Abstract.
Sensitive imaging techniques for small animals are needed to assess drug toxicity in preclinical studies. Optical coherence tomography (OCT) provides a noninvasive tool for high-resolution, depth-resolved visualization of drug-induced changes in tissue morphology. In a mouse model, we utilize OCT to assess vaginal tissue integrity following the application of topical microbicides (drugs used to prevent infection). Mice are challenged with herpes simplex virus-2 (HSV-2) to determine the correlation of tissue damage as quantified by OCT to increased susceptibility. The microbicide benzalkonium chloride (BZK) (0.02, 0.2, or 2%) or phosphate buffered saline control is administered intravaginally. In vivo OCT imaging and collection of tissue samples are performed after treatment. A quantitative OCT scoring system is applied to assess epithelial damage, and the results are compared with those of histology. A separate group of mice are treated similarly then challenged with HSV-2. Epithelial morphology quantified noninvasively by OCT and histology are dose-dependent (). The OCT scoring system detected a significant increase in epithelial damage with increasing BZK concentration (). These results paralleled an increase in HSV-2 susceptibility (). OCT can be used as a noninvasive tool to assess topical drug toxicity in a small animal model with potential to predict increased susceptibility to vaginal infection.
Keywords: optical coherence tomography, microbicides, mouse, benzalkonium chloride, drug, toxicity, vaginal tissue, damage, safety
1. Background
Sexually transmitted diseases (STDs), including AIDS, are global public health problems. In the United States alone, the Centers for Disease Control and Prevention (CDC) estimates that there are 19 million Americans infected each year by an STD pathogen.1 In addition, the number of HIV-infected people currently exceeds 30 million worldwide.2 Prevention strategies are important, including topical microbicides that can be used before or shortly after intercourse for prevention of STDs.3,4 There are promising candidates, such as tenofovir gel; however, there have been variable findings in protection from HIV acquisition in two separate studies using this gel vaginally (VOICE, CAPRISA), with reasons for this disparity not completely clear. It is well-known that there is an association between Nonoxynol-9 (N-9), a commonly used contraceptive spermicide, with induced vaginal lesions and increased HIV incidence. This demonstrates the complexity of developing safe and effective vaginally applied microbicides5 and underscores the importance of improving prescreening techniques and assays to determine microbicide safety profiles.6,7
To address the lack of reliable and sensitive methods to assess vaginal safety, we have applied high-resolution imaging methods and shown that they have increased sensitivity compared with standard white light (colposcopy) imaging in humans, sheep, and macaques, with the added benefit of noninvasive evaluation as compared to tissue biopsy.8–10 Optical coherence tomography (OCT) is a technique well suited for examination of superficial mucosal layers and close underlying stromal structures of tissues such as the vagina, cervix, and rectal epithelium,11–13 all of which may be exposed to microbicide products. Although we have previously shown that optical imaging can be used to detect microbicide-induced changes in these tissues, including thinning and disruption of the epithelial layer, the correlation of these image-based parameters to biological endpoints, such as susceptibility to pathogens and vaginal infection, has not been demonstrated.
Our previous work demonstrated that mice have increased susceptibility to intravaginal herpes simplex virus-2 (HSV-2) challenge within 16 h post-treatment with 0.2 and 2%, but not 0.02% benzalkonium chloride (BZK).14 In addition, our group has previously utilized colposcopy and high-resolution methods in the mouse model.14,15 The changes were induced by a single application of BZK14 or multiple applications of N-915 that were not readily apparent by colposcopic imaging. Thus noninvasive imaging techniques such as OCT, which can assess in-depth tissue integrity and morphology without invasive biopsy, would be a valuable asset to safety studies in both preclinical animal models and human clinical studies.
In this study, our objective was to determine the utility of OCT to detect structural and morphological changes induced in tissue following topical applications of vaginal agents. A secondary objective was to compare OCT findings to the biological endpoint of HSV-2 susceptibility. The mouse HSV-2 model is a well-documented in vivo model used extensively to assess microbicide toxicity and effects on pathogen susceptibility.16 The current study incorporates the high resolution imaging method of OCT to assess vaginal changes in a mouse model after treatment with BZK, a microbicide known to cause epithelial disruption in sheep,9 pigs,17 and increased susceptibility to HSV-2 in mice.18
2. Materials and Methods
2.1. Animal Model and Microbicide Treatments
The UTMB Institutional Animal Care and Use Committee approved all animal studies conforming to PHS Policy on Humane Care and Use of Laboratory Animals (OLAW) guidelines. Female Swiss-Webster mice () of 6 to 10 weeks of age (Harlen Spraque-Dawley, Houston, TX) were housed in an animal vivarium and allowed to acclimate for at least one week prior to enrollment in experimental procedures. Animals were housed five to a cage with a light/dark cycle of with food and water ad libitum. A total of 47 mice were used for OCT imaging, and 120 mice were used for genital herpes susceptibility testing. No mice were lost during experimental procedures, and thus no data points or measurements were excluded.
