Abstract
Adverse drug reactions are a common cause of patient morbidity and mortality. Type B drug reactions comprise only 20% of all drug reactions but they tend to be primarily immunologically mediated and less dependent on the drug’s pharmacological action and dose. Common Type B reactions seen in clinical practice are those of the immediate, IgE, Gell-Coombs Type I reactions, and the delayed, T-cell mediated, Type IV reactions. Management of these types of reactions, once they have occurred, requires careful consideration and recognition of the utility of routine diagnostic tests followed by ancillary specialised diagnostic testing. For Type I, IgE mediated reactions this includes prick/intradermal skin testing and oral provocation. For Type IV, T-cell mediated reactions this includes a variety of in vivo (patch testing) and ex vivo tests, many of which are currently mainly used in highly specialised research laboratories. The recent association of many serious delayed (Type IV) hypersensitivity reactions to specific drugs with HLA class I and II alleles has created the opportunity for HLA screening to exclude high risk populations from exposure to the implicated drug and hence prevent clinical reactions. For example, the 100% negative predictive value of HLA-B*5701 for true immunologically mediated abacavir hypersensitivity and the development of feasible, inexpensive DNA-based molecular tests has led to incorporation of HLA-B*5701 screening in routine HIV clinical practice. The mechanism by which drugs specifically interact with HLA has been recently characterised and promises to lead to strategies for pre-clinical screening to inform drug development and design.
Introduction
Adverse drug reactions (ADRs) by definition occur in association with a therapeutic dose of the drug. They represent a significant global burden and cost to the healthcare system and they have been cited as the fourth to sixth most common cause of death in some studies.1
Clinically and epidemiologically, ADRs can be classified into two broad types. Type A reactions, are common and predictable reactions that are largely dose dependent, are based on the drug’s pharmacological properties, and they are often preventable or reversible (Table 1).2 These Type A reactions are often influenced by factors that contribute to pharmacokinetic variability, for example organ dysfunction, underlying disease state, pregnancy, changes in drug exposure due to a genetic polymorphism in a drug metabolism or drug transporter gene or a drug-drug or food-drug interaction.
Table 1.
Features of pharmacological and hypersensitive ADRs. Adapted from Phillips.2
| Properties | Type A(Pharmacological ADRs) | Type B (Hypersensitivity ADRs) |
|---|---|---|
| Predictable | Yes | No |
| Dose dependent | +++ | + |
| Host Dependent (Genetic factors) | + | +++ |
| Immunological Basis | - | +++ |
- No known association,
low association,
medium association and
high association between the ADR (Adverse drug reactions) and properties listed.
Type B ADRs are less influenced by dosage and pharmacological action and are primarily immunologically mediated. Many of these types of drug ‘hypersensitivity’ reactions (HSRs) have been recently associated with genetic variability within the major histocompatibility complex (MHC) (Table 1).2 These can be further categorised into four types based on broad immunopathogenesis as outlined by the Gell-Coombs classification and later refined by Pichler et al. (Table 2).3–5 The focus in this review on testing for drug hypersensitivity syndromes will be on the more common and clinically relevant Type B ADRs of Gell-Coombs Type I and IV.
Table 2.
| Type of reaction | Immune reactant | Antigen | Effector | Clinical symptoms |
|---|---|---|---|---|
| Type I-immediate | IgE | Soluble antigen | Mast cells, basophils | Pruritus Angioedema, Urticaria, Bronchospasm |
| Type II- cytotoxic | IgG | Cell or matrix associated antigen | FcR positive cells (phagocytes, NK cells) | Thrombocytopaenia, Haemolytic anaemia |
| Type III- immune complex | IgG | Soluble antigen | FcR positive cells complement | Serum sickness |
| Type IV- delayed | T-cell mediated | |||
| IVa | Antigen presented by cells, or direct T-cell stimulation | (IVa)Macrophage | Contact dermatitis | |
| IVb | (IVb) Eosinophils | DRESS/DIHS/HSS | ||
| IVc | (IVc) T-cells | SJS/TEN, DILI | ||
| IVd | (IVd) Neutrophils | AGEP |
Ig: immunoglobulin, FcR: Fc receptor, NK cells: natural killer cells, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis, DRESS: drug reaction with eosinophilia and systemic symptoms, DIHS: drug-induced hypersensitivity syndrome, HSS: Hypersensitivity syndrome, AGEP: acute generalised exanthematous pustulosis, DILI: Drug induced liver injury.
Drug-induced IgE-mediated (Gell-Coombs Type I) HSR
Type I HSRs are immediate allergic reactions that typically occur within one hour of exposure to the allergen, but in some cases may not manifest for several hours (≤ 6 hours).6,7 Drugs and/or their metabolites are a frequent cause of Type I HSR and are mediated by drug-specific Immunoglobulin E antibodies (IgE). Accelerated reactions occurring out to 72 hours from drug exposure can be IgE mediated, however in general the more delayed a drug reaction is, the more likely it is to be mediated by an alternative mechanism. Drug-specific IgE may develop following exposure to the particular drug and once formed, these molecules bind to high affinity Fc (fragment, crystallisable) receptors on the surface of mast cells and basophils. Re-exposure to the causative drug or potentially to a cross-reacting related drug, leads to binding of the drug to these IgE molecules. Binding of the drug to two or more cell-bound IgE molecules causes cross-linking of the receptors and activation of the cell.8
The spectrum of clinical reactions in IgE mediated drug allergy ranges from relatively minor skin flushing and pruritus, through to more severe reactions with urticaria, angioedema, bronchospasm and anaphylaxis.6,7 These clinical features are the result of mast cell or basophil degranulation following cellular activation. During degranulation, vasoactive mediators such as histamine, proteases such as tryptase, and cytokines such as tumour necrosis factor (TNF) are released from preformed granules within the cytoplasm of the cell. Non-IgE-mediated immediate reactions, sometimes called pseudoallergic or histamine-release reactions, can present with similar clinical features to true IgE mediated reactions. These are most commonly dose-dependent and associated with drugs that directly stimulate the degranulation of mast cells and basophils, such as opiates, non-steroidal anti-inflammatory drugs (NSAIDs), radiocontrast media and vancomycin (‘red-man syndrome’).9–13
Diagnostic Testing for Immediate (Gell Coombs Type I, IgE-mediated) HSRs
The diagnostic evaluation of suspected IgE mediated drug HSR can involve both in vitro and in vivo testing methods. In vivo testing usually involves some combination of skin testing and oral provocation with the implicated drug. In vitro tests detect and quantify drug-specific IgE within the serum. The combination of in vivo tests, such as prick and intradermal skin testing and oral challenge, is generally considered the gold standard for diagnostic testing of immediate drug allergy. When skin testing and oral challenge are negative, this provides high level evidence that the patient can be safely re-exposed to the drug or drug class. Patients with a positive immediate skin test and oral challenge reaction, in keeping with an IgE mediated reaction, are usually advised to permanently avoid the drug or drug class in question, although if the drug is indicated, they may undergo a process known as rapid oral or intravenous desensitisation to the required drug. Unlike desensitisation to environmental allergens and bee venom, drug desensitisation is a temporary process, effective only while the patient is taking the drug, and which must be repeated every time treatment is interrupted.
In Vivo Testing
The utility of cutaneous testing and drug provocation tests (DPT) in the assessment of immediate hypersensitivity reactions has been demonstrated for numerous drugs, and in particular for beta-lactam antibiotics (Table 3). They can rapidly provide evidence of a biologic response to the drug in question. Due to the small risk of serious but reversible reactions associated with these procedures, patients should be evaluated by an experienced clinician prior to proceeding with these tests, which must be carried out in an appropriate clinical setting.11,12
Table 3.
Role of diagnostic/screening tests in immediate and delayed drug hypersensitivity reactions.
| In Vivo | Ex Vivo | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Phenotype | SPT/IDT | Patch test | Oral Challenge (DPT) | LTT | ELISpot ICS/other ex vivo | BAT | IgE/RAST | HLA Screen |
| IgE mediated | Yes | No | Yes | No | No | Not routine (specific not sensitive) | Yes-screen only (specific not sensitive) | No |
| Delayed Rash (MPE) | Yes -delayed IDT reading | Yes | Possible 5–7 day challenge | Yes | Yes | No | No | No |
| DRESS/DIHS | Yes - delayed IDT reading | Yes | No | Yes | Yes | No | No | Possible† |
| Abacavir HSS | No -risk severe reaction | Yes | No | Yes | Yes | No | No | Yes HLA-B*5701 |
| SJS/TEN | Low sensitivity | Low sensitivity | No | Yes | Yes | No | No | Possible† |
| AGEP | Yes -delayed IDT reading | Yes | No | Possible‡ | Possible | No | No | No |
| FDE | Yes- delayed IDT reading | Yes | No | Possible‡ | Limited data | No | No | No |
| DILI | No | No | No | Possible | Yes | No | No | Possible† |
Not routinely used globally in clinical practice as positive predictive value, negative predictive value and number needed to test to prevent one case varies amongst drugs and populations (see Table 4).
Limited data on the performance of LTT on AGEP and FDE. However along with a positive delayed IDT and patch test result as well as a good clinical diagnosis, a positive LTT can help in the identification of the culprit drug. 139 Yet diagnosis should take into account the possibility false positive and negative results, particularly in cases where multiply drugs are under suspicion.
HLA: human leukocyte antigen, LTT: Lymphocyte transformation test, ELISpot: enzyme-linked immunospot, ICS: intracellular cytokine staining, BAT: Basophil Activation Testing, Ig: Immunoglobulin, IDT: intradermal testing, SPT: skin prick testing, HSS: Hypersensitivity syndrome, DRESS: Drug reaction with eosinophilia and systemic symptoms, DIHS: Drug-induced Hypersensitivity syndrome, SJS/TEN: Stevens-Johnson syndrome / Toxic epidermal necrolysis, AGEP: acute generalised exanthematous pustulosis, DILI: Drug-induced liver injury, FDE: Fixed drug eruption.
Cutaneous Testing
Cutaneous testing usually uses a combination of both skin prick testing (SPT) and intradermal testing (IDT) using dilutions of the drug. Generally SPT is performed first, as this carries a very low risk of adverse reaction, and is then followed by IDT, which carries a slightly higher risk of serious adverse reactions. This process has been well validated for a number of different drugs including beta-lactam antibiotics. Some drugs can cause a non-specific irritant reaction with a wheal and flare response, therefore, a non-irritant testing concentration is required to avoid false positive results. Other drugs, such as opiates and vancomycin cause direct mast cell activation making testing infeasible, and fluoroquinolones must be tested at lower concentrations for this same reason. Positive (histamine) and negative (normal saline) controls are essential to ensure accurate interpretation of results. False negative and false positive results may also occur from incorrect intradermal skin testing technique. Antihistamines and other drugs with antihistaminic effects, such as antidepressants, can cause false negative results and are discontinued a minimum of two days prior to testing.11
The most extensive evidence-based literature for the use of cutaneous testing in immediate drug allergy exists for the penicillins. Studies investigating cutaneous testing for penicillin allergy have generally involved the use of the defined haptens, the major and minor determinants of penicillin, although the commercial availability of these preparations has been limited in the past. The combined use of the major determinant (benzylpenicilloyl poly-l-lysine or PPL) and the minor determinants mixture (MDM, which is a combination of benzylpenicillin, sodium benzylpenicillinoate and benzylpenicilloic acid) has a reported sensitivity of 70% and specificity of 97–100%. 11 The negative predictive value of cutaneous testing in patients with a history suggestive of immediate hypersensitivity to penicillin is reported to be 97–99%.14,15 The addition of other reagents such as amoxicillin has become increasingly important given the high community use of this drug and the increasing identification of patients who have selective side-chain mediated reactions to amoxicillin that can tolerate other penicillins and cephalosporins. Prick and intradermal skin testing to amoxicillin can detect additional patients that would otherwise be missed by the use of PPL and MDM, and have also identified patients with isolated positive cutaneous tests to amoxicillin. The utility of amoxicillin cutaneous testing varies between different studies, which may reflect differences in study populations as well as concentration of the solution used for testing.15
Testing has also been used for other drugs within the beta-lactam family, such as cephalosporins, which are structurally similar to penicillin in that they contain the four-member beta-lactam ring, but contain a six-member dihydrothiazine ring rather than a five-member thiazolidine ring. Cephalosporins also have two side chains (R1 and R2) compared to a single side chain (R1) in penicillins.
