow in myopathic LVs would demonstrate a smaller proportion of inflow volume passing directly to ejection and diminished the end-diastolic preservation of the inflow kinetic energy (KE).
Methods and results
In 10 patients with dilated cardiomyopathy (DCM) (49 ± 14 years, six females) and 10 healthy subjects (44 ± 17 years, four females), four-dimensional MRI velocity and morphological data were acquired. A previously validated method was used to separate the LV end-diastolic volume (EDV) into four flow components based on the blood's locations at the beginning and end of the cardiac cycle. KE was calculated over the cardiac cycle for each component. The EDV was larger (P = 0.021) and the ejection fraction smaller (P < 0.001) in DCM compared with healthy subjects; the SV was equivalent (DCM: 77 ± 19, healthy: 79 ± 16 mL). The proportion of the total LV inflow that passed directly to ejection was smaller in DCM (P = 0.000), but the end-diastolic KE/mL of the direct flow was not different in the two groups (NS).
Conclusion
Despite equivalent LVSVs, HF patients with mild LV remodelling demonstrate altered diastolic flow routes through the LV and impaired preservation of inflow KE at pre-systole compared with healthy subjects. These unique flow-specific changes in the flow route and energetics are detectable despite clinical compensation, and may prove useful as subclinical markers of LV dysfunction.
Keywords: 4D flow, Heart failure, Magnetic resonance imaging, Stroke volume
Introduction
Heart failure (HF) represents the end stage of the continuum of cardiovascular diseases. This disorder is common, and creates an enormous health-care burden. The prognosis of HF patients is dismal, with 5-year survival rates worse than many of the most common cancers.1
Cardiac remodelling is a key component of HF that progresses from adaptive to maladaptive as the disease worsens, and is associated with increased risks of symptoms and mortality.2 Patients with early or mild HF are often clinically compensated and their left ventricular (LV) stroke volume (SV) may be within the normal range. Despite normal LVSV, the prognosis and clinical outcomes of such patients are impaired compared with healthy comparators.
Blood flow through the heart and vessels is a fundamental aspect of cardiovascular function.3,4 In HF, altered intra-cardiac flow patterns have been recognized.5,6 Three-dimensional, time-resolved flow encoded MRI provides comprehensive velocity data of the intrinsically three-dimensional and time-varying (3D+time = 4D) flow patterns within the beating heart.7–15 Recently, the four-dimensional (4D) flow within the LV has been quantified and separated into different functional flow components.7,9,10 These components have been used to demonstrate component-specific routes and energetics in healthy hearts that may represent important aspects of normal ventricular function, including an improved preservation of LV inflow kinetic energy (KE) for effective and rapid systolic ejection.
We hypothesized that compensated HF patients, despite having comparable LVSVs to healthy subjects, would manifest their functional LV impairment as less LV diastolic inflow KE preservation and a diminished percentage of the flow component that transits the LV in a single cardiac cycle. Such discrepancies in LV diastolic–systolic coupling may represent important pathophysiological aspects of occult LV dysfunction. Accordingly, we measured LV 4D flow-specific measures in patients with compensated HF and compared them with those in healthy subjects.
Methods
Study population
Ten patients with clinically compensated dilated cardiomyopathy (DCM) and ten healthy subjects were included in the study (Table 1). At the time of diagnosis, DCM was defined as the presence of symptoms and signs of HF in the presence of echocardiographic findings of ventricular enlargement and systolic myocardial dysfunction by parameters of depressed ejection fraction and mitral annular descent. The patients were recruited from outpatients seen at the Department of Cardiology, Linköping University Hospital. Seven of the patients and seven of the controls were matched for age and gender. All subjects were in sinus rhythm. Inclusion criteria for (i) healthy subjects: normal electrocardiographic and echocardiographic examinations; and (ii) DCM patients: ≤65 years of age. Exclusion criteria for (i) all subjects: contraindications to MRI examination; (ii) normal subjects: a history of prior or current heart disease or the use of cardiac medication, and (iii) DCM patients: significantly irregular cardiac rhythm, a history of myocardial infarction, ≥moderate arterial hypertension, as well as ≥moderate valvular disorder, <mild LV dilatation, and <mild LV systolic dysfunction defined by echocardiography. Three of the DCM patients were included in a prior method validation study.9 All subjects gave written informed consent before participation and the study was approved by the regional ethical review board. The study complies with the declaration of Helsinki.
Table 1.
Demographical and clinical data of the study population
| Parameter | DCM (n = 10) | Healthy (n = 10) | P-value |
|---|---|---|---|
| Gender (female:male) | 6:4 | 4:6 | — |
| Age (years) | 49 ± 14 | 44 ± 17 | 0.54 |
| Weight (kg) | 82 ± 18 | 72 ± 8 | 0.172 |
| Heart rate (bpm) | 61 ± 11 | 68 ± 10 | 0.129 |
| Systolic BP (mmHg) | 122 ± 14 | 129 ± 11 | 0.269 |
| Diastolic BP (mmHg) | 77 ± 9 | 80 ± 4 | 0.439 |
| LV end-diastolic volume (mL) | 179 ± 33 | 147 ± 22 | 0.021 |
| LV sphericity index | 0.75 ± 0.12 | 0.57 ± 0.06 | 0.001 |
| LV ejection fraction | 42 ± 5 | 54 ± 6 | 0.000 |
| LV diastolic function according to echo Doppler indices | |||
| Normal diastolic function | 5 | 10 | — |
| Relaxation abnormality | 2 | — | — |
| Pseudonormal filling | 1 | — | — |
| Restrictive filling | 2 | — | — |
| Medication | |||
| ARB/ACE-I | 10 | 0 | — |
| Beta-blocker | 10 | 0 | — |
| Diuretic | 3 | 0 | — |
| Aldosterone I | 2 | 0 | — |
| Statin | 3 | 0 | — |
| Warfarin | 4 | 0 | — |
| Aspirin | 2 | 0 | — |
| Digoxin | 1 | 0 | — |
All values are mean ± 1 SD unless otherwise stated. P-values <0.05 are significant. LV, left ventricle; BP, blood pressure; ARB, angiotensin II receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor.