Medroxyprogesterone Acetate (Depo-provera™, Upjohn, Kalamazoo, MI) is a long-acting progestin hormone that temporarily stops the natural, four- to five-day mouse estrous cycle, and thins the vaginal epithelium to only two to four cell layers in thickness.16 Progestin-treated mice have increased susceptibility to infection with intravaginally administered STD pathogens, such as HSV-2 and Chlamydia trachomatis.16,19 Three nonhormone-treated mice were examined to evaluate imaging and histological differences between naturally cycling and progestin-thinned cervicovaginal epithelial tissues. All other mice enrolled in the study were subcutaneously injected with of Depo-provera™ seven days prior to commencement of experiments.16,18,20 One day prior to imaging, the vaginal tracts were swabbed using a wet alginate cotton-tipped swab to ensure that the tract was open and free of a mucous plug. Animals were randomly selected and given a single application of 25 μl of microbicide or control solution by intravaginal administration using a positive displacement pipette (Gilson Microman, Fisher Scientific, Pittsburgh, PA). Sixteen hours post-microbicide treatment the animals underwent OCT imaging by a technician masked to treatment. Animals were anesthetized with sodium pentobarbital () and placed in a supine position for OCT imaging. The vaginal tract was flushed with 1.5 ml phosphate buffered saline (PBS) using a 3-French (1 mm) silicone catheter (Solocath™, Harvard Apparatus, Holliston, MA) attached to a 3 cc syringe.
The microbicides evaluated were three aqueous concentrations of BZK (0.02, 0.2, or 2%) in 0.9% PBS. The PBS vehicle alone was used as a control solution. PBS, 0.02, 0.2, and 2% BZK groups contained 14, 12, 9, and 12 animals, respectively.
2.2. OCT Instrumentation
OCT imaging was performed as previously described8,9 using a NIRIS OCT system (Imalux Corp., Cleveland, OH). The NIRIS OCT system (Imalux Corp., Cleveland, OH) is a time-domain based imaging device that acquires single B-scan imaging data via a proprietary endoscopic fiber optic probe. The system operates at a center wavelength of and spectral bandwidth of 55 to 60 nm. The imaging system, used in conjunction with the probe, provides an axial (i.e., in-depth) and lateral spatial resolution (in air) of 10 to 20 μm and 25 to 35 μm, respectively. The endoscopic probe assembly is a “forward-viewing” (a.k.a. “forward-looking”) OCT probe. The “forward-viewing” probe design provides images that are two-dimensional (2-D) B-scans representing cross-sections of the tissue in depth. Each B-scan acquisition required approximately 1.5 to 2 s to collect. More specifications and details, including photographs, of an endoscopic imaging probe similar to the one utilized in this study have been previously reported.21
2.3. In Vivo OCT Imaging
For in vivo imaging of mice, the NIRIS system probe tip was gently introduced into the vaginal introitus was angled slightly to make contact with the vaginal wall while images were acquired. To ensure probe contact with the vaginal wall, mild pressure was applied externally to the midabdominal area of the animal by using an index finger. Five images were collected from each of the right, left, and posterior vaginal walls for a total of 15 images per animal. After imaging was completed, the animal was euthanized, and the vaginal tract was excised and placed in 10% neutral-buffered formalin fixative and submitted for routine histopathology processing.
2.4. OCT Image Scoring for Assessment of Vaginal Epithelial Integrity
An experienced OCT grader masked to treatment group evaluated all OCT images. For scoring, the images were displayed and viewed on a computer monitor using Presto 32 software (Optimec Ltd/BioMedTech LLC, Nizhny Novgorod, Russia) using an established quantitative grading system.8,9
The scoring system included three categories to assess the integrity of the cervicovaginal epithelium. Higher category scores indicated a greater degree of epithelial injury. Specifically, category 1 images had distinguishable, normal intact morphology that revealed a bi-layer structure of the epithelial and stromal layers. Category 2 images had mild to moderate abnormality with some epithelial/stromal layer structure visible and some areas of denuded epithelium or loss of architecture. Category 3 images had little structural morphology with no clear, distinct epithelial layer. Each image was graded as a category 1, 2, or 3 and all imaging sites (right, left, and posterior vagina) were averaged to provide a mean OCT score for the cervicovaginal tract of each animal.
2.5. In Vivo OCT Epithelial and Stromal Thickness Measurements
OCT images of the mouse cervicovaginal tract showed distinct layers within the vaginal wall, including the epithelial and stromal layers. Optical scattering property differences among epithelial and stromal tissues provide discrete transition sites for delineating tissue layer. Epithelial and stromal thickness was measured with a calibrated measuring tool in the Presto software program. Each vaginal OCT B-scan was measured at three randomly selected locations across the scan width to obtain mean epithelial and stromal thickness for that image.