Specific IgE may be directed against these side-chains rather than the beta-lactam ring, therefore a negative penicillin cutaneous test does not exclude cephalosporin reactivity. Some cephalosporins share a side-chain with other beta-lactams, e.g. cephalexin and ampicillin, and therefore may cross react.11,16 In patients with penicillin allergy, 5% or less will be cross-reactive to first generation cephalosporins and the risk for cross reactivity is much lower (<2%) for third and fourth generation cephalosporins.16,17 Up to 25% of patients with cephalosporin allergy may have evidence of sensitisation to penicillin, however in contemporary clinical practice this number is much lower, and side chain and selective reactivity to cephalosporins, such as the first generation cephalosporin, cephazolin, with tolerance of other penicillins and cephalosporins is more common.18 The reported sensitivity of cephalosporin cutaneous testing ranges between 30.7% and 69.7%; however as there is a lot of structural diversity amongst cephalosporins, the sensitivity depends on the implicated drug and which drugs are used for testing.19
Neuromuscular blocking agents (NMBA) are the most common cause of perioperative anaphylaxis and more than 50% of reactions are likely to be IgE mediated.11 Cutaneous testing for NMBA is a well established part of the assessment of suspected drug hypersensitivity reactions occurring in the perioperative period, and cutaneous testing for a wide range of NMBAs has been investigated. The sensitivity of cutaneous testing for NMBA is reported to be approximately 94–97%.20
Radiocontrast media can cause immediate hypersensitivity reactions as well as delayed reactions. The incidence of immediate reactions to ionic radiocontrast media is 1–3%, and for non-ionic radiocontrast media 0.5%.21 Immediate reactions to radiocontrast media had traditionally been thought to be non-IgE mediated reactions and pseudoallergic in nature, however the exact mechanisms are still not well understood and it is now acknowledged that at least some of these reactions are IgE-mediated. This hypothesis is supported by the finding that most patients with immediate radiocontrast media reactions had isolated positive results on cutaneous testing to the particular radiocontrast media responsible for their reaction, as well as in vitro evidence of sensitisation. Therefore cutaneous testing does appear to have a role in both diagnosis of radiocontrast media hypersensitivity and also in establishing potentially safe alternative radiocontrast media for future use.11,13 The negative predictive value of radiocontrast media cutaneous testing has been found to be high. In a study of 29 patients who had negative cutaneous tests and were rechallenged, the overall negative predictive value was 96%.21
Corticosteroids only rarely cause IgE mediated reactions, most commonly with injectable forms including methyl-prednisolone, hydrocortisone, triamcinolone and beta-methasone.22 In at least some of these cases, the reactions appear to be due to the stabilising agents used in injectable corticosteroid preparations, such as carboxymethylcellulose, rather than the corticosteroid itself. Cutaneous testing for corticosteroids is not well validated, but has been described in several case series.11
Drug Provocation Test (DPT)
Published evidence supports the utility of a controlled challenge with the drug suspected of causing an immediate hypersensitivity reaction and this approach is often considered to be the gold standard or the final step in confirming or excluding a drug allergy.23–26 Tests are considered positive if the same, or similar, signs and symptoms of the original reaction are reproduced by the challenge. The drug may be given as a single dose or in divided doses as a graded challenge, depending on the suspected drug and the severity of the reaction. A placebo may also be used if the symptoms of the reaction are very subjective or there is doubt over the results of the test. For practical reasons, oral administration of the drug is used, however some specialised clinics may give the drug by the same route used when the reaction occurred, if this is more appropriate. Since any challenge carries risk of recurrence of the original hypersensitivity, it should be conducted in an appropriately selected population by a specialist physician, and in an appropriate outpatient, preferably hospitalised, setting, where specific monitoring and treatment to rapidly reverse any such reactions can be applied.24
A position paper by the European Network for Drug Allergy (ENDA) has outlined four main indications for performing a DPT. The first two indications involve a challenge with the suspected drug to either exclude drug allergy in patients whose history is clearly not suggestive of a drug hypersensitivity reaction, or to consolidate the diagnosis in patients with a suggestive history but no evidence of sensitisation on testing. The other two indications involve challenge with an alternative to the responsible drug in patients with proven drug allergy, either to provide evidence of tolerance to unrelated medications for reassurance, or to exclude cross-reactions with structurally similar drugs.25
For assessment of immediate hypersensitivity reactions to beta-lactams, DPT is still considered necessary after in vivo and in vitro tests have been performed, even if these are both negative. In some reported case series, up to 55% of patients with negative cutaneous tests and negative specific IgE testing, have a positive DPT, although the reported negative predictive value of the in vivo and in vitro tests is much higher in other reported series. DPT itself has a high reported negative predictive value for beta-lactams of 94–98%.26
For other antibiotics such as sulphonamides, quinolones and macrolides, the use of DPT is essential as there are no reliable in vivo or in vitro tests available for diagnosis.24 As cutaneous testing for aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) is not reliable, DPT is a useful tool for the assessment of possible immediate hypersensitivity reactions. DPT in this setting is used as a means to ascertain whether patients have had a generalised COX-1 pharmacological reaction across aspirin and NSAIDs or an IgE mediated reaction to a specific NSAID. In this way DPT is commonly used to evaluate the tolerability of other NSAIDs in patients with a strong history of immediate reaction to a single NSAID. Cutaneous testing for immediate reactions to corticosteroids has low sensitivity, therefore DPT is often required to confirm tolerance after negative cutaneous testing.25,26
In Vitro Testing
Detection of specific IgE in the serum provides evidence of sensitisation to a particular allergen, and in combination with a reliable history of an allergic reaction can support the diagnosis of allergy. In vitro testing methods for drug allergy have the advantage over in vivo testing methods of posing no direct risk to the patient. However, although many have been shown to be highly specific, they lack sensitivity compared to in vivo testing (Table 3).27 The lack of sensitivity has limited the clinical utility of these tests which in some settings have been used as a screening tool to select patients appropriate for SPT and IDT.
Specific IgE Testing
Drug-specific IgE can be detected in the serum using several immunoassay methods. These include radioallergosorbent testing (RAST), enzyme linked immunosorbent assay (ELISA) or fluoroenzyme immunoassay (FEIA).28 RAST has now been largely superseded by the ELISA and FEIA methods. In these tests the allergen, which may be bound to a carrier protein, is embedded in a solid phase polymer. The patient’s serum is then incubated with the allergen and if specific IgE is present in the serum, it will bind to allergen. This can then be detected using anti-human IgE molecules that have been labelled with an enzyme or a radioactive or fluorescent label depending on the method used.
Although methods of detecting specific IgE in serum generally have high specificity, their main limitation is a lack of sensitivity. When used in the appropriate clinical setting, these tests can be a very useful tool in diagnosing allergy to various foods and airborne allergens, with high reported positive predictive values when an appropriate cut-off is used.29 However, in drug allergy testing, the problem with sensitivity is more evident, particularly for beta-lactam antibiotics.30 In one study comparing methods in penicillin allergy testing, depending on the initial clinical manifestations, the reported sensitivity for RAST was 42.9–62.5% and for FEIA was 12.5–25%.27 For aminopenicillins, the reported sensitivity ranges from 28.6–64.3% and specificity 72.7–100%. 27 Cefaclor is the only cephalosporin that has a commercially available serum specific IgE test available. Testing for other cephalosporins has been performed using in-house assays for research purposes only, but these are not commercially available.31 The reported sensitivity of specific IgE testing ranges from 6.7–40% with a specificity of 75%.27
Specific IgE can be measured for a limited number of other drugs, including muscle relaxants and opiates. The reported sensitivity and specificity using FEIA are 68% and 93% for rocuronium, 60% and 100% for suxamethonium, 88% and 100% for morphine and 86% and 100% for pholcodine.30
Basophil Activation Testing
The Basophil Activation Test (BAT) is a method of measuring drug-induced activation of basophils. Alterations in the cell surface expression of basophil activation markers, such as CD63 or CD203c, can be measured using cytometry.32 The utility of BAT has been shown for a number of different IgE-mediated allergies, including airborne allergens (pollens, house dust mite, and animal dander), various foods and hymenoptera venom. BAT is not routinely used in clinical practice at present, but has been investigated as an adjunct to cutaneous testing and specific IgE testing in assessing drug allergy, especially where equivocal results have been obtained. As more than one drug can be tested for at one time, it is also possible to examine for potential cross reactivity with related drugs.33 However, despite having good specificity, the reported sensitivity for BAT in drug allergies is low.
The use of BAT has only been studied in a limited number of drugs, primarily NMBA, beta-lactam antibiotics and NSAIDs. For NMBA, the reported sensitivity ranges from 36% to 86%, with reported specificity from 81% to 100%. For beta-lactam antibiotics, the reported sensitivity varies between 22% and 55%, and specificity between 79% and 100%. NSAIDs have an even wider range of reported results, with sensitivities ranging from 17% to 70%, and specificities ranging from 40% to 100%.32
Drug-Induced Delayed (Gell-Coombs Type IV) HSRs
Type IV delayed HSRs are generally T-cell mediated and can manifest as mild skin reactions to more severe and potentially fatal multisystem reactions. Although these reactions can occur anywhere from 1–2 days to 8 weeks following drug initiation, they are most commonly seen during the second week of first drug exposure. At the more severe end of the spectrum, delayed HSRs can lead to continuing hospitalisation, sometimes requiring intensive care and patients may need to use alternative drugs which are less effective and/or more expensive.