Data acquisition
All subjects underwent MRI examination to acquire 4D flow data and morphological images. The 4D flow data were acquired during free-breathing, using an ECG-triggered, retrospectively navigator-gated, three-dimensional, three-directional, time-resolved phase contrast MRI sequence on a clinical 1.5 T Philips Achieva scanner (Philips Healthcare, Best, the Netherlands). The acquisition and post-processing of the 4D flow data were performed as described previously.9 Scan parameters included: velocity encoding (VENC) = 100 cm/s, flip angle = 8°, echo time = 3.7 ms, repetition time = 6.3 ms, parallel imaging (SENSE) speed-up factor = 2, and k-space segmentation factor = 2. These settings gave a temporal resolution of 50.4 ms. The spatial resolution was 3 × 3 × 3 mm3 and the field-of-view (FOV) was adjusted for each subject to cover the left heart. The mean scan time, including the navigator efficiency and arrhythmia rejection for the Cartesian 3DcinePC sequence, was 31 min (range 16–57, median: 30, std: 8). The mean time required to acquire all k-space lines required for the 4D flow data, excluding navigator efficiency and arrhythmia rejection, was ∼12 min (std: 2, median: 12, and range: 9–18). After post-processing, where corrections were made for concomitant gradient fields, phase-wraps, and background phase errors, the first of a set of data quality control steps was applied as in Eriksson et al.9 This consists of emitting pathlines continuously throughout the cardiac cycle from an emitter grid positioned in the left atrium. A pathline shows the path that an imaginary mass-less particle would take through the time resolved velocity field. If pathlines leave the blood pool at locations incompatible with the actual blood flow, e.g. leaving the LV through the ventricular apex, this is an indication of data imperfections. All data sets passed this quality control.
Morphological long-axis and two stacks of short-axis images were acquired in 30 time frames during end-expiratory breath holds, using cine-balanced steady-state free-precession. Slice thickness was 8 mm. The acquired and reconstructed pixel size for the short-axis images was 2.19 × 1.78 and 1.37 × 1.37 mm2, respectively.
Echocardiography was performed using a Vivid 7 scanner and a 2.0 MHz probe (GE, Vingmed Ultrasound, Horten, Norway), and used for enrollment criteria. In addition, LV diastolic function at rest was characterized according to conventional echo Doppler indices.16
Data analysis
All data sets were analysed using a previously presented and validated method.7,9 In short, this consists of a manual segmentation of the LV from short-axis images at time of end-diastole (ED) and end-systole (ES) using freely available software (Segment).17 The segmentation at ED is resampled to give a volume with isotropic voxels of the same size as the flow data. From the centre of each voxel in the LV segmentation, a pathline is emitted. Pathlines are created backwards and forwards in time until the preceding or subsequent ES, respectively. In combination, these forwards and backwards pathlines represent the entire LV end-diastolic volume (EDV) tracked over one complete cardiac cycle. The positions of all pathlines at the time of ES relative to the cardiac chambers defined by the ES segmentation are then used to separate them into four different flow components: direct flow, retained inflow, delayed ejection flow, and residual volume, per definitions listed in Table 2 and Figure 1.9,10 Further, the inflow components direct flow and retained inflow were divided into early and late inflow as in Eriksson et al.7 The early and late diastolic phases were defined as the interval from onset diastole until mid-diastasis and the interval from mid-diastasis until ED, respectively. The time of mid-diastasis was defined visually as the time frame at which the lowest number of pathlines crossed the mitral valve plane. As each pathline represents a volume of blood with a calculable mass (by using the density of blood, ρblood = 1060 kg/m3) and a known velocity at every point in time, the KE for each pathline can be calculated at every point in time by 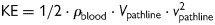 , where Vpathline is the volume that one pathline represents and vpathline is the velocity of the pathline at a given point in time. Each component's KE is the sum of the KE for each of its pathlines.7 The KE for each component was then divided by its volume. The KE of each component's pathlines at the time of ED were compared.
, where Vpathline is the volume that one pathline represents and vpathline is the velocity of the pathline at a given point in time. Each component's KE is the sum of the KE for each of its pathlines.7 The KE for each component was then divided by its volume. The KE of each component's pathlines at the time of ED were compared.
Table 2.
Definition of LV blood flow components
| Component | Definition |
|---|---|
| Direct flow | Blood that enters the LV during diastole and leaves the LV during systole in the analysed heart beat; component of both inflow and ejected volume |
| Retained inflow | Blood that enters the LV during diastole but does not leave during systole in the analysed heart beat; component of inflow volume only |
| Delayed ejection flow | Blood that starts and resides inside the LV during diastole and leaves during systole in the analysed heart beat; component of ejected volume only |
| Residual volume | Blood that resides within the LV for at least two cardiac cycles; not a component of inflow or ejected volume |
Figure 1.
