Abstract
Though aphasia is primarily characterized by impairments in the comprehension and/or expression of language, research has shown that patients with aphasia also show deficits in cognitive-linguistic domains such as attention, executive function, concept knowledge and memory (Helm-Estabrooks, 2002 for review). Research in aphasia suggests that cognitive impairments can impact the online construction of language, new verbal learning, and transactional success (Freedman & Martin, 2001; Hula & McNeil, 2008; Ramsberger, 2005). In our research, we extend this hypothesis to suggest that general cognitive deficits influence progress with therapy. The aim of our study is to explore learning, a cognitive process that is integral to relearning language, yet underexplored in the field of aphasia rehabilitation. We examine non-linguistic category learning in patients with aphasia (n=19) and in healthy controls (n=12), comparing feedback and non-feedback based instruction. Participants complete two computer-based learning tasks that require them to categorize novel animals based on the percentage of features shared with one of two prototypes. As hypothesized, healthy controls showed successful category learning following both methods of instruction. In contrast, only 60% of our patient population demonstrated successful non-linguistic category learning. Patient performance was not predictable by standardized measures of cognitive ability. Results suggest that general learning is affected in aphasia and is a unique, important factor to consider in the field of aphasia rehabilitation.
1. Introduction
While we have some understanding of how individuals with post-stroke aphasia relearn language, why some patients respond to treatment while others do not remains a looming question in the field of aphasia rehabilitation (Best & Nickels, 2000; Kelly & Armstrong, 2009). Much progress has been made in the field, such that clinicians and researchers are equipped with means of assessing aphasia (Spreen & Risser, 2003), model frameworks of language processing and impairment that help describe the nature of deficits and guide therapy (Howard & Hatfield, 1987), as well as multiple therapies and tasks that studies have demonstrated are efficacious in improving language function in patients with aphasia (Holland, Fromm, DeRuyter & Stein, 1996; Kiran & Sandberg, 2011). In spite of this progress, we still do not fully understand the mechanisms of therapy (Ferguson, 1999) nor are we able to prescribe the most appropriate treatments for patients based on their language deficits and cognitive profiles (Best & Nickels, 2000; Kelly & Armstrong, 2009). We suggest that while research has progressed in terms of developing assessments and therapies for aphasia, learning is a process that is integral to relearning language and therefore to rehabilitation, yet is insufficiently represented.
Traditional research in aphasia has predominantly focused on the role of brain regions specialized for language, however a growing body of lesion and neuroimaging research now recognizes that language is part of an extensive network of connected brain regions that subserve not only language, but processes such as working memory and cognitive control (Tomasi & Volkow, 2012; for review Turken & Dronkers, 2011). Accordingly, an increasing number of studies in aphasia rehabilitation acknowledge the important contribution of multiple factors of cognition to therapy outcomes and communicative success (Fridriksson, Nettles, Davis, Morrow, & Montgomery, 2006; Helm-Estabrooks, 2002; Keil & Kaszniak, 2002; Ramsberger, 2005). Researchers have identified skills that might be important towards constructing and retrieving language, such as attention (Erickson, Goldinger & LaPointe, 1996; Hula & McNeil, 2008; Lesniak, Bak, Czepiel, Seniow, & Czlonkowska, 2008; Murray, 2012; Peach, Rubin & Newhoff, 1994), executive function (Keil & Kaszniak, 2002; Lesniak et al., 2008; Ramsberger, 2005; Zinn, Bosworth, Hoenig, & Swartwelder, 2007), concept knowledge (Chertkow, Bub, Deaudon & Whitehead, 1997) and memory (Helm-Estabrooks, 2002; LaPointe & Erickson, 1991).