Clinical Syndromes
Delayed Rash Without Other Clinical Symptoms
Delayed rash or exanthems, without other clinical symptoms or systemic features, represent the majority of immunologically mediated drug reactions or Type B ADRs. They usually appear in the second week of first drug exposure and when rechallenged, the response can be rapid and more severe. Although a variety of clinical rash phenotypes are described, the eruption most commonly consists of erythematous macules or papules, sometimes referred to as morbilliform which appear first on the face and torso, often becoming generalised. These reactions can occur in association with any drug, however, they are most associated with aminopenicillins, allopurinol, anti-retroviral agents (abacavir, non-nucleoside reverse transcriptase inhibitors) and antibacterial sulphonamides.34,35 When continued treatment with the drug is important, and the rash is of mild to moderate severity and not associated with fever, internal organ or mucosal involvement, it is safe to attempt to treat with symptomatic management such as antihistamines and topical corticosteroids. In most cases the rash will attenuate and disappear within a few days despite continued treatment. Although some evidence suggests that this is mediated through immunological tolerance to drugs potentially through expansion of suppressive T-cell populations such as regulatory T cells, the specific cellular mechanisms leading to tolerance have not been elucidated. Alternatively, if treatment has already been interrupted, a process of desensitisation or graded reintroduction can be undertaken where the drug is reintroduced starting with very small doses increased over a period of days to weeks. In some instances, exanthem reactions have been associated with a possible drug-viral interaction, for example, the interaction between aminopenicillins and Epstein-Barr Virus (EBV).36 It has been well established that viral infections can increase the incidences of drug-induced exanthema reactions.36 Patients with infectious mononucleosis, an EBV induced disease, have a increased risk of developing a severe morbilliform reaction when given the antibiotic, ampicillin.36 The majority of patients who experience an ampicillin/mononucleosis morbilliform reaction can later tolerate ampicillin and other aminopenicillins when the mononucleosis has resolved. The pathological mechanisms behind this viral-drug interaction are largely unknown, therefore, those with mononucleosis should avoid ampicillin and other beta-lactam antibiotics of the aminopenicillins family.36
Fixed Drug Eruption
Fixed drug eruption (FDE) usually consists of erythematous round lesions, red to dusky purple or brown colour and sometimes features blisters. One or more lesions appear and the patient may complain of a burning and/or itchy sensation in the affected area.36 Occurring days to weeks after taking the causative drug, more commonly affected areas include specific areas of the skin, like the hands and feet as well as mucosal surfaces.36 The site of the eruption is fixed, meaning when the patient is re-exposed to the drug, the eruption occurs in exactly the same area. The eruption usually heals within 7–10 days after the causative drug has been removed, although hyperpigmentation may be permanent.36 The immunopathogenesis of FDE is thought to be primarily driven by resident intraepidermal CD8+ T cells with an effector memory phenotype. Weak two digit HLA associations have been described, however more contemporary studies utilising high resolution four digit HLA typing have not been reported (Table 4).54 FDE has been described with several drugs and classes of drugs; common medications that cause fixed drug eruptions include antibiotics such as sulfamethoxazole and tetracyclines as well as NSAIDs.37,38 Patch testing, applied at the site of the original reaction, has been reported to be positive in 30–50% of those with FDE, however this is drug dependent with patch testing to NSAIDs having a higher sensitivity than antibiotics.39,40
Table 4.
Pharmacogenetic HLA-associated drug hypersensitivity and related drug-induced syndromes. Adapted from Pavlos.54
| Syndrome and Drug | HLA Allele/genetic associations | Populations |
|---|---|---|
| SJS/TEN (SCAR) | ||
| Allopurinol | B*5801 (or B*58 haplotype) | Han Chinese, Thai, European, Italian, Korean98–103 |
| Carbamazepine | B*1502 | Han Chinese, Thai, Malaysian, Indian91,104–108 |
| B*1511 | Korean, Japanese86,109 | |
| B*1518, B*5901 and C*0704 | Japanese110 | |
| A*3101 | Japanese, northern European, Korean86–88 | |
| Oxcarbazepine | B*1502 and B*1518 | Han Chinese, Taiwanese90,111 |
| Lamotrigine | B*1502 | Han Chinese111 |
| B*38 | European102 | |
| Phenytoin | B*1502, B*1301, Cw*0801 and DRB1*1602 | Han Chinese92,111 |
| Sulfamethoxazole | B*38 | European102 |
| Methazolamide | B*5901, Cw*0102 alleles and B*5901–Cw*0102–A*2402 haplotype | Korean and Japanese112,113 |
| Sulphonamides | A*29, B*12 and DR7 | European112 |
| Oxicam | B*73, A*2 and B*12 | European102 |
|
| ||
| HSS/DIHS/DRESS | ||
| Abacavir | B*5701 | European, African80 |
| Allopurinol | B*5801 (or B*58 haplotype) | Han Chinese, Korean, Japanese, Thai, European99–101,103 |
| Nevirapine (hepatitis/low CD4+) | DRB1*0101 | Australian, European114,115 |
| DRB1*0102 | South African116 | |
| Nevirapine (DIHS/DRESS) | Cw*8 or CW*8–B*14 haplotype | Italian, Japanese117,118 |
| Cw*4 and DRB1*15 | Han Chinese119 | |
| B*3505 | Asian115 | |
| B*3501 and B*15/DRB1*15 | Australian57 | |
| Carbamazepine | 8.1 AH (HLA A*0101, Cw*0701, B*0801, DRB1*0301, DQA1*0501, DQB1*0201) | Caucasians 120 |
| A*3101 | Northern European, Korean and Japanese86–88 | |
| A*11 and B*51 (weak) | ||
|
| ||
| Delayed Rash (nonsystemic) | ||
| Efavirenz | DRB1*01 | French121 |
| Nevirapine | DRB1*01 | French 121 |
| Cw*04, B*3505; rs1576*G CCHCR1 status (GWAS) | African, Asian, European, Thai115,122–124 | |
| Aminopenicillins | A*2 and DR*52 | Italian125 |
| Carbamazepine (MPE) | A*3101 | Han Chinese, northern European88,126 |
| Oxcarbazepine-induced MPE | B*1502 | Han Chinese127 |
|
| ||
| Fixed Drug Eruption | ||
| Feprazone | B*22 | Italian37,128 |
| Sulfamethoxazole | A*30–B*13–Cw*6 haplotype | Turkish38 |
|
| ||
| DILI | ||
| Amoxicillin–clavulanate | DRB1*1501, DRB107 protective, A*0201, DQB1*0602 and rs3135388, a tag SNP of DRB1*1501–DQB1*0602 | European129–131 |
| Lumiracoxib | DRB1*1501–DQB1*0602–DRB5*0101– DQA1*0102 haplotype | International multicentre132 |
| Ximelagatran | DRB1*07 and DQA1*02 | Swedish133 |
| Diclofenac | ABCB11, C-24T, UGT2B7*2, IL-4 C-590-A | European134–136 |
| Isoniazid | NAT2 slow acetylator, CYP2E1*5 and *1B | European135,136 |
| Flucloxacillin | B*5701, DRB1*0107–DQB1*0103 | European137 |
| Lapatinib | DRB1*0701–DQA2*0201–DQB1*0202/0202 | International, multicentre138 |
| Ximelagatran | DRB1*07 and DQA1*02 | European133 |
DIHS: Drug-induced hypersensitivity syndrome, DILI: Drug-induced liver disease, DRESS: Drug reaction with eosinophilia and systemic symptoms, GWAS: Genome-wide association study, HSS: Hypersensitivity syndrome, MPE: Maculopapular eruption, SJS: Stevens–Johnson syndrome, TEN: Toxic epidermal necrolysis. SCAR: severe cutaneous adverse reaction, HLA human leukocyte antigen A, B, C, DRB1, DQA1, DQA2, DQB1 genes, CD4 (cluster of differentiation 4).
Single Organ Involvement HSRs
DILI (drug-induced liver injury) is the major cause of drug withdrawal during clinical and pre-clinical development.41 Although hepatitis can occur as a part of a systemic hypersensitive reaction, DILI is distinct from this and only incorporates the liver as a single organ HSR. DILI frequently manifests without an accompanying rash or fever. It is commonly hypothesised that for some drugs, DILI is driven by metabolic, immune and genetic factors. Medications commonly associated with DILI type single organ involvement include the beta-lactam antimicrobials flucloxacillin, amoxicillin-clavulanate, tetracycline antimicrobials and anti-tuberculous agents. Other examples of single organ involvement HSR include pancreatitis and tubulointerstitial nephritis.
Severe Cutaneous Adverse Reactions (SCARs)
Reactions of most concern in clinical practice include drug-reaction, eosinophilia and systemic symptoms (DRESS) sometimes referred to as drug-hypersensitivity induced syndrome (DHIS) or hypersensitivity syndrome (HSS), acute generalised exanthematous pustulosis (AGEP) and the most severe of the cutaneous adverse reactions which comprise a spectrum of severity: Stevens-Johnson-syndrome (SJS) and toxic epidermal necrolysis (TEN).
DRESS/DHIS/HSS
By definition DRESS/DIHS/HSS is characterised by fever, acute widespread maculopapular rash, white cell abnormalities and multi-organ involvement. Lymphadenopathy is common and DRESS/DIHS/HSS is often mistaken for a viral illness. Approximately 80% of DRESS/DIHS cases involve the liver, 40% involve the kidneys, and 38% cause injury to lungs, heart and/or pancreas.42 The reactivation of human herpes viruses (HHVs) particularly HHV-6 and cytomegalovirus (CMV), has been shown to occur with some, but not all drugs associated with DRESS/DIHS/HSS, and a re-emergence of any spectrum of the hypersensitivity symptoms can occur up to 2 weeks (or longer) after treatment with the drug has been stopped.43,44 Laboratory evidence of autoimmunity is common with up to 30–40% of patients developing anti-thyroglobulin or antithyroid peroxidase antibodies although few develop symptoms of thyroiditis or hypothyroidism.45 Less commonly overt autoimmune thyroiditis or other autoimmune diseases such as systemic lupus erythematosis (SLE) occur, often months after the resolution of DIHS/DRESS/HSS symptoms.42,46 Drugs used in clinical practice that are frequently implicated in DIHS/DRESS/HSS type reactions, include allopurinol, aromatic amine anticonvulsants, antibacterial sulphonamides and the antiretroviral (ART) drugs abacavir and nevirapine.46,47
SJS/TEN
SJS/TEN are a spectrum of severe skin disease with high associated morbidity and mortality that is usually drug related. The mortality of TEN is highest and can reach 30–50%. SJS involves ≤10% or less of body surface area; SJS/TEN overlap 10–30% and TEN ≥30%.48–50 The initial rash of SJS/TEN can appear non-specific, however is often characterised by pain, rapid progression and associated systemic features such as fever and malaise.48,49 Buccal, ocular, and genital mucosal surfaces and in some cases, respiratory, and gastrointestinal tracts become inflamed with blister formation, and serious obstruction and sepsis can ensue. Epidermal necrosis and detachment occurs, and in patients with TEN, look similar to a serious burn requiring aggressive supportive and intensive management, ideally in a dedicated tertiary care burns unit.49,50 Drugs linked to SJS and TEN overlap with DIHS/DRESS and include allopurinol, aromatic anticonvulsants, nevirapine, NSAIDs and sulphur antimicrobials.48–51
AGEP
AGEP typically involves an acute fever, above 38°C and the cutaneous eruption of small non-follicular pustules, forming within an acute, widespread erythema.52–53 AGEP has been reported to involve the mucous membranes in up to 25% but when it does, the symptoms are generally mild. Patients often present with acute fever, neutrophilia and sometimes oedema of the face and hands and mild eosinophilia, but internal organ involvement such as hepatitis, although reported, is not common.52 Drugs commonly associated with AGEP syndrome include aminopenicillins and other beta-lactams, NSAIDs, quinolones, macrolides, calcium channel blockers as well as antimalarial drugs such as chloroquine and (hydroxy-) chloroquine.52–54 There have been no convincing class I or class II HLA allele associations with AGEP.53
Genetic Associations and Delayed HSRs
The interaction between the HLA or MHC cell surface proteins and T-cell receptors is central to T-cell mediated immune responses. In keeping with this and the transition from serologic HLA typing to sequence based typing resolved to four digits, there have been numerous associations with high odds ratios described between various phenotypes of delayed drug HSR and class I and II HLA alleles over the past 10 years (Table 4).54 The MHC chromosomal region is an approximately 3.6 mega base pair section of DNA, located on the short arm of the chromosome 6 in humans (6q21.3). The MHC region contains a large number of highly polymorphic genes characterised by high linkage disequilibrium; these include the HLA class I, II and III genes. The three major HLA class I genes include HLA-A, HLA-B, and HLA-C. class II includes HLA-DR, HLA-DP and HLA-DQ while class III contains many other genes associated with the immune system like the Tumour Necrosis Factor (TNF) gene, lymphotoxin alpha (LTA), heat shock proteins as well as a variety of non-immune related genes.55,56 That there are up to 5,190 different HLA class I and II alleles, show the highly polymorphic nature of this region, HLA-B being the most polymorphic, with over 1,800 alleles. HLA alleles are often ethnically associated which can correspond with the varying prevalence of given delayed HSRs in a specific population. Carbamazepine associated SJS/TEN, for instance, has increased prevalence in areas such as Southeast Asia where the carriage rate of the associated HLA-B*1502 allele is high (10–15%) compared to <1% carriage rate in Caucasian European populations (Table 5).57,58 This polymorphic nature is important in the adaptability of the immune system. The HLA class I and II genes encode cell-surface protein receptors that present peptides to receptors on the surface of T-cells (TCR). MHC class I molecules are expressed by most nucleated cells and are responsible for presenting peptides to cytotoxic or killer T-cells which positively present the co-receptor CD8 (CD8+ T-cells) and in turn which mount a cytotoxic T-cell response if the TCR recognises the peptide presented as a foreign peptide i.e. pathogen-derived. MHC class II molecules are expressed by antigen presenting cells (APCs) only, which are responsible for presenting peptides to helper or regulatory T-cells, that positively present the co-receptor CD4 (CD4+ T-cells), similarly a response is mounted if a foreign peptide is presented. Often severe immune diseases or responses are a result of the corruption of this process.