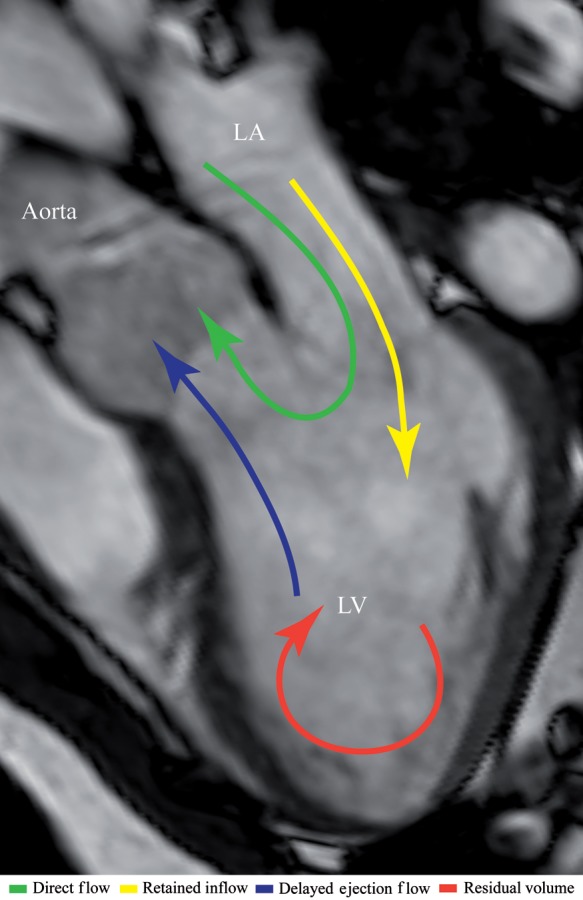
Blood flow component definitions: illustration showing the components defined in Table 2. Direct flow, green; retained inflow, yellow, delayed ejection flow, blue; and residual volume, red. LA, left atrium; LV, left ventricle.
Statistical analysis
Inter-group comparisons of inflow and ejected volumes and of parameters in Table 1 were made using two-sample t-tests; a P-value <0.05 was considered significant. Inter-group comparisons of component volumes (Figure 3) and end-diastolic KE (Figure 5) were made using two-sample t-tests, Bonferroni corrected for multiple comparisons, and hence a P-value <0.0125 was considered significant. For intra-group comparison of components, a two-way analysis of variance with the Tukey post hoc test was used, and a P-value <0.05 was considered significant. For comparisons between early (E) and late (A) inflow (Figure 6), the following was done: for intra-group comparison, i.e. E vs. A in the same subject, Bonferroni-corrected paired t-tests were used and a P-value <0.025 was considered significant. For inter-group comparison, Bonferroni-corrected two-sample t-tests were used, and a P-value <0.0125 was considered significant. Minitab 16.2 was used for statistical calculations.
Figure 3.
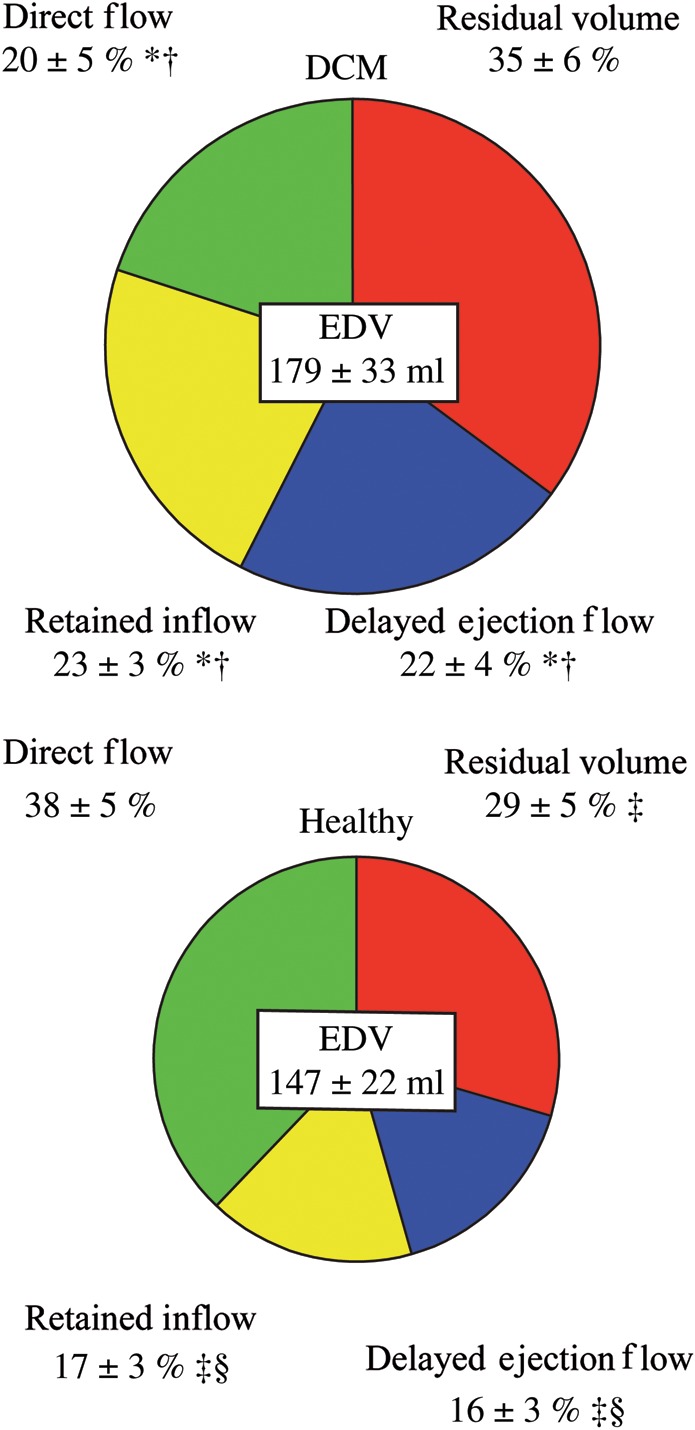
Blood flow components: blood flow components presented as a percentage of end-diastolic volume (mean ± SD). Top panel: DCM patients (n = 10); lower panel: healthy subjects (n = 10). *Components with a P-value <0.0125 compared with the corresponding component in the healthy group. Intra-group comparison: †P-value ≤0.0002 among DCM patients vs. residual volume. ‡P-value ≤0.005 vs. direct flow in healthy subjects. §P-value =0.000 vs. residual volume in healthy subjects.