In a 1997 study, for instance, Chertkow et al. examined sentence comprehension in aphasia and drew attention to a subset of patients with aphasia who showed semantic deficits that extended into nonverbal domains of object representation and concept knowledge. Jefferies and Lambon Ralph (2006), Jefferies, Patterson and Lambon Ralph (2008) and Noonan, Jefferies, Corbett and Lambon Ralph (2009) further explored this question comparing the behavior of patients with semantic dementia (SD) with patients with semantic aphasia (SA), their results suggesting that in many cases SA patients have preserved conceptual knowledge, but impaired executive function, this impairment impacting their control over semantic activation. Studies exploring new verbal learning in aphasia have shown that learning ability is related to patients’ profiles of linguistic (Grossman & Carey, 1987; Gupta, Martin, Abbs, Schwartz & Lipinski, 2006) and cognitive (Freedman & Martin, 2001) strengths and deficits. Patient phonological and semantic short-term memory skills, for example, appear to influence patients’ abilities to engage in phonological learning (word translation learning) and semantic learning (new definition learning, Freedman & Martin, 2001).
With respect to verbal and non-verbal tasks in aphasia, many studies have demonstrated a disparity between language skills and non-linguistic ability (Basso, De Renzi, Fagolioni, Scotti, & Spinnler, 1973; Chertkow et al., 1997; Helm-Estabrooks, 2002), illustrating that patients with aphasia can have differing degrees of impairment in both verbal and nonverbal domains. Though degrees of impairment can differ in these domains, they remain related, researchers postulating a contribution of non-linguistic cognitive impairments to the online construction of language (Hula & McNeil, 2008) and to transactional success in functional communication in aphasia (Ramsberger, 2005). In addition, some researchers have found that treatment related outcomes are best predicted by non-linguistic skills such as executive function and monitoring, rather than by language ability (Fillingham, Sage, & Lambon Ralph, 2005a, 2005b). Studies such as these draw attention to the interconnectedness of cognitive, non-linguistic factors and language, and to the importance of exploring nonverbal domains as a means of better characterizing and understanding the deficits that surface in aphasia.
We suggest that not only are nonverbal cognitive-linguistic processes important to the retrieval and construction of language in conversation, but that nonverbal cognitive processes might be important in the relearning or reaccess to language that is brought about through therapy. More specifically, we identify learning as a critical process involved in language relearning subsequent to stroke. Support for this hypothesis comes from recent neuroimaging studies in aphasia that explore the association between treatment related changes and neural structures and activation. Menke et al. (2009), for example, found evidence for a relationship between short-term improvements with therapy and bilateral activation of the hippocampus, a structure critical to memory. Shortly thereafter in a diffusion tensor imaging study, Meinzer et al. (2010) showed a correlation between success with language therapy and the structural integrity of the hippocampus and surrounding fiber tracts. Studies that explore novel lexical, semantic and syntactic learning in healthy individuals have shown the engagement of similar structures (Breitenstein et al., 2005; Maguire & Frith, 2004; Optiz & Friederici, 2003) suggesting that comparable mechanisms may underlie the processes of language rehabilitation and novel learning in healthy individuals (Menke et al., 2009; Rijntjes, 2006). Goldenberg and Spatt (1994) examined the correlation between success with therapy and lesion location and volume, and found that patients who showed limited improvements in therapy had lesions that were close to, or that included portions of the entorhinal cortex, an important structure in the relay of information between the neocortex and the hippocampus (Squire, 1992) considered critical to learning and memory (Eichenbaum, Otto, & Cohen, 1992; Squire, 1992). While we do not know the exact mechanisms by which aphasia rehabilitation leads to functional outcomes, researchers concluded that results demonstrate the involvement of explicit learning in aphasia rehabilitation (Goldenberg & Spatt, 1994). For these reasons, we aim to use nonverbal learning in aphasia as a window into learning, proposing that a better understanding of these mechanisms could be essential in the diagnostic characterization of patients with aphasia.