Table 5.
The negative and positive predictive values and the number needed to test to prevent one specific drug reaction. The numbers shown are indicative of abacavir-induced HSS, allopurinol and carbamazepine associated SJS/TEN and flucloxacillin-associated DILI.Adapted from Phillips.57,85
| Drug and associated reaction | HLA Allele | HLA Carriage rate | Prevalence of Diagnosis | Negative Predictive Values | Positive Predictive Values | NNT to prevent ‘one’ case |
|---|---|---|---|---|---|---|
| Abacavir-HSS | B*5701 | 6–8% Caucasian <1% Asian/African 2.5% in African American |
8% (3% true HSR 2-7% false positive diagnosis) |
100% (patch test confirmed) | 55% | 13 |
| Allopurinol-SJS/TEN | B*5801 | 9–11% Han Chinese 1-6% Caucasian |
1/250–1/1000 | 100% in Han Chinese | 3% | 250 |
| Carbamazepine-SJS/TEN | B*1502 | 10–15% Han Chinese <0.1% Caucasian |
<1–6/1000 | 100% in Han Chinese | 3% | 1000 |
| Flucloxacillin-DILI | B*5701 | As for Abacavir | 8.5/100,000 | 99.99% | 0.12% | 13819 |
HLA: Human leukocyte antigen, NNT: Number Needed to Test, HHS: Hypersensitivity syndrome, SJS: Stevens–Johnson syndrome; TEN: Toxic epidermal necrolysis, DILI: Drug-induced Liver Injury.
Screening and Diagnostic Testing for Delayed (Gell-Coombs Type IV) HSRs
Molecular and genetic sequencing tests have been useful in screening for populations at risk and excluding them from a specific drug (Table 3). HLA-B*5701 screening, prior to abacavir prescription, is the most common example, and it is now used in routine HIV clinical practice (Figure 1).54 It is important to realise that there is no test that has 100% sensitivity for the diagnosis of Type IV or delayed HSR. The correct clinical diagnosis is typically based on the appropriate clinical picture combined with supportive laboratory tests and histopathology. Furthermore, in view of the fact that many of these syndromes are associated with high morbidity and mortality which can worsen on re-exposure, it is often a clinical decision to give patients a MedicAlert for the drug in question and any other drugs for which there could be immunological cross-reactivity based on their chemical structure. Diagnostic in vivo and/or ex vivo testing can however provide important ancillary information, particularly on the propensity to react to structurally related drugs and information on differential reactivity when the patient was taking more than one drug at the time of development of the hypersensitivity reaction.
Figure 1.
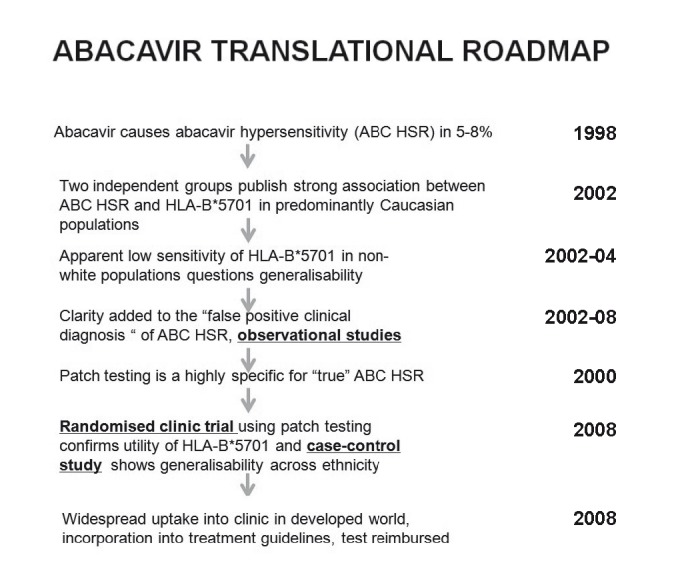
Abacavir translational roadmap. The abacavir example provides a ‘roadmap’ of how a pharmacogenetic (HLA-B*5701) test can be translated into routine clinical practice. Adapted from Pavlos et al.54
Use of HLA Typing in Screening for Drug Hypersensitivity
Methods for HLA Allele Identification
Over the past 10 years with the widespread availability of full allelic high resolution (four digit) sequence based HLA typing, primarily in specialty transplant laboratories, many associations have been found between drug-induced severe cutaneous adverse reactions (SCARs) and other severe immunologically mediated delayed HSRs such as DILI and key class I and II HLA alleles (Table 4).54 HLA typing by next generation sequencing methods in research settings is further improving the ease, accuracy, cost and turn-around-time of high resolution HLA typing. Prior to this, HLA molecules, even in specialised transplant laboratories, were largely typed by serological responses to HLA antigens or other DNA based assays that provided lower levels of accuracy and resolution.58–59
Once an association between a specific HLA allele and clinical phenotype has been established to have 100% negative predictive value, suggesting some clinical utility, then an allele specific test can be designed. The experience with abacavir and HLA-B*5701 has exemplified the development of feasible and rapid allele specific molecular and flow cytometric methods that can be used by many routine diagnostic laboratories. Laboratory validation of a HLA-B*5701 specific molecular test against sequence based HLA typing was built into the PREDICT-1 study, a double blinded randomised study whose primary aim was to examine the clinical utility of HLA-B*5701 to prevent clinical and immunologically confirmed abacavir hypersensitivity and other similar molecular tests have since been developed including an allele-specific PCR and melting curve assay.60 HLA alleles related to HLA-B*5701 such as HLA-B*5702 and HLA-B*5703 are distinguishable from B*5701 due to the inability to be amplified by either primer set. This method provides a cheaper alternative to expensive SBT methods as well as a higher specificity compared to standard serological typing methods.60,61 A simple Taqman assay has also been developed based on HCP5 rs2395029 which is a HLA-B*5701 haplospecific marker in strong but not complete linkage disequilibrium with HLA-B*5701. Cases of patch-test positive abacavir hypersensitivity have occurred in patients positive for HLA-B*5701 but negative for HCP5 rs2395029 suggesting some caution for this test which would have slightly less than 100% negative predictive value.62,63 Flow cytometric based techniques have also been developed based on a B17 monoclonal antibody and this has the advantage of a quick turnaround time by being performed on fresh cells and aligning with other tests routinely performed in the HIV population such as CD4+ T cell count.60
In Vivo Testing
Patch and Intradermal Cutaneous Testing for Delayed (Type IV) Reactions
Patch testing has traditionally been the diagnostic procedure of choice for delayed hypersensitivity reactions including contact dermatitis to non-drug substances such as metals, latex and preservatives. The procedure of patch testing involves preparing the drug in the appropriate vehicle (typically petrolatum) at the highest non-irritating concentration and leaving the patch tape affixed to the skin generally for a minimum of 48 hours (although 24 hours was sufficient for abacavir). Readings are then done at a minimum of 48 and 96 hours and sometimes 7 days and read according to a standardised grading scale. In view of the theoretical risk of a false negative reaction during immunological response of an acute delayed drug hypersensitivity, reaction performance of patch testing is typically delayed for at least 4–6 weeks following the onset of the drug reaction. False positive patch testing results can also occur and maybe related to an excipient of the drug or the vehicle used for testing itself. The vehicle (e.g. petrolatum without drug) should always be used as a negative control. In general, irritant or false positive reactions are often indicated by erythema without induration that appears within 24 hours and rapidly disappears. The sensitivity of patch testing varies considerably, based on the specific clinical phenotype of the delayed reaction and the implicated drug. It is believed for some drugs that active metabolism to the implicated metabolite in the skin is needed and this would be dependent on the expression of specific drug metabolism enzymes such as various cytochrome P450 isoforms in the appropriate compartment of the skin. Reactions associated with patch testing are limited to the site of the drug application and more generalised reactions are very rare.61,64 One case reported a patch test induced exfoliative dermatitis, however it was reported that the test was performed using a solution of crushed tablets in petrolatum and therefore the final drug concentration was not adequately controlled.61,64,65
The sensitivity of patch testing has varied according to phenotype and drug, but is generally <70% with higher sensitivities reported for AGEP, abacavir HSS, carbamazepine SJS/DRESS and fixed drug eruption (Table 3)65,66 Patch testing has been a very useful research tool to identify true immunologically-mediated abacavir HSS. Patch testing was used as a co-primary endpoint (with clinical diagnosis) in the PREDICT-1 and the SHAPE studies to show that HLA-B*5701 testing has a 100% negative predictive value for true immunologically mediated abacavir HSS.54 From the PREDICT-1 study, it could be ascertained that the diagnostic sensitivity of patch testing for abacavir HSS was 87%. Early studies demonstrated that 100% of patch test positive patients carried HLA-B*5701 and cutaneous biopsies from the positive patch test showed identical histopathology to the rash of acute abacavir HSS patients with an abundance of CD4+ and CD8+ T-cells. This suggests the reproduction of a localised HSS in the skin, which is illustrated by local erythema and induration (Figures 2a and 2b).67,68Figure 2b illustrates the positive response to an abacavir patch test with increasing concentrations from 0.1% to 25% w/w (abacavir/petrolatum).