2.6. Histology Epithelial and Stromal Thickness Measurements
Paraffin-embedded histology samples were cut longitudinally to the vaginal lumen to reveal both vaginal lumen and wall for morphological analysis. Several sections from each tissue sample were stained with Hematoxylin and Eosin (H&E). All slides were screened by light microscopy in order to find a section that was midway through the cervicovaginal tract. Microphotographs of the vaginal epithelium were collected using a color digital camera (Spot RT Slider, Diagnostic Instruments, Sterling Heights, MI) at a magnification of . A calibrated measuring tool within the software program (Spot Advanced software, Diagnostic Instruments, Sterling Heights, MI) was used to measure epithelial and vaginal wall thickness at ten sites on each microhistograph.
2.7. Genital Herpes Susceptibility Testing
HSV-2 susceptibility studies have been previously described.22 Briefly, four groups of mice () were administered 2.0, 0.2, and 0.02% BZK solution or PBS and 16 h later were challenged with infectious dose 50 () of HSV-2. Animals were evaluated for infection and for the development of genital herpes disease. The study was conducted in duplicate, which provided a total number of 30 mice for each BZK and PBS treatment group.
2.8. Statistical Methods
The PROC Mixed (OCT scoring and morphology data) or GLM (Histology data) program of the SAS system (SAS Institute: SA/STAT: User’s Guide, Version 9: Cary, NC: SAS Institute) was used to calculate least squares means, confidence interval, standard error, and values. A type 3 test of fixed effects was performed to determine statistical significance. Comparisons between OCT and histology were analyzed with an unpaired -test to obtain two-tailed values. Significance for HSV-2 susceptibility was assessed using a Fishers’ Exact Test (Graphpad Prism). values of 0.05 or less were considered significant for all statistical tests.
3. Results
3.1. In Vivo OCT Imaging
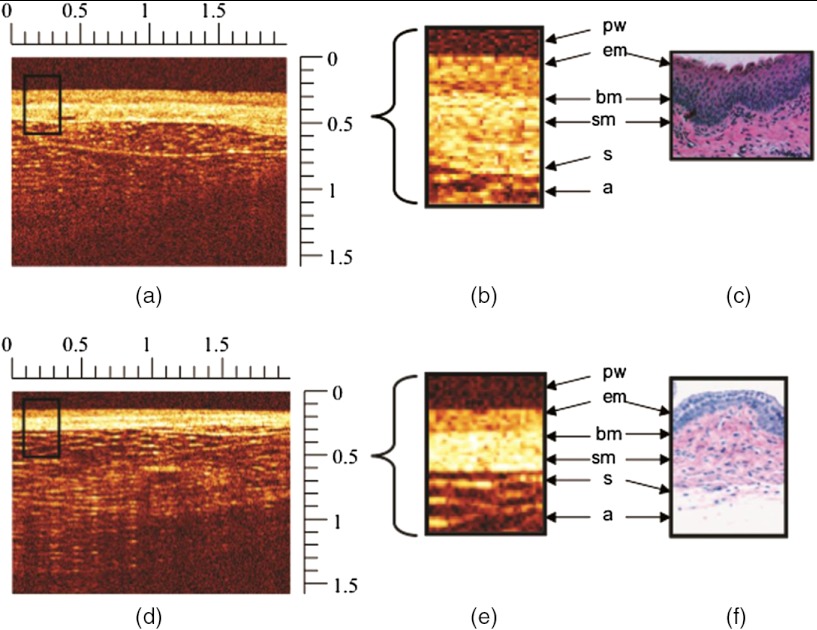
Figure 1 shows representative OCT images from the vagina of a naturally cycling (without progestin pretreatment) and a progestin-treated mouse. OCT images of the vaginal wall revealed a bilayer structure that was verified by histology. Adipose, connective, and colorectal (not shown) tissue could also be observed beyond the vaginal serosa. The microscopic difference in tissue and cellular composition and abrupt transitions between the epithelium, stroma, and adipose layers makes delineation of these layers possible with OCT. In natural-cycling mice, the epithelial layer appears as a slightly darker band relative to the brighter stromal layers (see Fig. 1). This exhibits increased light scatter attributed to structural proteins, such as collagen, elastin, and muscle fibers.
Fig. 1.