Research in other patient populations, such as Parkinson’s disease, Alzheimer’s disease, frontotemporal dementia and amnesia, has emphasized the importance of understanding subtleties of learning ability in patients with brain damage (Filoteo, Maddox, Ing, Zizak & Song, 2005; Knowlton & Squire, 1993; Knowlton, Squire & Gluck, 1994; Koenig, Smith, & Grossman, 2006; Koenig, Smith, Moore, Glosser, & Grossman, 2007; Shohamy et al, 2004) that we suggest is also essential in aphasia. Knowlton et al. (1994), for example, conducted an experiment exploring the ability of patients with amnesia to learn stimulus outcome associations between geometric cards and weather conditions. Previous research had shown that despite deficits in episodic memory, patients with amnesia were capable of learning some types of information. Knowlton et al. (1994) found that an alternate means of instruction administered through gradual trial-by-trial feedback, allowed amnesic patients to overcome memory deficits and learn probabilistic card-condition pairings as well as controls. This study demonstrated that for the case of amnesia, characteristics of the to-be-learned material were not the factor confounding learning; rather, it was the method of instruction and the way in which memory systems were recruited to support learning that facilitated success. While differential patient success with language learning might very well be affected by semantic, phonological and grammatical characteristics of target material, additional cognitive mechanisms that are independent of verbal processing skills might also contribute to language learning. One study of high pertinence to the methods of the current paper is by Koenig et al. (2006) examining learning in patients with frontotemporal dementia (FTD). In their study, researchers explored participants’ abilities to learn to categorize novel animals, comparing rule-based and similarity-based paradigms. Researchers found that different profiles of learning arose among patients with semantic dementia (SD) and patients with progressive nonfluent aphasia (PNFA), the PNFA group showing impaired rule-based learning. Aphasia associated with frontotemporal dementia is distinct from stroke-related aphasia; however we draw attention to this study because researchers implemented nonverbal learning as a means of isolating learning in patients with language impairments, and drew further attention to the distinct processes involved in different methods of learning.
Despite the breadth of research dedicated to nonverbal learning in other populations with brain damage, and the identified impact of instruction method on success with learning, no recent study has explored nonverbal learning in stroke-related aphasia. An exploration into nonverbal learning offers the potential to determine whether patients with aphasia experience language deficits that are supported by an intact cognitive foundation for learning, or whether deficits in language occur in the context of degraded cognitive architectures to support learning. If patients learn novel nonverbal information as well as controls, results will suggest that the observed variability in learning in aphasia is directly linked to the integrity of the language system and to linguistic demands. If, in contrast, patients with aphasia show deficits in learning novel nonverbal information, results will suggest that, in addition to cognitive-linguistic deficits, deficits in the cognitive architecture supporting general learning affect patients’ abilities to learn or relearn language. If the latter is true, in the long-term, measures of nonverbal learning ability can be included into diagnostic characterizations of patients; such measures presenting a gateway towards language treatments that are selected for and/or tailored to individuals.
To this end, in the current study we take a nonverbal approach in the exploration of learning in aphasia and seek to determine whether patients learn novel non-linguistic tasks similarly to healthy age-matched controls. In addition, we are interested in exploring whether differences in nonverbal learning arise following different methods of instruction. For these purposes, we have developed two tasks in which participants learn to categorize novel animals as belonging to one of two categories. The two tasks have shared stimuli, and in both tasks, participants learn to categorize novel animals as belonging to one of two categories. We compare learning following instruction that is paired associate in nature and instruction administered through trial-by-trial feedback, paradigms similar in design to those implemented in aphasia (Breitenstein, Kamping, Jansen, Schomacher & Knecht, 2004) and in healthy and brain damaged populations (Knowlton & Squire, 1993; Knowlton et al., 1994; Poldrack et al., 2001; Zeithamova, Maddox, & Schnyer, 2008). Research has shown variable engagement of neural structures during paired associate and feedback-based categorization that interact both competitively and cooperatively (Maddox, Love, Glass & Filoteo, 2008; Poldrack & Packard 2003 for review), however previous experiments suggest that trial-by-trial feedback-based learning relies heavily on cortico-striatal loops of the basal ganglia and on nondeclarative memory systems (Poldrack et al., 2001; Seger & Miller, 2010 for review). In contrast, paired associate learning in the absence of feedback is likely to have a greater dependence on medial temporal lobe declarative memory systems (Poldrack et al., 2001). While the present study does not specifically examine the neural underpinnings of feedback or paired associate learning, the behavioral manifestations following these different learning methods may be informative towards our understanding of learning in aphasia.