Figure 2.
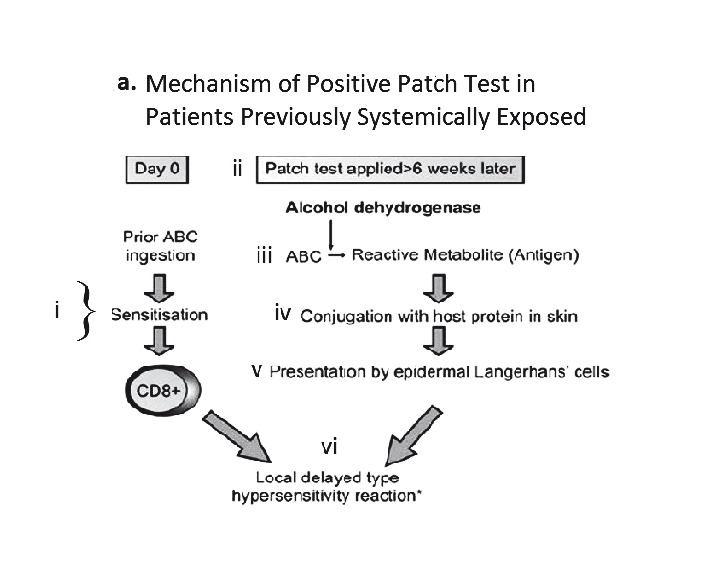
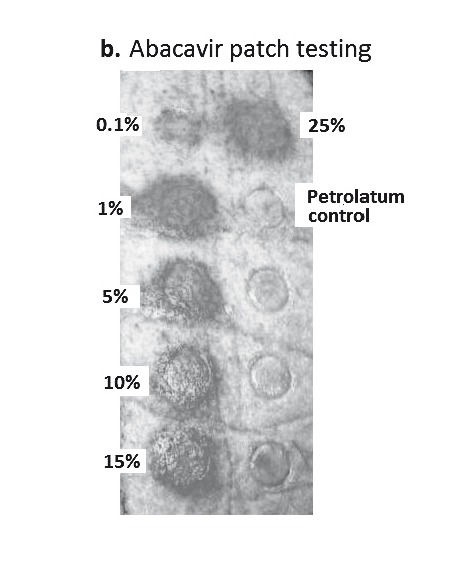
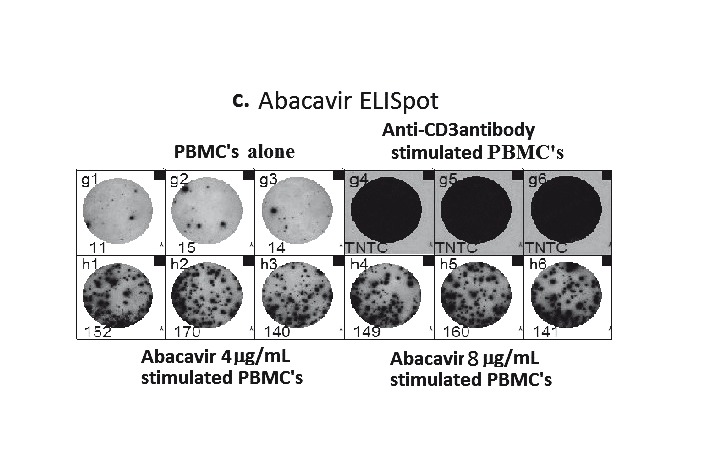
A. Proposed mechanism of positive patch test in patients previously systemically exposed. (i) After sensitisation and immunological priming, (ii) the patch test requires abacavir from the patch to penetrate into the skin (epidermal layer). (iii) Abacavir is then metabolised into a reactive metabolite, by the enzyme alcohol dehydrogenase (ADH). (iv) The metabolite then conjugates with host proteins to form an antigen. (v) Once recognised by MHC expressing, antigen-presenting epidermal Langerhans cells (LCs), they are processed and presented to effector cells of the immune system. (vi)This in turn triggers peptide-specific CD8+ T-cells to migrate into the skin, proliferate, and release proinflammatory cytokines and chemokines causing a localised response. B. Abacavir patch test with positive patch tests in an HLA-B*5701 positive patient 2 months following abacavir HSS. Documented with increasing concentrations from 0.1% to 25% w/w (abacavir/petrolatum) and negative petrolatum control (below the 25% abacavir test patch square). C. ABC-induced IFN-γ responses by ELISpot assay (shown as spot forming units (SFU)/200,000). Clockwise from top left - Incubation of PBMCs from an HLA-B*5701 positive patient 72 months following abacavir HSS. In 4 and 8 µg/mL of abacavir (pharmacologically relevant concentrations) produces INF-γ responses in the form of (blue) spots; PBMCs only, no INF-γ responses were detected; and lastly the addition of anti-CD3 antibodies to PBMCs acts as a positive control.
In the case of fixed drug eruption where resident CD8+ T cells may be important in the immunopathogenesis, the patch needs to be applied over the exact location where the reaction previously occurred. In drug induced SJS/TEN cases however, positive patch tests results are rare, depending highly on the drug being tested, associated allele and the patient group being tested.69
Intradermal testing with delayed readings has also been studied in the diagnosis of delayed cutaneous drug hypersensitivity syndromes and in some studies has shown a higher sensitivity than patch testing.70 This type of testing is generally limited to drugs available parenterally and testing is conducted using the same methods as used for intradermal skin testing for immediate reactions except that the highest non-irritating concentration of the drug is used and readings are performed at 24 hours and later. Given the severity of some delayed cutaneous hypersensitivity reactions and the less than 100% sensitivity of patch and intradermal skin testing for delayed reactions, a negative patch test is usually not used as the basis for oral provocation with the implicated drug. Patch and intradermal testing, however, have the advantage of being able to be performed on multiple drugs simultaneously, and/or, on structurally related medications, that may have been given concurrently when the patient developed a delayed hypersensitivity reaction.
In Vitro (Ex Vivo)
Lymphocyte Transformation Testing (LTT)
LTT has been studied for over 20 years, mainly in the diagnosis of delayed drug hypersensitivity reactions (Table 3).28 Lymphocytes isolated from peripheral blood mononuclear cells (PBMCs) of a patient with a specific delayed HSR are cultured with pharmacologic concentrations of the drug in question. After 5–7 days the amount of incorporated 3H-thymidine is determined and the result is expressed as a stimulation index. Enhanced proliferative responses in the presence of a drug are interpreted as drug-specific T-cell sensitisation. The specificity of the LTT response has been confirmed in studies that have generated drug specific T-cell lines and clones from LTT culture. The LTT can be performed with a variety of different drugs and can produce positive results in different clinical manifestations of ADRs.71 Similar to patch testing, a positive LTT can support a clinical diagnosis and may help but can also help pinpoint the responsible drug if the patient is receiving several different medications at the time of the original clinical reaction.72 The sensitivity of the LTT varies widely between studies, is related to both the drug and phenotype of clinical reaction and is still considered a research based testing method.73 Some drugs may affect T-cell stimulation and transformation of lymphocytes by pharmacological and not immunological means. Prostaglandin E2 (PGE2), which has a suppressive effect on interleukin-2 (IL-2), is produced by macrophages in cell cultures. PGE2 inhibitors therefore reduce the suppression of IL-2 which leads to increased T-cell proliferation. Many drugs associated with delayed hypersensitivity reactions are oxidised in the liver by enzymes of the cytochrome P450 system and, if it is the metabolite that is important in mediating the drug reaction, these will not be detected by conventional LTT because of insufficient production of the metabolites in cell culture.71 False positives may also occur in patients showing enhanced proliferative response in LTT to drugs they have tolerated.74,75 Similar to patch testing, a negative result does not rule out any drug tested but a positive result in the correct clinical context provides useful information. The optimal time to perform a LTT is somewhat controversial with some studies suggesting, for certain clinical phenotypes, such as SJS/TEN, it should be performed within the first week after onset of symptoms and others suggesting that early testing is associated with false positives.73,75 For DRESS/DIHS, many agree that the LTT should be performed in the recovery period, five to six weeks after onset of cutaneous symptoms.73,75
ELISpot and Intracellular Cytokine Staining
Similar to LTT, the enzyme-linked immunospot (ELISpot) assay and intracellular cytokine staining (ICS) have been used in the research setting to study a variety of phenotypes of Type IV delayed HSRs (Table 3).76 Both ELISpot and ICS are ex vivo assays that are used to measure the production and release of a target cytokine(s) by a population of T-cells in relation to exposure to pharmacological concentrations of the suspected drug or drug metabolite. Although these assays provide a potentially promising and rapid means of examining ex vivo responses to drugs, they are currently research tools since like other in vivo and ex vivo tests used for drug hypersensitivity, their sensitivity is less than 100% and the expected durability of responses in relation to the original drug reaction is unknown for most drugs and clinical phenotypes of delayed drug reactions. The ELISpot method involves adding PBMCs to a filter-bottom 96-well plate pre-coated with a specific anti-cytokine antibody and the drug in question. The anti-cytokine antibody used depends greatly on the cytokine being detected, which in essence serves as an immunocorrelate for the T-cell response under analysis, e.g. examining CD8+ T-cell mediated reactions, such as abacavir HSR, the cytokine output measurement of choice is interferon-gamma (INF-γ).76–78 Positive drug-free controls, include anti-CD3 antibodies which can stimulate the release of a variety of T cell cytokines, including INF-γ, and/or, a multiple peptide construct derived from a variety of cytomegalovirus (CMV), Epstein-Bar Virus (EBV) and influenza virus strains. The HLA class I restricted epitopes in the CMV-EBV-FLU (CEF) control are designed to release IFN-γ from CD8+ T cells in individuals with certain highly frequent HLA types. Negative drug-free controls are also used to ascertain the effect of background immune activation. The plate is then cultured for 24–48 hours to allow cytokine secretion which are captured by the anti-cytokine antibodies. Following incubation, the cells are washed off, detector antibody is added followed by an enzyme substrate. The cytokine-secreting cells are then identified as spots of secreted cytokine.76,78Figure 2c illustrates the abacavir-induced IFN-γ responses by ELISpot assay, with responses seen in the form of (blue) spots in the PBMCs stimulated with the pharmacological significant concentration of 4 μg/mL of abacavir (top left). Nothing is seen in the negative control of PBMCs only (top right) and responses seen in the positive control of anti-CD3 antibody stimulated PBMCs (bottom).
ICS by flow cytometry allows for the analysis of individual cells in a mixed population. ICS relies upon the stimulation of T-cells in the presence of an inhibitor of protein transport, in order to retain the cytokines inside the cell. Cells are first stimulated with pharmacological concentrations of the drug in question. An inhibitor of protein transport is added to prevent cytokines leaving the cell. After washing the cells, membranes are made porous or permeable and anti-cytokine antibodies are added. Cells producing a cytokine are measured using fluorescent labelled ntibodies. ICS by flow cytometry incorporates laser-based, biophysical technology to detect biomarkers and protein expression. Each fluorophore has a characteristic peak excitation and emission wavelength. By attaching different fluorophores to several different antibodies, a single assay can be designed to target multiply proteins of interest, in either an assay to identify cell surface proteins of interest, or to assess the production of several cytokines within target cells.