Representative optical coherence tomography (OCT) and histograph images of the cervicovaginal tract from naturally cycling (a–c) and progestin-treated (d–f) mice. High- and low-signal intensities in OCT images are denoted by white and black, respectively, and rulers are in millimeters; (a) shows an OCT image of the vaginal wall (black boxed region) of a naturally cycling mouse that is to 250 mm in thickness. The boxed region of interest (ROI) is expanded (b) to reveal the vaginal structural detail compared with a representative histology photomicrograph (c). In this comparison, several anatomical features can be resolved and identified, such as epithelium (em), basement membrane (bm), submucosa (sm), serosa (s), and adipose tissue (a). The location of the endoscopic probe tip window (pw) is also denoted in (b) and (d). An OCT image (d) from a progestin-treated mouse which has hormonally thinned epithelium and an overall vaginal wall thickness of . The vaginal wall ROI (black boxed region) is further expanded (e) and shown next to a representative histology microphotograph (f), revealing the ability of OCT to resolve the epithelium (em) and submucosal (sm) layers of the vaginal wall, which are approximately 35 and 150 μm in thickness, respectively.
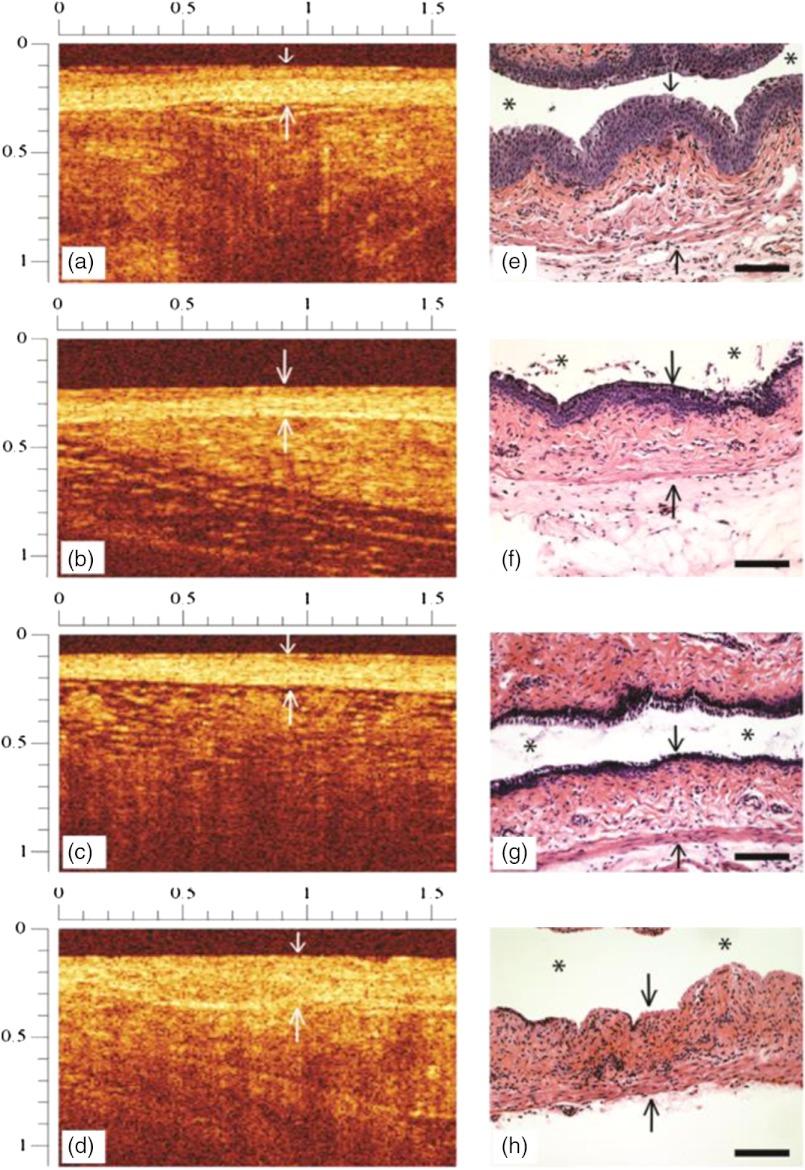
Figure 2(a)–2(h) shows representative OCT and histology images from the vaginal tract of animals treated with 0.02, 0.2, and 2% BZK or PBS. Figure 2(a) and 2(b) are examples of a OCT category “1” score. Figure 2(c) and 2(d) illustrate OCT scores of “2” and “3,” respectively.
Fig. 2.
Representative OCT images (a–d) and corresponding histology (e–h) from mice treated with phosphate buffered saline (PBS) (a, e), 0.02% benzalkonium chloride (BZK) (b, f), 0.2% BZK (c, g), or 2% BZK (d, h). Downward-pointing white arrows in the upper dark region of the OCT images (a–d) indicate the transition point between OCT probe tip window and the vaginal epithelial surface. Black arrows denote the epithelial surface in the histology microphotographs (e–h). The lower, upward directed arrows indicate the location of the stromal margin. The intravaginal lumen is indicated by asterisks (*) in the histology photomicrographs. Top and left OCT image rulers are in mm. In histology microphotographs, .