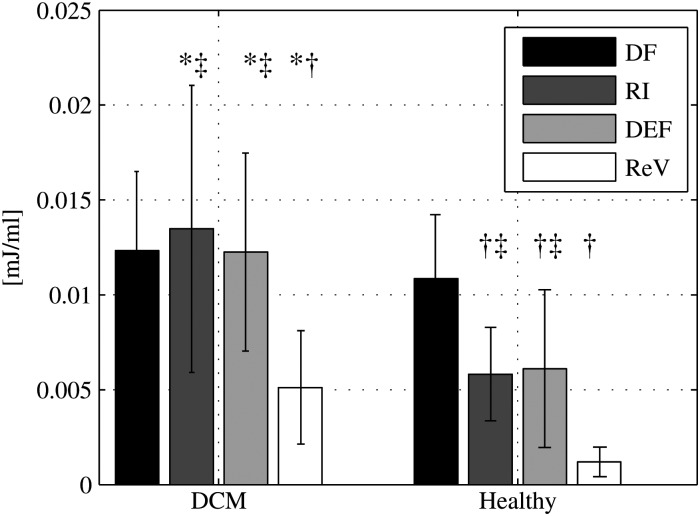
Figure 5.
Pre-systolic kinetic energy: the kinetic energy (KE) at end-diastole, in mJ/mL, for each flow component in DCM patients (left) and healthy subjects (right). Bars show group mean and standard deviation. *P-value <0.0125 vs. the corresponding component in healthy subjects. Intra-group comparison: †P-value ≤0.0001 vs. direct flow. ‡P-value ≤0.0001 vs. residual volume.
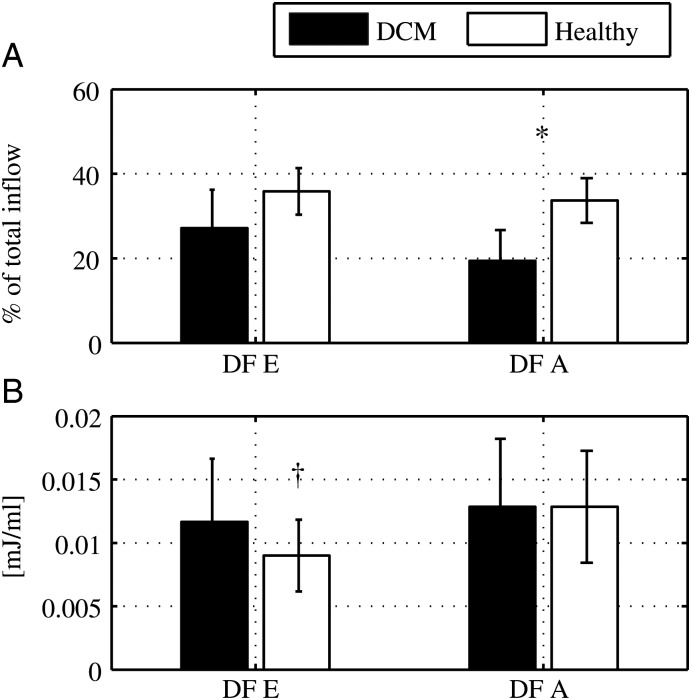
Figure 6.
Early vs. late inflow: characteristics of direct flow during early (E) and late (A) diastole. At end-diastole (A) the direct flow volume as a percentage of the total inflow for E and A. The sum of the values at E and A, for each group, gives the direct flow to total inflow ratio for the entire diastolic filling phase (DCM: 46 ± 9% vs. healthy: 70 ± 6%, P = 0.000). (B) The kinetic energy at E and A. Data are presented as mean and standard deviation. (A) *P-value =0.000 for direct flow A in DCM vs. direct flow A in healthy subjects. (B) †P-value =0.003 for direct flow E in healthy subjects vs. direct flow A in healthy subjects.