Based on experiments using similar tasks, we predict that healthy controls will learn categories equally well following both methods of instruction. With respect to patients, we conceive of two potential outcomes. One hypothesis is that patients with aphasia will demonstrate non-linguistic category learning that is parallel to learning observed in healthy controls. Previous studies have demonstrated that patients with aphasia are capable of new learning (Breitenstein et al., 2004; Freedman & Martin, 2001; Gupta et al., 2006; Kelly & Armstrong, 2009; Marshall, Neuburger & Phillips, 1992; Tuomiranta et al., 2011), therefore in the context of non-linguistic material normal learning can be expected. On the other hand, based on research in populations with amnesia and Parkinson’s disease that demonstrate disrupted nonverbal learning subsequent to brain damage, we hypothesize that patients with aphasia may also have deficits in nonverbal learning. Learning in aphasia may be attributable to both language and cognitive processing, such that patients will show impaired category learning relative to healthy controls, even when learning is non-linguistic. If this is the case, we anticipate that patients with greater impairments in executive function may show more disordered learning, as some studies have found executive function to be a predictor of therapy outcomes (Filloteo et al., 2005a, 2005b).
2. Materials and Methods
2.1 Participants
Twenty patients (ten men) with single left hemisphere strokes (M = 61.40, SD = 11.98, ranging from 33.7 – 86.8 years of age) participated in the study. Upon enrollment, patients had completed between 3 and 21 years of education (M = 14.84, SD = 4.08). Patients were recruited from a patient pool at the Sargent College of Health and Rehabilitation Sciences. All patients were premorbidly right handed and were tested at least six months after the onset of their stroke. At the time of testing, patients had no concomitant medical problems. Western Aphasia Battery (WAB, Kertesz, 1982) aphasia quotients (AQs) ranged from 24.8 – 98 encompassing Broca’s and Wernicke’s aphasia types, Conduction, Transcortical motor and Anomic aphasia. Table 1 provides a breakdown of patient demographic information, aphasia type and aphasia characteristics. One patient was dropped following testing (see results) and is not included in Table 1. Another patient did not fully complete the WAB and therefore could not be assigned an aphasia type or aphasia quotient.
Table 1.
Control (Cn) participant information
| Control | Cn1 | Cn2 | Cn3 | Cn4 | Cn5 | Cn6 | Cn7 | Cn8 | Cn9 | Cn10 | Cn11 | Cn12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 32.9 | 56.8 | 57.6 | 57.2 | 61.2 | 59.7 | 60.6 | 65.4 | 69.5 | 70 | 72.6 | 58.7 |
| Gender | F | M | F | F | M | F | F | F | M | M | F | M |
| Education | 19 | 16 | 18 | 16 | 21 | 19 | 16 | 12 | 16 | 16 | 16 | 18 |
Thirteen control participants (five men, see Table 2) with no known history of neurological disease, psychiatric disorders or developmental speech, language or learning disabilities took part in the study (M = 60.18, SD = 10.17, ranging from 32.9 – 72.6 years of age). One control participant was left-handed. The control group and patient group did not differ in age or in education level (mean years of education for controls = 17.00, SD = 1.91). One control participant had to be dropped after testing (see results) and is not included in Table 2. All participants provided consent according to Boston University’s IRB. Participants received $5 for every hour of their time.
Table 2.