As seen in Fig. 2(a) and 2(b), the animals treated with PBS and 0.02% BZK show little or no visible morphological abnormalities by OCT. However, in Fig. 2(c) and 2(g), after treatment with 0.2% BZK, there was thinned epithelium visible in both the OCT and histology images. Figure 2(d) and 2(h) show the vaginal epithelium as fully disrupted after treatment with 2% BZK. In addition, the characteristic, normal bilayer morphology was absent, and the stroma was altered with respect to scattering intensity.
3.2. OCT Image Scoring
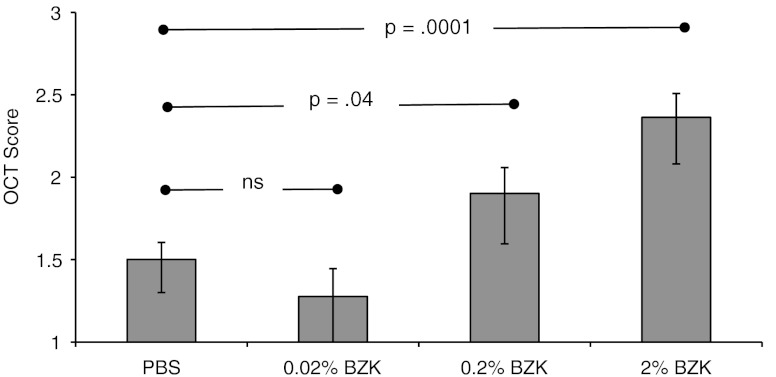
The OCT data set for the BZK treated animals included 15 images per mouse, with five images obtained at each of three different sites within the vagina (right, left, and posterior walls). Figure 3 shows the results of the grading assessment as the least squares mean scores with confidence intervals. The scores indicate an increase in abnormal tissue morphology quantified by increasing scores, associated with increasing BZK concentration. The scores for both 0.2% () and 2.0% () BZK groups were significantly higher than the PBS control group, signifying tissue injury. In contrast, the 0.02% BZK treated group was not significantly different than the PBS controls ().
Fig. 3.
Mean OCT Scores for PBS, 0.02% BZK, 0.2% BZK, and 2% BZK treatment groups. Data is shown as least squares means with confidence intervals (1.96*SE). (*, ^ compared with PBS controls).
3.3. OCT and Histology Thickness Measurements
Measurements of vaginal epithelium and stromal layer thickness were made from OCT and histology images. As shown in Fig. 1(a), under in vivo conditions, OCT allowed for resolving the epithelial and stromal layers in normal, naturally cycling mice. Figure 4 shows a comparison of the epithelial and stromal thicknesses obtained from OCT and histology images. Both OCT () and histology () detected significant differences in treatment groups 16 h after treatment with BZK as compared with PBS. Histology was found to be more sensitive than OCT at detecting small changes (sometimes on the order of a single cell layer or ) to epithelial thickness in response to BZK treatment.
Fig. 4.
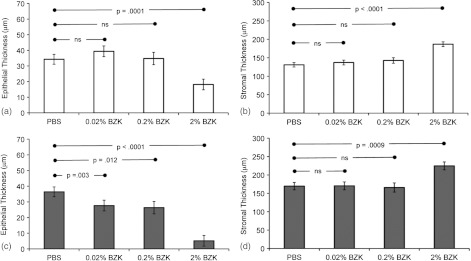
Mean epithelial (a, c) and stromal (b, d) thickness measured by OCT (a, b) and histology (c, d) 16 h after a single treatment with PBS or BZK Data is shown as least squares means with standard error of the mean. Epithelial thickness was decreased in the 2% BZK treatment group by OCT measurements and in all BZK treatment groups by histology measurements when compared to the PBS control group (^, *).
In mice treated with 2% BZK, OCT detected a significant decrease () in epithelial thickness relative to PBS treated animals. The decrease was also apparent on histology (). OCT did not detect significant differences in epithelial thickness in the 0.02% BZK () or 0.2% BZK () treatment groups compared to the PBS control group. In contrast, epithelial thickness measured from histology microphotographs revealed significant differences in 0.02% and 0.2% BZK treatment groups compared to the PBS control group ().
In PBS treated animals, the mean epithelial thickness obtained by OCT () and histology () was not significantly different. However, this observation was not present in the BZK-treated groups. The means for epithelial thickness obtained from in vivo OCT images were significantly thicker () than those obtained using histopathologic technique performed on fixed tissue samples for all animals treated with BZK. One potential factor in this difference was loss of the superficial layer of epithelium during histological processing as evidenced by the presence of debris in the lumen [Fig. 2(f) and 2(g)]. Furthermore, it should be recognized that the resolution of the current system is marginally acceptable for the analysis reported in the current work due to the baseline-thinned epithelium in these progestin-treated mice.
Stromal thickness was similar in all treatment groups except after treatment with 2% BZK, in which the stromal layer was thickened as measured by both OCT and histology when compared to the PBS treated group [Fig. 4(b) and 4(d)]. For all treatment groups, the mean stromal thickness obtained from OCT images was consistently thinner (range 30 to 40 μm) than those obtained from histology photomicrographs, a finding likely due to tissue compression of this highly vascular and compressible layer.