Results
The analysis of clinical data at rest (Table 1) shows that there was no statistically significant difference between the groups for age, heart rate, weight, or blood pressure. There were differences in LV morphological characteristics and global systolic function: the EDV and the sphericity index were higher and the ejection fraction was lower in the DCM patients compared with healthy subjects. All healthy subjects and half of the patients showed normal LV diastolic function. All patients were in NYHA class II. Two of the patients had a left bundle branch block.
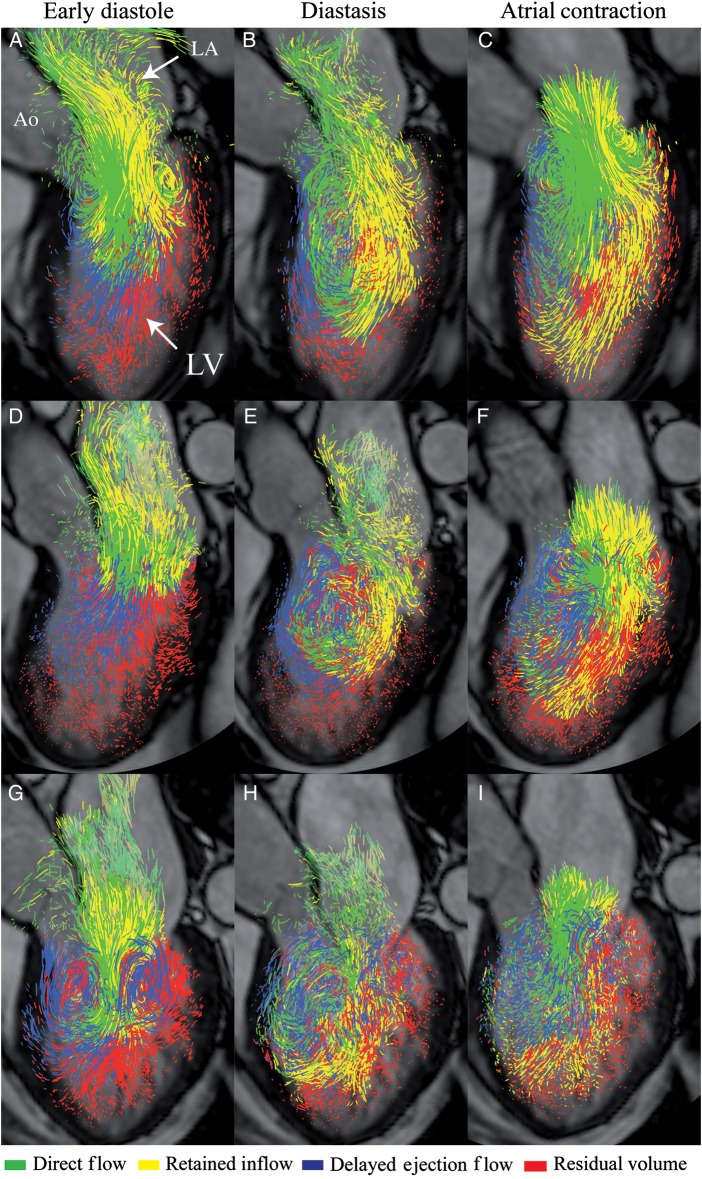
Visualizations were created of all flow data sets. These were inspected as an initial data quality assurance check. Confirmation of adequate data quality required pathline constraint to anatomical structures via visual inspection (Figure 2 and Supplementary data online, Movies S1–S3). A second data quality check was performed by comparison of quantitative inflow and outflow LV volumes to assure equivalence. No data sets were rejected based on visualization, and there was no significant difference in inflow volume vs. ejected volume in either the DCM patients or the healthy subjects (P = 0.99 and P = 0.73, respectively).
Figure 2.
Blood flow visualization: pathline visualization of the four flow components (direct flow, retained inflow, delayed ejection flow, and residual volume). (A–C) a healthy 50-year-old woman with normal LV diastolic function; (D–F) a 62-year-old male with DCM and LV relaxation abnormality; (G–I) a 61-year-old female with DCM and restrictive LV filling. Semi-transparent three-chamber images provide anatomical orientation. Ao, aorta; LA, left atrium; LV, left ventricle.