For SJS/TEN, measurement of other outputs in ex vivo tests such as granulysin may be useful. Granulysin is a 15-kDa cytotoxic protein excreted by CD8+ T cells and CD56+ NK cells that is now thought to be essential in the immunopathogenesis of SJS/TEN. It is also found in the blister fluid during the acute reaction which has quantitatively been associated with SJS/TEN disease severity and it is a marker for NK and T-cell activation.78 Granulysin may be helpful in differential diagnosis of bullous skin diseases, as granulysin is highly elevated in SJS/TEN, distinctively separating it from other bullous skin diseases like bullous pemphigoid. The percentages of apoptotic target cells can be assessed by fluorescence microscopy or flow cytometry.79 Currently, an immunochromatographic test for serum granulysin has shown predictability in the early stages of SJS/TEN 2–4 days prior to typical mucosal and cutaneous symptoms.79
Impact of Screening and Diagnostic Testing for Immediate and Delayed Drug HSRs
Immediate Drug HSRs
Combinations of testing approaches, including prick and intradermal skin testing and oral provocation, are useful in the clinic for clarification of the nature of the drug reaction, and in particular, for either clarifying or removing the label of an immediate drug reaction and giving the patient specific information on what drugs and classes of drug can be safely taken in the future (Table 3). Clinical studies have reinforced that most patients labelled with an immediate drug allergy can tolerate the drug or class of drugs in question. The most common reason for this would be that the initial symptoms were not IgE mediated but it is also known that the propensity to mount an IgE mediated allergic reaction is not permanent and that there is significant attrition with time. For instance, approximately 10% of patients per year will lose skin test reactivity to penicillin and it is estimated that 80% of patients over 5 years will have lost skin test reactivity to amoxicillin. A label of drug allergy can be associated with fewer therapeutic options which may translate into the need to use more expensive and toxic drugs. This has been exemplified in the case of penicillin allergy where use of less effective antibiotics and adverse clinical outcomes may result. In the case that a patient has a positive skin test, a MedicAlert can be issued and specific information given with regards to which drugs or class of drugs can be taken in the future. If the drug is necessary and alternative therapeutic options lacking, patients with positive skin tests suggestive of an IgE mediated reaction can still receive the drug in question through a procedure called rapid oral or intravenous desensitisation whereby increasing doses of the drug are administered over several hours. The exact mechanism by which desensitisation works is unknown, but once successful, the procedure must be repeated every time the drug is ceased or dosing is interrupted.
HLA Screening for Delayed Drug Hypersensitivity Reactions
The utility of HLA screening to predict and prevent specific drug hypersensitivity syndromes is most clearly highlighted by the abacavir example which has provided the practical roadmap of how discovery of a specific pharmacogenetic test can fully traverse the T1→T4 translational pathway into routine use in HIV clinical practice (Figure 1).54 Abacavir is a guanosine analogue reverse transcriptase inhibitor currently used in combination therapy of HIV. The major treatment limiting side effect of abacavir, identified in the pre-marketing phase of drug development, was a drug hypersensitivity syndrome characterised by fever, malaise and in 70% of patients, a cutaneous rash. Abacavir HSR occurred in 5–8% of predominantly Caucasian populations, starting the drug an average of 8 days into abacavir treatment with rapid reversal of symptoms within 72 hours of drug discontinuation; however rechallenge was reported to result in hypotension, shock and even death.62,63 A genetic link to abacavir hypersensitivity was first highlighted by the observation that HSS occurred at a lowered frequency within Asian and African ethnic populations and one report of familial association.63,80 The discovery of a strong association between HLA-B*5701 and abacavir HSS by two independent groups in 2002 raised hopes for translation into a screening test, however the identification of patients clinically diagnosed with abacavir HSS who did not carry HLA-B*5701 raised safety concerns. It was later determined that these cases represented false positive clinical diagnosis, which was further supported by randomised clinical trials demonstrating that abacavir HSS was diagnosed in 2–3% of those patients in the study arm not receiving abacavir. Skin patch testing was developed and used to identify patients with true immunologically-mediated abacavir HSS.80,81 A large randomised clinical study helped confirm the clinical utility of HLA-B*5701 screening to eliminate true immunologically mediated abacavir HSS, whilst other studies confirmed the generalisability of HLA-B*5701 testing across ethnicity as well as its effectiveness in clinical practice.54,80,82,83 In parallel to the clinical studies, a body of scientifically researched evidence converged to support abacavir HSS as an exclusively HLA-B*5701 restricted CD8+ T cell dependent reaction. Abacavir specific CD8+ T-cell responses can be reproduced in cell culture, using PBMCs derived from healthy donors who are HLA-B*5701 positive and abacavir naive.51,54,84 It could be determined from PREDICT-1 study that the positive predictive value of HLA-B*5701 for abacavir HSS was 55% (Table 5).57,85 Currently the mechanism by which 45% of HLA-B*5701 positive individuals would tolerate abacavir is not understood but from a safety and cost-effectiveness standpoint, it currently makes sense to exclude all HLA-B*5701 positive individuals from abacavir treatment. HLA-B*5701 screening is now routinely done prior to abacavir prescription and in most cases as part of routine baseline HIV care in most of the developing world and its use has been endorsed by all major HIV guideline bodies.
The translation of HLA-B*5701 from discovery to routine use to prevent abacavir HSS is a success story however there have been challenges and hurdles to the translation of other HLA alleles in routine screening (Table 3). In 2004, SJS/TEN associated with the aromatic amine anticonvulsant, carbamazepine, was found to be strongly associated with the HLA-B*1502 allele in the Han Chinese population where this allele has a prevalence of 10–15%.51,54 In Southeast Asian populations, studies suggest a 100% negative predictive value for HLA-B*1502 for carbamazepine associated SJS/TEN. However, unlike abacavir HSS and HLA-B*5701, this does not appear to generalise across ethnicity and in Caucasians where HLA-B*1502 carriage is rare (<1%) studies have suggested potential associations of other alleles with carbamazepine SJS/TEN such as HLA-A*3101.86–88 Currently the FDA has recommended routine HLA-B*1502 screening in high risk individuals of Southeast Asian origin. HLA-B*1502 has also been associated with SJS/TEN related to oxcarbazepine and phenytoin, an aromatic amine anticonvulsant, structurally associated with carbamazepine, and it would be recommended that all other aromatic amine anticonvulsants (phenytoin, phenobarbital, oxcarbazepine and probably lamotrigine) be avoided in those who have experienced carbamazepine associated SJS/TEN or who carry HLA-B*1502.89–92 In predominantly Caucasian populations, given the low prevalence of HLA-B*1502, it can be estimated that 10,000 or more individuals would need to be screened to prevent one case of SJS/TEN. In addition, the less than 100% negative predictive value of this allele for development of SJS/TEN in non-Southeast Asian populations, raises important safety considerations. The implications of the prevalence of an allele in a given population, the prevalence of the HSR disease in question, the positive predictive value of the specific HLA allele for the HSR disease and the impact on the number needed to test to prevent one case for common HLA HSR associations is shown in Table 5.57,85 More recent insights which may explain, in part, why most HLA-B*1502 positive individuals will tolerate carbamazepine was published by Ko et al. who examined the T-cell receptor repertoire in CD8+ T cells in patients with SJS/TEN associated with carbamazepine. They found that a dominant T-cell clone VB-11-ISGSY was present in 84% of patients with SJS/TEN but absent in 100% of carbamazepine tolerant patients.93
Current Research and Future Perspectives
It was previously postulated that most delayed hypersensitivity HSRs occurred through the hapten model whereby a small molecule such as a drug covalently bound to, and permanently altered a host protein which was then recognised through an immune response. Despite this widely accepted hapten model which was originally proposed several decades ago, antibodies to the parent drug and/or metabolite were never found for most drugs and Pichler proposed the pharmacological-interaction model suggesting that a drug could directly interact with the MHC and/or T-cell receptor. More recently, the altered peptide repertoire model was proposed and postulated, that for many drugs, there is rapid and non-covalent binding between the drug and HLA, with the drug occupying anchor sites within the antigen binding cleft and altering the repertoire of self-peptide ligands that can be bound and presented to T-cells. Thus the drug mediating the HSR, in essence, creates a novel HLA type, which in turn creates an allograft reaction.94,95 Current studies, recently published, suggest that the altered peptide repertoire model could explain the immunopathogenesis of abacavir HSS and carbamazepine SJS/TEN.95–97 Based on this model, the crystal structure of abacavir-HLA-B*5701-peptide has been solved and explains the exquisite specificity of HLA-B*5701 for abacavir and why other HLA alleles such as HLA-B*5801 and HLA-B*5703 which differ by only two amino acids in the HLA-B F binding pocket do not bind abacavir (Figure 3).95 This has created a very exciting and important area of future research, since understanding the structural and biochemical basis of how drugs interact with HLA molecules, the functional consequences, and the pathogenesis of the incomplete positive predictive value and varying clinical phenotypes, may provide a strategy to improve the safety and cost-effectiveness of drug development. In particular, this could provide a roadmap for the pre-clinical screening to inform the design of drugs which do not interact with HLA and hence would be unlikely to cause hypersensitivity reactions and exclude high risk drugs from development before use in man.
Figure 3.
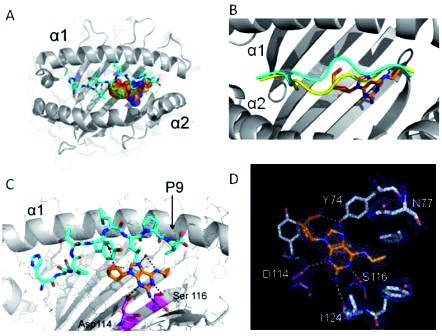
Crystal structure of the abacavir-MHC-peptide complex solved to resolution of 0.2 nm (nanometres). A. HLA-B*5701 is shown in gray and abacavir as spheres with orange for carbon, blue for nitrogen and red for oxygen and peptide V is shown in cyan. B. As per the altered peptide repertoire model drug binding influences the peptide backbone conformation by shifting the main chain (peptide bound in the absence of abacavir is shown in yellow and that bound to abacavir and HLA-B*5701 is shown in cyan). C. H-bond interactions (black dashes) between abacavir and the peptide and HLA-B*5701 are shown. The specific residues that distinguish abacavir sensitive HLA-B*5701 from abacavir insensitive HLA-B*5703 are shown in magenta (carbon), blue (nitrogen) and red (oxygen). D. Experimental electron density corresponding to abacavir in a Fo-Fc difference map with blue mesh showing the final 2Fo-Fc electron density map of abacavir in the antigen-binding cleft of HLA-B*5701 and showing H-bond interactions between abacavir and HLA-B*5701 as yellow dashed lines. Adapted from Ostrov et al.95
Conclusions
Type B or immunologically mediated drug reactions cause significant patient morbidity and mortality that cannot be predicted based on the pharmacological structure of the drug. Type I (IgE mediated, immediate) and Type IV (T-cell mediated, delayed) are the most common Type B reactions seen in clinical practice. Careful clinical history and phenotyping is the key to diagnosis and risk stratification of patients who have experienced potential Type I and Type IV allergic drug reactions and help guide choice of diagnostic tests, which in turn can be useful in identifying patients with true immunologically mediated drug reactions to a specific drug and providing important drug safety information. The gold standard for diagnostic evaluation of suspected Type I, IgE mediated, immediate drug allergy involves primary in vivo testing such as prick and intradermal skin testing with validated reagents and/or oral provocation. Type IV delayed HSRs are T-cell mediated with a variety of effector cell phenotypes and involve a spectrum of clinical severity from mild skin reactions without systemic features to more severe and potentially fatal cutaneous disease often with multisystem involvement. In vivo tests such as patch testing and intradermal testing with delayed readings and ex vivo research tests such as LTT and ELISpot are specific tests that have been useful to define patients with true immunologically mediated delayed reactions to a specific drug. However, the diagnostic sensitivity of these tests is significantly less than 100%, and given the severity of these reactions, patients are typically advised to avoid a specific drug, class of drugs or multiple drugs that might be implicated by clinical history, even if diagnostic testing is negative. More recently, several Type IV reactions such as abacavir HSS, DILI, DRESS/DIHS and SJS/TEN, have shown strong associations with specific HLA class I and II alleles. In the case of the association with abacavir and HLA-B*5701 this has led to the translation of HLA-B*5701 as a screening test to exclude positive patients from treatment with abacavir. Inexpensive DNA-based molecular tests that could be incorporated into routine diagnostic laboratories were instrumental in this translation. Recent research implicates the altered peptide model in the immunopathogenesis of abacavir HSS and carbamazepine SJS/TEN, and future research is focusing on defining specific interactions between HLA and drugs that could help define pre-clinical strategies to develop safer drugs.