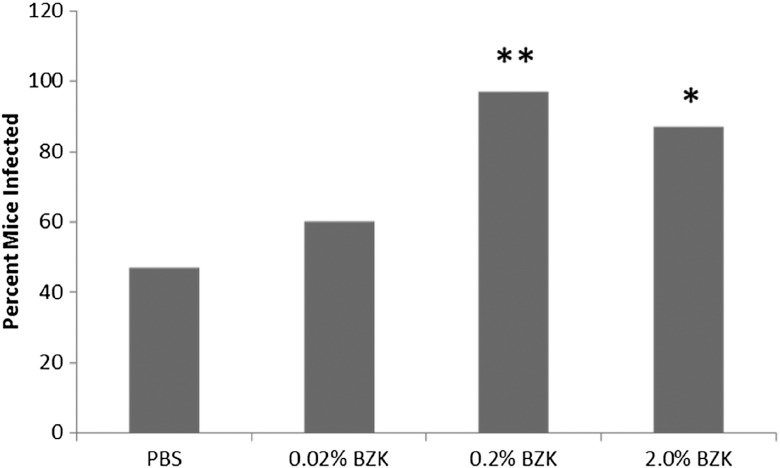
3.4. HSV-2 Susceptibility
The results of the susceptibility testing have been previously reported and are reprinted with permission here.14 Mice treated with 0.2 or 2.0% BZK had increased susceptibility to HSV-2 challenge when compared with PBS-treated mice. Furthermore, there were no statistically significant differences between the group treated with 0.02% BZK and PBS control mice, see Fig. 5.
Fig. 5.
HSV-2 susceptibility in mice. Percent of mice in each treatment group ( per group) that were identified as having HSV-2 infection after HSV-2 challenge (data used with permission from sexually transmitted diseases (STD).14 * versus PBS, Fishers’ exact test; ** versus PBS Fishers’ exact test.
4. Discussion
In this study, we demonstrated the ability of OCT to visualize drug-induced changes in structural features of the vaginal epithelium and underlying stroma in a small animal model, which cannot be visualized using conventional white-light imaging techniques such as colposcopy. These studies demonstrate the utility of OCT for assessment of drug-induced vaginal epithelial integrity. In vivo findings of this nature have not been previously reported in mice. Because of increasing published studies relating pathogen transmission with epithelial integrity, the ability to accurately assess these parameters is important for screening the potential toxicity of vaginal microbicides designed for STD prevention.
Assessment of epithelial integrity provides information about the effectiveness of the natural protective barrier of the vagina, in which vaginal epithelial thickness plays a leading role. Disruption or thinning of the epithelium compromises this protective barrier leading to enhanced susceptibility to infectious agents such as HIV, HSV-2, or human papillomavirus (HPV). Since susceptibility studies require large numbers of mice, an image-based screening tool could help direct drug development. Thus, OCT characterization of tissue injury in a limited number of small animals may yield a useful image-based metric to initially evaluate the risk of microbicide-induced increased susceptibility prior to conducting safety studies in large animal models and human.
Epithelial layer integrity was determined based on a previously reported and validated OCT visual grading system8–10,23 with higher scores indicative of epithelial disruption. The 0.2 and 2.0% BZK-treated mice had increased scores when compared with PBS-treated mice. These findings are consistent with the observed increased susceptibility to HSV-2 infection endpoints. These results confirm the ability of the scoring system to detect drug-induced changes in the epithelial integrity in small animal models, findings that were consistent with the dose response to an HSV-2 challenge after treatment with BZK.
The current study utilized a range of BZK concentrations to establish the detection limits of the OCT system in an animal model with hormonally thinned epithelium. The progestin-treated mouse vaginal tract is thin (25 to 40 μm) relative to those of nonprogestin-treated mice (up to 100 μm) and humans (250 to 320 μm)24 and large animal models, such as the macaque (192 to 248 μm)8 or sheep (60 to 90 μm).9 After progestin treatment, the mouse vaginal epithelium is two to four cell layers thick (25 to 40 μm) and consists of a single superficial layer of columnar cells overlying a few stratified squamous-type epithelial cells.19 OCT imaging allowed visualization of the thin epithelial layer in progestin-treated animals. Using the currently commercially available OCT endoscopic system, it was not possible to differentiate the columnar layer from the underlying squamous cell layer in these animals because the cell layer thickness is comparable with the resolution of the system (). This observation has been made previously for an OCT system similar to the NIRIS system.25 Our evaluation of epithelial layer thickness in vivo using OCT was similar to that obtained using histology in the progestin-thinned PBS treated animals. A mean epithelial thickness of about was observed in both OCT images and histology samples. However, in the BZK treated mice, the epithelial layer is thinned as shown in histology measurements, reaching thicknesses comparable or less than the axial resolution of the current OCT system.