LV stroke volumes
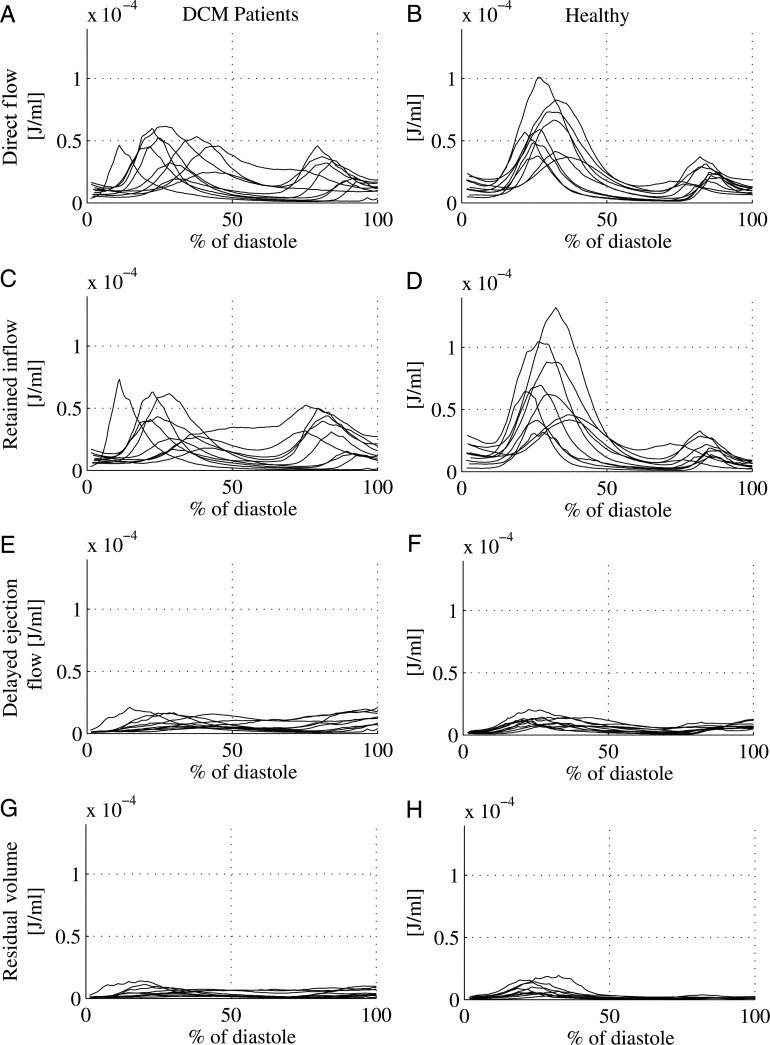
While the LVSVs in the DCM group and the healthy group were equal (inflow volume: 77 ± 18 mL in DCM vs. 80 ± 17 mL in healthy; ejected volume: 77 ± 19 mL in DCM vs. 79 ± 16 mL in the healthy, P = 0.73 and P = 0.81, respectively) the direct flow was significantly smaller in DCM compared with healthy (35 ± 11 vs. 56 ± 14 mL, P = 0.002), resulting in a smaller direct flow to total inflow ratio in DCM compared with healthy subjects (46 ± 9 vs. 70 ± 6%, P = 0.000). The KE was calculated over time for all components (Figure 4). At ED there was no significant difference between the KE/mL of the direct flow in DCM patients compared with healthy subjects. The KE/mL for the other three components was significantly larger in the DCM group than in normals (Figure 5).
Figure 4.
Kinetic energy over diastole: for each individual in the study kinetic energy per mL of blood (J/mL) is shown over the diastolic interval, normalized by the length of diastole, left panels: DCM patients; and right panels: healthy subjects. Direct flow (A and B), retained inflow (C and D), delayed ejection flow (E and F), and residual volume (G and H).
The direct flow volume was divided into early and late inflow in order to study the impact of atrial contraction.7 The ratio of early diastolic direct flow/total inflow volume was equal in the groups, whereas the late diastolic direct flow volume/total inflow volume ratio was smaller in the DCM group compared with healthy (P < 0.001) (Figure 6A). The direct flow KE/mL at ED was smaller for the early diastolic sub-volume in healthy subjects (P = 0.003), whereas there was no difference between the diastolic phases in DCM (NS) (Figure 6B).
LV residual volume
Although the EDV was significantly larger in the DCM group (P = 0.021) (Table 1), the amount of the residual volume relative to the EDV did not differ between the DCM group and the healthy subjects (NS) (Figure 3). The residual volume possessed a significantly larger KE/mL at ED in DCM patients compared with healthy subjects (P = 0.002, Figure 5).
Discussion
It is straightforward to assume that ventricular remodelling and dysfunction in patients with HF would be associated with alterations in flows through the heart chambers. In this study, we apply a previously validated 4D flow method9 in patients with compensated HF and an equal number of healthy subjects. This novel method allows the separation of the total LV volume into functionally distinct components. Comparison of the volume, timing, and KE of those components in these two states clarifies those differences, and yields some surprises.
The present study shows that, despite mild LV remodelling associated with DCM, the SV in these compensated HF patients is similar to normals. The composition of the SV is different, however: a smaller proportion of the flow transits the LV in a single cardiac cycle (direct flow). Although the volume of the direct flow is lower in these HF patients, on a per mL basis its KE at the time of ED does not differ from normal subjects. The percentage of the total EDV that consists of a re-circulating residual volume was also similar between DCM patients and normals, but in contrast, on a per mL basis the residual volume had a greater end-diastolic KE compared with normals. Finally, the late diastolic inflow in normal LVs had a larger proportion of the direct flow compared with DCM.
These observed differences may be relevant to the function and remodelling of the LV. The normal interaction between flowing blood and the developing heart in utero stimulates a continuous positive remodelling process that creates an optimal geometry for efficient flow.3,4,18,19 The same responses to flow-induced forces may also play a role in the pathophysiology of HF.2,18,20
The KE of inflowing blood has several possible consequences in the receiving ventricle. The KE of the entering blood may be (i) translated into motion of the blood already residing in the LV, (ii) converted into potential energy stored in the myocardium, (iii) dissipated as heat, or (iv) decelerated with the elevation of LV diastolic pressure. Less deceleration of flow in the LV may be an important component of normal diastolic function. A computational fluid dynamics study of the vortical flow in the right ventricle during filling, for example, suggested that the early diastolic vortices' KE prevented inflow-impeding pressure rise within the RV.21 Higher KE of intra-ventricular blood resulting from altered LV myocardial properties, such as impaired active relaxation or increased stiffness, will likely lead to elevated filling pressures as elevated velocities decelerate.