Footnotes
Competing Interests: CR and JB declare no competing interests. EP has received honaria/expenses from Merck Pty Ltd, ViiV Healthcare, Pfizer, and Janssen-Cilag, and Gilead Sciences.
References
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Phillips E, Mallal S. HLA and Disease. In: Mehra N, editor. The HLA Complex in Biology and Medicine: A Resource Book. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2010. pp. 333–49. [Google Scholar]
- 3.Pichler WJ. Drug Hypersensitivity Reactions: Classification and Relationship to T-Cell Activation. In: Pichler W, editor. Drug Hypersensitivity. 1st ed. Basel, Switzerland: S Karger Pub; 2007. pp. 168–89. [Google Scholar]
- 4.Pichler WJ, Adam J, Daubner B, Gentinetta T, Keller M, Yerly D. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. Med Clin North Am. 2010;94:645–64. xv. doi: 10.1016/j.mcna.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Gell PGH, Coombs RRA. The classification of allergic reactions underlying disease. In: Gell PGH, Coombs RRA, editors. Clinical Aspects of Immunology. 2nd ed. Oxford: Black well Scientific; 1963. pp. 317–37. [Google Scholar]
- 6.Bircher A, Scherer Hofmeier K. Drug hypersensitivity reactions: Inconsistency in the use of the classification of immediate and nonimmediate reactions. J Allergy Clin Immunol. 2012;129:263–4. doi: 10.1016/j.jaci.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Uzzaman A, Cho SH. Chapter 28: Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33(Suppl 1):S96–9. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 8.Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84:268–72. doi: 10.4065/84.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldo BA, Pham NH. Histamine-releasing and allergenic properties of opioid analgesic drugs: resolving the two. Anaesth Intensive Care. 2012;40:216–35. doi: 10.1177/0310057X1204000204. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA and GA2LEN/HANNA. Allergy. 2011;66:818–29. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 11.Kränke B, Aberer W. Skin testing for IgE-mediated drug allergy. Immunol Allergy Clin North Am. 2009;29:503–16. doi: 10.1016/j.iac.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Liccardi G, D’Amato G, Canonica GW, Salzillo A, Piccolo A, Passalacqua G. Systemic reactions from skin testing: literature review. J Investig Allergol Clin Immunol. 2006;16:75–8. [PubMed] [Google Scholar]
- 13.Brockow K, Ring J. Anaphylaxis to radiographic contrast media. Curr Opin Allergy Clin Immunol. 2011;11:326–31. doi: 10.1097/ACI.0b013e32834877c3. [DOI] [PubMed] [Google Scholar]
- 14.Sogn DD, Evans R, 3rd, Shepherd GM, Casale TB, Condemi J, Greenberger PA, et al. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152:1025–32. [PubMed] [Google Scholar]
- 15.Fox S, Park MA. Penicillin skin testing in the evaluation and management of penicillin allergy. Ann Allergy Asthma Immunol. 2011;106:1–7. doi: 10.1016/j.anai.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Torres MJ, Blanca M. The complex clinical picture of beta-lactam hypersensitivity: penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med Clin North Am. 2010;94:805–20. xii. doi: 10.1016/j.mcna.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Greenberger PA. Chapter 30: Drug allergy. Allergy Asthma Proc. 2012;33(Suppl 1):S103–7. doi: 10.2500/aap.2012.33.3563. [DOI] [PubMed] [Google Scholar]
- 18.Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126:994–9. doi: 10.1016/j.jaci.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 19.Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64:183–93. doi: 10.1111/j.1398-9995.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 20.Mertes PM, Aimone-Gastin I, Guéant-Rodriguez RM, Mouton-Faivre C, Audibert G, O’Brien J, et al. Hypersensitivity reactions to neuromuscular blocking agents. Curr Pharm Des. 2008;14:2809–25. doi: 10.2174/138161208786369704. [DOI] [PubMed] [Google Scholar]
- 21.Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(Suppl 2):S126–37. doi: 10.1016/j.jaci.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273–9. doi: 10.1097/ACI.0b013e32833b1f34. [DOI] [PubMed] [Google Scholar]
- 23.Messaad D, Sahla H, Benahmed S, Godard P, Bousquet J, Demoly P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann Intern Med. 2004;140:1001–6. doi: 10.7326/0003-4819-140-12-200406150-00009. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet PJ, Gaeta F, Bousquet-Rouanet L, Lefrant JY, Demoly P, Romano A. Provocation tests in diagnosing drug hypersensitivity. Curr Pharm Des. 2008;14:2792–802. doi: 10.2174/138161208786369731. [DOI] [PubMed] [Google Scholar]
- 25.Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. European Network for Drug Allergy (ENDA) EAACI interest group on drug hypersensitivity Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–63. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 26.Rerkpattanapipat T, Chiriac AM, Demoly P. Drug provocation tests in hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2011;11:299–304. doi: 10.1097/ACI.0b013e328348a4e9. [DOI] [PubMed] [Google Scholar]
- 27.Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate β-lactam allergy. Allergy. 2007;62:47–52. doi: 10.1111/j.1398-9995.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 28.Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012;4:251–63. doi: 10.4168/aair.2012.4.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Mayorga C, Sanz ML, Gamboa PM, García BE, Caballero MT, García JM, et al. Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol. 2010;20:103–9. [PubMed] [Google Scholar]
- 31.Guéant JL, Guéant-Rodriguez RM, Viola M, Valluzzi RL, Romano A. IgE-mediated hypersensitivity to cephalosporins. Curr Pharm Des. 2006;12:3335–45. doi: 10.2174/138161206778194060. [DOI] [PubMed] [Google Scholar]
- 32.Leysen J, Sabato V, Verweij MM, De Knop KJ, Bridts CH, De Clerck LS, et al. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev Clin Immunol. 2011;7:349–55. doi: 10.1586/eci.11.14. [DOI] [PubMed] [Google Scholar]
- 33.Hausmann OV, Gentinetta T, Bridts CH, Ebo DG. The basophil activation test in immediate-type drug allergy. Immunol Allergy Clin North Am. 2009;29:555–66. doi: 10.1016/j.iac.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–9. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Hunziker T, Künzi UP, Braunschweig S, Zehnder D, Hoigné R. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388–93. doi: 10.1111/j.1398-9995.1997.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee A, Thomson J. Drug-induced skin reactions. In: Lee A, editor. Adverse Drug Reactions. 2nd ed. London, UK: Pharmaceutical Press; 2006. pp. 126–55. [Google Scholar]
- 37.Pellicano R, Lomuto M, Ciavarella G, Di Giorgio G, Gasparini P. Fixed drug eruptions with feprazone are linked to HLA-B22. J Am Acad Dermatol. 1997;36:782–4. doi: 10.1016/s0190-9622(97)80347-7. [DOI] [PubMed] [Google Scholar]
- 38.Ozkaya-Bayazit E, Akar U. Fixed drug eruption induced by trimethoprim-sulfamethoxazole: evidence for a link to HLA-A30 B13 Cw6 haplotype. J Am Acad Dermatol. 2001;45:712–7. doi: 10.1067/mjd.2001.117854. [DOI] [PubMed] [Google Scholar]
- 39.Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions--a 20-year review. Contact Dermatitis. 2011;65:195–201. doi: 10.1111/j.1600-0536.2011.01946.x. [DOI] [PubMed] [Google Scholar]
- 40.Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316–21. doi: 10.1097/ACI.0b013e32832cda4c. [DOI] [PubMed] [Google Scholar]
- 41.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–99. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 42.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36:6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- 43.Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–9. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- 44.Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed] [Google Scholar]
- 45.Gupta A, Eggo MC, Uetrecht JP, Cribb AE, Daneman D, Rieder MJ, et al. Drug-induced hypothyroidism: the thyroid as a target organ in hypersensitivity reactions to anticonvulsants and sulfonamides. Clin Pharmacol Ther. 1992;51:56–67. doi: 10.1038/clpt.1992.8. [DOI] [PubMed] [Google Scholar]
- 46.Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- 47.Kano Y, Shiohara T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin North Am. 2009;29:481–501. doi: 10.1016/j.iac.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 49.Solensky R, Khan DA, Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlos R, Phillips EJ. Individualization of antiretroviral therapy. Pharmacogenomics Pers Med. 2012;5:1–17. doi: 10.2147/PGPM.S15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau J-C. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 53.Beylot C, Doutre M-S, Beylot-Barry M. Acute generalized exanthematous pustulosis. Semin Cutan Med Surg. 1996;15:244–9. doi: 10.1016/s1085-5629(96)80037-x. [DOI] [PubMed] [Google Scholar]
- 54.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–306. doi: 10.2217/pgs.12.108. [DOI] [PubMed] [Google Scholar]
- 55.Bodmer WF, Bodmer JG. Evolution and function of the HLA system. Br Med Bull. 1978;34:309–16. doi: 10.1093/oxfordjournals.bmb.a071518. [DOI] [PubMed] [Google Scholar]
- 56.Stenzel A, Lu T, Koch WA, Hampe J, Guenther SM, De La Vega FM, et al. Patterns of linkage disequilibrium in the MHC region on human chromosome 6p. Hum Genet. 2004;114:377–85. doi: 10.1007/s00439-003-1075-5. [DOI] [PubMed] [Google Scholar]
- 57.Phillips EJ, Keane N, Blyth C. Both HLA class I restricted CD8+ and class II restricted CD4+ T Cells are implicated in the pathogenesis of nevirapine hypersensitivity. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011. p. H1-1399c. [DOI] [PubMed] [Google Scholar]
- 58.Dunckley H. HLA typing by SSO and SSP methods. Methods Mol Biol. 2012;882:9–25. doi: 10.1007/978-1-61779-842-9_2. [DOI] [PubMed] [Google Scholar]
- 59.Little AM. An Overview of HLA Typing for Hematopoietic Stem Cell Transplantation. In: Beksac M, editor. Methods in Molecular Medicine: Bone Marrow and Stem Cell Transplantation. Totowa, NJ: Humana Press; 2007. pp. 35–49. [DOI] [PubMed] [Google Scholar]
- 60.Hammond E, Mamotte C, Nolan D, Mallal S. HLA-B*5701 typing: evaluation of an allele-specific polymerase chain reaction melting assay. Tissue Antigens. 2007;70:58–61. doi: 10.1111/j.1399-0039.2007.00840.x. [DOI] [PubMed] [Google Scholar]
- 61.Profaizer T, Eckels D. HLA alleles and drug hypersensitivity reactions. Int J Immunogenet. 2012;39:99–105. doi: 10.1111/j.1744-313X.2011.01061.x. [DOI] [PubMed] [Google Scholar]
- 62.Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11:973–87. doi: 10.2217/pgs.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nolan D, Thorborn D, Schaefer M, Laird R, Rauch A, Montaner J, et al. Genetic factors predicting abacavir hypersensitivity and tolerance in HLA-B*5701 positive individuals. Eur J Dermatol. 2008;18:247. [Google Scholar]
- 64.Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dumé L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234–6. doi: 10.1111/j.1600-0536.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 65.Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254–7. doi: 10.1111/j.1600-0536.1993.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 66.Osawa J, Naito S, Aihara M, Kitamura K, Ikezawa Z, Nakajima H. Evaluation of skin test reactions in patients with non-immediate type drug eruptions. J Dermatol. 1990;17:235–9. doi: 10.1111/j.1346-8138.1990.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 67.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–5. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi N, Bauer TW, Togawa D, Lieberman IH, Sakai H, Fujishiro T, et al. A molecular gram stain using broad range PCR and pyrosequencing technology: a potentially useful tool for diagnosing orthopaedic infections. Diagn Mol Pathol. 2005;14:83–9. doi: 10.1097/01.pas.0000162753.38284.1a. [DOI] [PubMed] [Google Scholar]