OCT measurements of the stromal layer in the vaginal wall differed from the values obtained by histology by approximately 30 to 40 μm. This is likely attributed to compression of the vascular stromal layer by contact with the OCT probe.8,9 The pressure induced by the application of a contact probe can compress tissue,25 and therefore layer thickness interpretations can be affected. However, there are also benefits to application of probe pressure, including enhancement of delineation of colorectal mucosa from underlying stromal tissue25,26 and epidermis from dermis in the skin.27 The influence of pressure from the use of a contact probe can be beneficial for outlining structures, such as for monitoring the morphology of the epithelial layer as quantified by the scoring system. Conversely, pressure can be a limitation in measurement of highly compressible tissue layers, such as that in the stroma of the vagina or the mucosa of the intestine. Despite the effect of compression in the current study, the discrepancy observed between OCT and histology stromal measurements remained consistent throughout all treatment groups.
In previous studies, we have shown that OCT can delineate vaginal epithelium from stromal tissue in nonhuman primate and ovine animal models as well as in women, detecting changes in epithelial thickness as a result of Nonoxynol-9 (N-9) and BZK treatments.8,10,15 However, women and the larger animal models have thicker vaginal epithelial layers than the progestin-treated mice. Recent developments in OCT technology could improve the ability to visualize the mouse epithelium as newer systems are capable of providing axial, in-depth resolutions approaching a few microns,28 substantially better resolution (5 to ) than that employed in this study with the NIRIS system. Unfortunately, at this time, OCT systems commercially available for soft tissue imaging typically have specific configurations (i.e., time-domain versus spectral-domain) and/or data acquisition interfaces (i.e., data collection probes/optics) that are designed for use in specific clinical disciplines (i.e., ophthalmology, cardiovascular, OB/GYN, and GI) in humans. To further improve on the initial work performed in this study, a better probe designed specifically for use in the mouse cervicovaginal compartment would be ideal for future studies. Specially designed probes previously reported with rotational and lateral viewing capabilities that are to 2.5 mm () in size would be well suited for use in mice; however, they are not commercially available.11–13
5. Conclusion
Noninvasive, high-resolution imaging modalities, such as OCT, used in small animal models have the potential to screen candidate microbicides prior to initiating larger, more expensive, nonhuman primate and human studies. OCT provides unique information that cannot be obtained with any other in vivo imaging modality. Use of this imaging modality in the murine HSV-2 pathogen model enables direct comparisons between in vivo imaging findings (OCT scoring system) and a well-established screening model of STD pathogen susceptibility. Comprehensive in vitro and in vivo testing methods are now warranted for candidate microbicide development programs and OCT shows promise in playing a future role in the screening process for determining microbicide safety.
Acknowledgments
We like to acknowledge Heather Lander for her invaluable assistance in editing and preparation of the manuscript. The authors would like to thank Alai Tan for assistance with Biostatistics, Rebecca Johnston for technical assistance, and the NIH/NIAID (R21 AI076062, NO1 HD53407, and R33AI07606205) for sponsoring this research. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Centers for Disease Control and Prevention Department U.S. of Health and Human Services, “Sexually transmitted disease surveillance, 2008,” (2009), http://www.cdc.gov/std/stats08/surv2008-complete.pdf (accessed 28 March 2013).
- 2.UNAIDS and WHO, “AIDS epidemic update, 2009,” Geneva, UNAIDS (2009), http://www.unaids.org/en/dataanalysis/epidemiology/2009aidsepidemicupdate (accessed 28 March 2013).