The differences in the volumes and diastolic energetics of the four functional flow components observed between myopathic and normal LVs can be considered in the light of the diastolic impact of the KE. The volume of the direct flow component is significantly less in DCM than normals, so that while the end-diastolic KE/mL is equivalent in both groups, the total direct flow KE at ED is smaller in the myopathic LVs. As this is volume destined for ejection during the subsequent systole, a lower KE at ED may indicate that incremental contribution from systolic contraction will be required for its ejection. In contrast, the retained inflow has a larger KE/mL at ED in DCM compared with normal LVs. This suggests a shift in the specific effects of inflow KE on the myopathic ventricle, with increased interaction between the inflowing blood and the resident volume (delayed ejection flow and residual volume) and surrounding myocardium. Separation of inflow into early and late diastolic phases demonstrates that the end-diastolic KE/mL of the direct flow volume was smaller for the early diastolic portion compared with the late diastolic portion in healthy subjects, whereas there was no difference between the diastolic phases in DCM. This may reflect impaired active relaxation of the myocardium in myopathic LVs. Late diastolic differences were also observed between the two groups: the proportion of the direct flow following atrial contraction was smaller in DCM compared with normal LVs. This shift might correlate with impaired diastolic–systolic coupling since the direct flow contributed by atrial contraction in normal hearts preserves inflow KE particularly well. The loss of organized atrial contraction, which is clinically related to increased symptoms in HF patients, may eliminate this advantageous KE preservation. The degree to which late diastolic inflow augments LV outflow might be a parameter that could influence target heart rates and optimal pacing strategies also in patients with HF.
The residual volume outlines the periphery of the LV chamber and appears to provide a fluid–fluid interface that impacts the flow paths of the retained inflow and delayed ejection flow. Although there was no significant difference in the relative amount of the non-exchanging residual volume between the groups, the exchanging components (retained inflow and delayed ejection flow) were relatively larger in the DCM patients. This is in agreement with preliminary data suggesting that decompensated HF patients have a larger residual volume than compensated patients.12 In the current study, the KE/mL of the residual volume at ED was higher in patients compared with healthy subjects, which may reflect the impact of stiffer and less compliant myocardium. Moreover, converting motion of inflowing blood to motion of the residual volume may offer advantages in terms of less diastolic pressure rise, as well as in avoidance of thrombosis. A large amount of the residual volume with low velocities may create conditions promoting intra-ventricular thrombus formation. This is supported by an echo-Doppler study that demonstrated lower apical blood velocities in DCM patients with LV thrombus compared with DCM patients without thrombus.22
Study limitations
Meticulous quality control of the post-processed data was fundamental in this study and evidence of aberrant pathlines indicating data imperfections was carefully sought.9 Accuracy of pathline computation is dependent on a large number of factors, such as temporal and spatial resolution, VENC, field strength, MRI hardware, and intra-voxel dephasing due to turbulence. Errors in pathlines computation will propagate from one time frame to the next and accumulate over time. Eddy currents and concomitant gradient fields may also hamper the accuracy of 4D PC-MRI flow data. The potential effects of these factors were anticipated and minimized by the acquisition design and by tailored post-processing.
The data acquisition was optimized for the blood flow through the left ventricle, with VENC targeted to the range of diastolic inflow velocities. As systolic velocities downstream from the aortic valve can exceed the upper limit of the velocity scale used, aliasing artefacts may occur there and data from that region were not studied.
The scan times in this study were relatively long. However, new MRI sequences23 as well as improvements in MRI hardware continue to decrease scan times. The morphologic images and the 3DcinePC velocity data were acquired in two separate acquisitions; hence, there is a risk of mismatch due to patient movement. Mismatch would be detectable as a large difference in inflow and outflow volumes, and visible offsets between the morphologic images and the contrast poor magnitude data taken from the flow data acquisition. This problem was addressed by acquiring morphological images both before and after the 3DcinePC velocity data. In the future, registration algorithms might improve matching of the 3DcinePC velocity data to the underlying morphological data and further reduction in the potential for this type of error.
The present findings relate only to a relatively small number of patients and healthy subjects in the supine position at rest and in sinus rhythm with no significant valvular disorder. It is reasonable to assume that different flow patterns and energetics would be seen with altered rhythm, valve disease, and at stress with higher heart rates and different loading conditions. The residual volume is likely to be the most inaccurately assessed component, due to partial volume effects at the chamber boundaries and imperfect segmentation, which may inadvertently include some myocardium. This fact may partly explain the relatively low flow-based LV ejection fraction values observed in the healthy subjects. The same methodology was used in both groups, however.
Conclusion
Although total LVSV is equal in healthy subjects and HF patients with mild LV remodelling, the SV's transventricular flow paths to ejection and diastolic energetics are significantly different in the two states. Late filling facilitates a direct transit of blood through the normal LV, but this aspect of diastolic–systolic coupling is less pronounced in myopathic LVs. These multi-dimensional flow-based abnormalities are detectable despite clinical compensation, and may prove useful as subclinical markers of impaired LV function.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
Funding
This study was funded by The Swedish Research Council, The Swedish Heart and Lung Foundation, and The Emil and Wera Cornell foundation.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors appreciate the assistance provided by Jan Engvall and Johan Kihlberg.