- 69.Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254–7. doi: 10.1111/j.1600-0536.1993.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 70.Barbaud A, Reichert-Penetrat S, Tréchot P, Jacquin-Petit MA, Ehlinger A, Noirez V, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49–58. doi: 10.1046/j.1365-2133.1998.02313.x. [DOI] [PubMed] [Google Scholar]
- 71.Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80–90. [PubMed] [Google Scholar]
- 72.Rozieres A, Hennino A, Rodet K, Gutowski M-C, Gunera-Saad N, Berard F, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 73.Nagao-Dias AT, Teixeira FM, Coelho HL. Diagnosing immune-mediated reactions to drugs. Allergol Immunopathol (Madr) 2009;37:98–104. doi: 10.1016/s0301-0546(09)71112-7. [DOI] [PubMed] [Google Scholar]
- 74.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–20. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 75.Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–44. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 76.Scheibenbogen C, Lee K-H, Stevanovic S, Witzens M, Willhauck M, Waldmann V, et al. Analysis of the T cell response to tumor and viral peptide antigens by an IFNgamma-ELISPOT assay. Int J Cancer. 1997;71:932–6. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 77.McCutcheon M, Wehner N, Wensky A, Kushner M, Doan S, Hsiao L, et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods. 1997;210:149–66. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 78.Scheibenbogen C, Romero P, Rivoltini L, Herr W, Schmittel A, Cerottini J-C, et al. Quantitation of antigen-reactive T cells in peripheral blood by IFNgamma-ELISPOT assay and chromium-release assay: a four-centre comparative trial. J Immunol Methods. 2000;244:81–9. doi: 10.1016/s0022-1759(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 79.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 80.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–32. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 81.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–2. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 82.Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, et al. Study of Hypersensitivity to Abacavir and Pharmacogenetic Evaluation Study Team High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–8. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 83.Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006;43:99–102. doi: 10.1086/504874. [DOI] [PubMed] [Google Scholar]
- 84.Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822–32. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 85.Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011;127(Suppl):S60–6. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SH, Lee KW, Song WJ, Kim SH, Jee YK, Lee SM, et al. Adverse Drug Reaction Research Group in Korea Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 87.Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20:1034–41. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- 88.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillips EJ, Chung WH, Mockenhaupt M, Roujeau J-C, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011;127:S60–6. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin LC, Lai PC, Yang SF, Yang RC. Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. Kaohsiung J Med Sci. 2009;25:82–6. doi: 10.1016/S1607-551X(09)70045-2. [DOI] [PubMed] [Google Scholar]
- 91.Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol. 2009;75:579–82. doi: 10.4103/0378-6323.57718. [DOI] [PubMed] [Google Scholar]
- 92.Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48:1015–8. doi: 10.1111/j.1528-1167.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 93.Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128:1266–76. e11. doi: 10.1016/j.jaci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 94.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–8. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 95.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109:9959–64. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–8. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 97.Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57:01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012;26:F21–9. doi: 10.1097/QAD.0b013e328355fe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan SH, Tan T. HLA and allopurinol drug eruption. Dermatologica. 1989;179:32–3. doi: 10.1159/000248097. [DOI] [PubMed] [Google Scholar]
- 99.Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 101.Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Adverse Drug Reaction Research Group in Korea Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–7. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 102.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. RegiSCAR study group A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 103.Dainichi T, Uchi H, Moroi Y, Furue M. Stevens-Johnson syndrome, drug-induced hypersensitivity syndrome and toxic epidermal necrolysis caused by allopurinol in patients with a common HLA allele: what causes the diversity? Dermatology. 2007;215:86–8. doi: 10.1159/000102045. [DOI] [PubMed] [Google Scholar]
- 104.Wang Q, Zhou JQ, Zhou LM, Chen ZY, Fang ZY, Chen SD, et al. Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure. 2011;20:446–8. doi: 10.1016/j.seizure.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 106.Kulkantrakorn K, Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Prabmechai N, Vannaprasaht S, et al. HLA-B*1502 strongly predicts carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Thai patients with neuropathic pain. Pain Pract. 2012;12:202–8. doi: 10.1111/j.1533-2500.2011.00479.x. [DOI] [PubMed] [Google Scholar]
- 107.Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Chen P, Lin SY, Chen WH, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51:926–30. doi: 10.1111/j.1528-1167.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 108.Then SM, Rani ZZ, Raymond AA, Ratnaningrum S, Jamal R. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol. 2011;29:290–3. [PubMed] [Google Scholar]
- 109.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. JSAR research group HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51:2461–5. doi: 10.1111/j.1528-1167.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 110.Ikeda H, Takahashi Y, Yamazaki E, Fujiwara T, Kaniwa N, Saito Y, et al. HLA class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia. 2010;51:297–300. doi: 10.1111/j.1528-1167.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 111.Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11:349–56. doi: 10.2217/pgs.09.162. [DOI] [PubMed] [Google Scholar]
- 112.Roujeau JC, Huynh TN, Bracq C, Guillaume JC, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987;123:1171–3. [PubMed] [Google Scholar]
- 113.Kim SH, Kim M, Lee KW, Kim SH, Kang HR, Park HW, et al. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics. 2010;11:879–84. doi: 10.2217/pgs.10.54. [DOI] [PubMed] [Google Scholar]
- 114.Martin AM, Nolan D, James I, Cameron P, Keller J, Moore C, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–9. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 115.Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, et al. Nevirapine Toxicogenomics Study Team Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–80. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phillips E, Bartlett J, Sanne I, Lederman M, Hinkle J, Rousseau F, et al. Associations between HLA-DRB1*0102, HLA-B*5801 and Hepatotoxicity in Patients who Initiated Nevirapine Containing Regimens in South Africa. 18th Conference on Retroviruses and Opportunistic Infections; MA, USA. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gatanaga H, Yazaki H, Tanuma J, Honda M, Genka I, Teruya K, et al. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007;21:264–5. doi: 10.1097/QAD.0b013e32801199d9. [DOI] [PubMed] [Google Scholar]
- 118.Littera R, Carcassi C, Masala A, Piano P, Serra P, Ortu F, et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–6. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- 119.Gao S, Gui XE, Liang K, Liu Z, Hu J, Dong B. HLA-dependent hypersensitivity reaction to nevirapine in Chinese Han HIV-infected patients. AIDS Res Hum Retroviruses. 2012;28:540–3. doi: 10.1089/AID.2011.0107. [DOI] [PubMed] [Google Scholar]
- 120.Alfirevic A, Jorgensen AL, Williamson PR, Chadwick DW, Park BK, Pirmohamed M. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics. 2006;7:813–8. doi: 10.2217/14622416.7.6.813. [DOI] [PubMed] [Google Scholar]
- 121.Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–1. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 122.Likanonsakul S, Rattanatham T, Feangvad S, Uttayamakul S, Prasithsirikul W, Tunthanathip P, et al. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res Ther. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–46. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 124.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, et al. Genome-wide association study identifies variations in 6p21.3 associated with nevirapine-induced rash. Clin Infect Dis. 2011;53:341–8. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 125.Romano A, De Santis A, Romito A, Di Fonso M, Venuti A, Gasbarrini GB, et al. Delayed hypersensitivity to aminopenicillins is related to major histocompatibility complex genes. Ann Allergy Asthma Immunol. 1998;80:433–7. doi: 10.1016/s1081-1206(10)62997-3. [DOI] [PubMed] [Google Scholar]
- 126.Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 127.Hu FY, Wu XT, An DM, Yan B, Stefan H, Zhou D. Phenytoin-induced Stevens-Johnson syndrome with negative HLA-B*1502 allele in mainland China: two cases. Seizure. 2011;20:431–2. doi: 10.1016/j.seizure.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Pellicano R, Ciavarella G, Lomuto M, Di Giorgio G. Genetic susceptibility to fixed drug eruption: evidence for a link with HLA-B22. J Am Acad Dermatol. 1994;30:52–4. doi: 10.1016/s0190-9622(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 129.Hautekeete ML, Horsmans Y, Van Waeyenberge CD, Demanet C, Henrion J, Verbist L, et al. HLA association of amoxicillin-clavulanate—induced hepatitis. Gastroenterology. 1999;117:1181–6. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 130.Lucena MI, Molokhia M, Shen YU, Urban TJ, Aithal GP, Andrade RJ, et al. Spanish DILI Registry. EUDRAGENE. DILIN. DILIGEN. International SAEC Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–47. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–20. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010;42:711–4. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- 133.Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008;8:186–95. doi: 10.1038/sj.tpj.6500458. [DOI] [PubMed] [Google Scholar]
- 134.Daly AK, Aithal GP, Leathart JB, Swainsbury RA, Dang TS, Day CP. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology. 2007;132:272–81. doi: 10.1053/j.gastro.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 135.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009;29:400–11. doi: 10.1055/s-0029-1240009. [DOI] [PubMed] [Google Scholar]
- 136.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Drug Metab Rev. 2012;44:116–26. doi: 10.3109/03602532.2011.605790. [DOI] [PubMed] [Google Scholar]
- 137.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, et al. DILIGEN Study. International SAE Consortium HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–9. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 138.Spraggs CF, Budde LR, Briley LP, Bing N, Cox CJ, King KS, et al. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011;29:667–73. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 139.Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(Suppl):S21–3. doi: 10.1016/j.jaad.2006.02.032. [DOI] [PubMed] [Google Scholar]