- 3.Rosenthal S. L., Cohen S. S., Stanberry L. R., “Topical microbicides. Current status and research considerations for adolescent girls,” Sex. Transm. Dis. 25(7), 368–377 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Coggins C., “Avoiding HIV transmission: women need more options,” Sex. Transm. Infect. 75(6), 374–375 (1999). [PubMed] [Google Scholar]
- 5.World Health Organization, “WHO/CONRAD technical consultation on nonoxynol-9, World Health Organization, Geneva, 9-10 October 2001: summary report,” Reprod. Health Matters 10(20), 175–181 (2002). 10.1016/S0968-8080(02)00085-X [DOI] [PubMed] [Google Scholar]
- 6.The International Working Group on Vaginal Microbicides, “Recommendations for the development of vaginal microbicides. International Working Group on Vaginal Microbicides,” AIDS 10(8), 1–6 (1996). [PubMed] [Google Scholar]
- 7.Moscicki A. B., “Vaginal microbicides: where are we and where are we going?” J. Infect. Chemother. 14(5), 337–341 (2008). 10.1007/s10156-008-0630-3 [DOI] [PubMed] [Google Scholar]
- 8.Vincent K. L., et al. , “Application of optical coherence tomography for monitoring changes in cervicovaginal epithelial morphology in macaques: potential for assessment of microbicide safety,” Sex. Transm. Dis. 35(3), 269–275 (2008). 10.1097/OLQ.0b013e31815abad8 [DOI] [PubMed] [Google Scholar]
- 9.Vincent K. L., et al. , “High resolution imaging of epithelial injury in the sheep cervicovaginal tract: a promising model for testing safety of candidate microbicides,” Sex. Transm. Dis. 36(5), 312–318 (2009). 10.1097/OLQ.0b013e31819496e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent K. L., et al. , “Optical coherence tomography compared with colposcopy for assessment of vaginal epithelial damage: a randomized controlled trial,” Obstet. Gynecol. 118(6), 1354–1361 (2011). 10.1097/AOG.0b013e318238f563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto J. G., et al. , “Optical biopsy and imaging using optical coherence tomography,” Nat. Med. 1(9), 970–972 (1995). 10.1038/nm0995-970 [DOI] [PubMed] [Google Scholar]
- 12.Pitris C., et al. , “High-resolution imaging of gynecologic neoplasms using optical coherence tomography,” Obstet. Gynecol. 93(1), 135–139 (1999). 10.1016/S0029-7844(98)00375-5 [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto J. G., et al. , “Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy,” Neoplasia 2(1–2), 9–25 (2000). 10.1038/sj.neo.7900071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent K. L., et al. , “Benzalkonium chloride causes colposcopic changes and increased susceptibility to genital herpes infection in mice,” Sex. Transm. Dis. 37(9), 579–584 (2010). 10.1097/OLQ.0b013e3181dac410 [DOI] [PubMed] [Google Scholar]
- 15.Vargas G., et al. , “Use of high-resolution confocal imaging of the vaginal epithelial microstructure to detect microbicide toxicity,” J. Infect. Dis. 199(10), 1546–1552 (2009). 10.1086/599993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushic C., et al. , “Progesterone increases susceptibility and decreases immune responses to genital herpes infection,” J. Virol. 77(8), 4558–4565 (2003). 10.1128/JVI.77.8.4558-4565.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Cruz O. J., Erbeck D., Uckun F. M., “A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate,” Toxicol. Pathol. 33(4), 465–476 (2005). 10.1080/01926230590959866 [DOI] [PubMed] [Google Scholar]
- 18.Cone R. A., et al. , “Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission,” BMC Infect. Dis. 6, 90 (2006). 10.1186/1471-2334-6-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achilles S. L., et al. , “Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis,” Sex. Transm. Dis. 29(11), 655–664 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Bourne N., et al. , “Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection,” Antimicrob. Agents Chemother. 49(3), 1181–1183 (2005). 10.1128/AAC.49.3.1181-1183.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zagaynova E., et al. , “Endoscopic OCT with forward-looking probe: clinical studies in urology and gastroenterology,” J. Biophoton. 1(2), 114–128 (2008). 10.1002/(ISSN)1864-0648 [DOI] [PubMed] [Google Scholar]
- 22.Vincent K. L., et al. , “Benzalkonium chloride causes colposcopic changes and increased susceptibility to genital herpes infection in mice,” Sex. Transm. Dis. 37(9), 579–584 (2010). 10.1097/OLQ.0b013e3181dac410 [DOI] [PubMed] [Google Scholar]
- 23.Vincent K. L., et al. , “Monitoring vaginal epithelial thickness changes noninvasively in sheep using optical coherence tomography,” Am. J. Obstet. Gynecol. 208(4), 282.e1–282.e7 (2013). 10.1016/j.ajog.2013.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimaraes J. V., et al. , “Thickness of the cervical epithelium of autopsied patients with acquired immunodeficiency syndrome,” Ann. Diagn. Pathol. 11(4), 258–261 (2007). 10.1016/j.anndiagpath.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 25.Dolin L. S., et al. , “Fundamentals of OCT and clinical applications of endoscopic OCT,” in Handbook of Coherent Domain Optical Methods, Tuchin V. V., Eds., pp. 255–256, Kluwer Academic Publishers, Dordrecht, the Netherlands: (2004). [Google Scholar]
- 26.Agrba P. D., et al. , “Compression as a method for increasing the informativity of optical coherence tomography of biotissues,” Opt. Spectrosc. 107(6), 853–858 (2009). 10.1134/S0030400X09120042 [DOI] [Google Scholar]
- 27.Kirillin M. Y., Agrba P. D., Kamensky V. A., “In vivo study of the effect of mechanical compression on formation of OCT images of human skin,” J. Biophoton. 3(12), 752–758 (2010). 10.1002/jbio.v3.12 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Padilla J. A., et al. , “High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy,” Arch. Ophthalmol. 125(6), 775–780 (2007). 10.1001/archopht.125.6.775 [DOI] [PMC free article] [PubMed] [Google Scholar]