References
- 1.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. doi:10.1016/S1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;58:1370–80. doi: 10.1056/NEJMra072139. doi:10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 3.Richter Y, Edelman ER. Cardiology is flow. Circulation. 2006;113:2679–82. doi: 10.1161/CIRCULATIONAHA.106.632687. doi:10.1161/CIRCULATIONAHA.106.632687. [DOI] [PubMed] [Google Scholar]
- 4.Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000;404:759–61. doi: 10.1038/35008075. doi:10.1038/35008075. [DOI] [PubMed] [Google Scholar]
- 5.Mohiaddin RH. Flow patterns in the dilated ischemic left ventricle studied by MR imaging with velocity vector mapping. J Magn Reson Imaging. 1995;5:493–8. doi: 10.1002/jmri.1880050503. doi:10.1002/jmri.1880050503. [DOI] [PubMed] [Google Scholar]
- 6.Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging. 2008;1:705–17. doi: 10.1016/j.jcmg.2008.06.008. doi:10.1016/j.jcmg.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson J, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T, Carlhall CJ. Quantification of presystolic blood flow organization and energetics in the human left ventricle. Am J Physiol Heart Circ Physiol. 2011;300:H2135–41. doi: 10.1152/ajpheart.00993.2010. doi:10.1152/ajpheart.00993.2010. [DOI] [PubMed] [Google Scholar]
- 8.Wigström L, Ebbers T, Fyrenius A, Karlsson M, Engvall J, Wranne B, et al. Particle trace visualization of intracardiac flow using time-resolved 3D phase contrast MRI. Magn Reson Med. 1999;41:793–9. doi: 10.1002/(sici)1522-2594(199904)41:4<793::aid-mrm19>3.0.co;2-2. doi:10.1002/(SICI)1522-2594(199904)41:4<793::AID-MRM19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson J, Carlhäll CJ, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T. Semi-automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson. 2010;12:9. doi: 10.1186/1532-429X-12-9. doi:10.1186/1532-429X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolger AF, Heiberg E, Karlsson M, Wigström L, Engvall J, Sigfridsson A, et al. Transit of blood flow through the human left ventricle mapped by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:741–7. doi: 10.1080/10976640701544530. doi:10.1080/10976640701544530. [DOI] [PubMed] [Google Scholar]
- 11.Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:7. doi: 10.1186/1532-429X-13-7. doi:10.1186/1532-429X-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlhäll CJ, Bolger A. Passing strange: flow in the failing ventricle. Circ Heart Fail. 2010;3:326–31. doi: 10.1161/CIRCHEARTFAILURE.109.911867. doi:10.1161/CIRCHEARTFAILURE.109.911867. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson AG, Zajac J, Eriksson J, Dyverfeldt P, Bolger AF, Ebbers T, et al. 4-D blood flow in the human right ventricle. Am J Physiol Heart Circ Physiol. 2011;301:H2344–50. doi: 10.1152/ajpheart.00622.2011. doi:10.1152/ajpheart.00622.2011. [DOI] [PubMed] [Google Scholar]
- 14.Markl M, Geiger J, Kilner PJ, Foll D, Stiller B, Beyersdorf F, et al. Time-resolved three-dimensional magnetic resonance velocity mapping of cardiovascular flow paths in volunteers and patients with Fontan circulation. Eur J Cardiothorac Surg. 2011;39:206–12. doi: 10.1016/j.ejcts.2010.05.026. doi:10.1016/j.ejcts.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson M, Heiberg E, Toger J, Arheden H. Quantification of left and right ventricular kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. Am J Physiol Heart Circul Physiol. 2012;302:H893–900. doi: 10.1152/ajpheart.00942.2011. doi:10.1152/ajpheart.00942.2011. [DOI] [PubMed] [Google Scholar]
- 16.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–6. doi: 10.1016/j.jacc.2005.09.032. doi:10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. doi:10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–7. doi: 10.1038/nature01282. doi:10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 19.Taber LA. Mechanical aspects of cardiac development. Prog Biophys Mol Biol. 1998;69:237–55. doi: 10.1016/s0079-6107(98)00010-8. doi:10.1016/S0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 20.Colucci WS. Molecular and cellular mechanisms of myocardial failure. Am J Cardiol. 1997;80:15L–25. doi: 10.1016/s0002-9149(97)00845-x. doi:10.1016/S0002-9149(97)00845-X. [DOI] [PubMed] [Google Scholar]
- 21.Pasipoularides A, Shu M, Shah A, Womack MS, Glower DD. Diastolic right ventricular filling vortex in normal and volume overload states. Am J Physiol Heart Circ Physiol. 2003;284:H1064–72. doi: 10.1152/ajpheart.00804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maze SS, Kotler MN, Parry WR. Flow characteristics in the dilated left ventricle with thrombus: qualitative and quantitative Doppler analysis. J Am Coll Cardiol. 1989;13:873–81. doi: 10.1016/0735-1097(89)90230-1. doi:10.1016/0735-1097(89)90230-1. [DOI] [PubMed] [Google Scholar]
- 23.Sigfridsson A, Petersson S, Johan Carlhall C, Ebbers T. Spiral readouts for 4D flow MRI. J Cardiovasc Magn Reson. 2012;14:1–2. doi:10.1186/1532-429X-14-S1-W31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.