Patient (Pt) participant information
| Patient | Age | Gender | Education | Months post onset | Aphasia Type | Comprehension | Attention | Memory | Executive Function | Visuo spatial | BNT | AQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | 33.7 | F | 14 | 6 | Con. | 91 | WNL | Sev | Mod | WNL | 0 | 25 |
| Pt 2 | 49.7 | F | 18 | 24 | An. | 185 | WNL | WNL | WNL | WNL | 100 | 94 |
| Pt 3 | 52.7 | F | 12 | 24.8 | Wern. | 116 | WNL | Sev | Mod | WNL | 6.67 | 41 |
| Pt 4 | 52.7 | M | 16 | 106.8 | Con./Wern. | 142 | Mild | Sev | WNL | WNL | 6.67 | 48 |
| Pt 5 | 61 | M | 13 | 6 | An. | 192 | Mild | Mild | Sev | Mild | 80 | 91 |
| Pt 6 | 63.7 | F | 18 | 18 | An. | 143 | Mild | Sev | Sev | Mild | 13.3 | 68 |
| Pt 7 | 65.7 | F | 18 | 41.5 | Bro. | 120 | Mild | Sev | Sev | Mild | 0 | 28 |
| Pt 8 | 69.5 | M | 21 | 27.5 | Wern. | 78 | Mild | Sev | Mild | WNL | 0 | 34 |
| Pt 9 | 75.7 | M | 3 | 15.3 | - | - | Mild | Mod | Mod | Mild | 2 | - |
| Pt 10 | 77.2 | F | 16 | 93.6 | An. | 200 | WNL | WNL | WNL | WNL | 98.3 | 98 |
| Pt 11 | 86.8 | M | 12 | 13.2 | An. | 185 | Mild | Mod | Mild | Mild | 58.3 | 88 |
| Pt 12 | 49.3 | M | 12 | 162 | Brocas | 137 | Mild | Sev | Mod | Mild | 58.33 | 58.2 |
| Pt 13 | 51.9 | M | 11 | 259.6 | An. | 175 | Mod | Sev | Mild | Mild | 31.67 | 61.3 |
| Pt 14 | 57.3 | F | 16 | 68.4 | An. | 170 | Mild | Mod | Sev | Mod | 56.67 | 80.2 |
| Pt 15 | 59.5 | M | 19 | 26.5 | An. | 178 | WNL | Mod | WNL | WNL | 78.33 | 82.8 |
| Pt 16 | 61 | M | 16 | 44.6 | Cond. | 168 | WNL | WNL | WNL | WNL | 43.33 | 67.9 |
| Pt 17 | 63.6 | F | 16 | 64.7 | An. | 174 | WNL | Mod | Mod | WNL | 30 | 69.1 |
| Pt 18 | 67.5 | F | 12 | 28.4 | TCM | 179 | Mod | Sev | Mod | Sev | 83.3 | 82.2 |
| Pt 19 | 68 | M | 19 | 13.2 | An. | 74.3 | Mild | Mild | Mod | Mild | 30 | 74.3 |
Note. For patients, composite scores of attention, memory, executive functions (Executive) and visuospatial skills (Visuospatial) as obtained with the CLQT, Boston naming test (BNT) scores and aphasia quotients (AQ) are reflected. Aphasia types are abbreviated as follows: conduction (Cond.), anomic (An.), Wernicke’s (Wern.), Broca’s (Br.) and transcortical motor (TCM). WNL indicates scores within normal limits. Italics indicate patients classified as learners during our experiment.
2.2 Stimuli
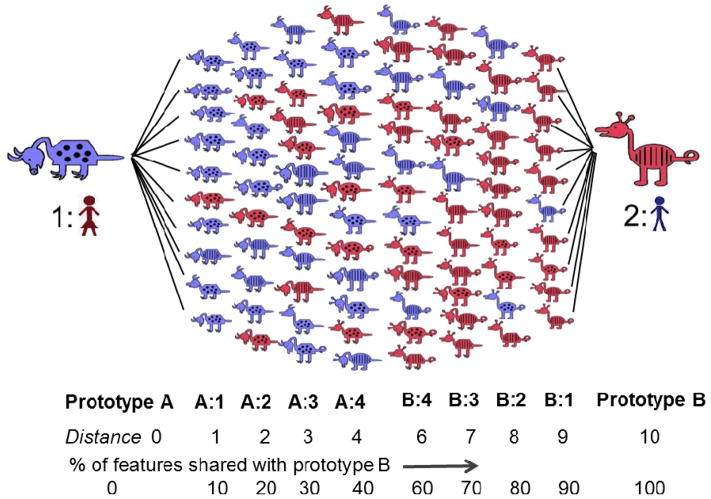
Stimuli for the experiment were two sets of 1024 cartoon animals developed by Zeithamova et al. (2008) that vary on ten binary dimensions (neck length, tail shape, feet, etc.). For each set, one stimulus was selected as prototype A with each other animal identified in terms of the number of features by which it differed from the prototype. This difference was defined as an animal’s distance from the prototype. In other words, animals at a distance of three from the prototype all differed from prototype A by three features, and thus had seven features in common with prototype A. Only one animal differed from prototype A by all ten features (distance of 10) and was therefore selected as prototype B. In this manner two category extremes, or prototypes, were established for each stimulus set.
All animals that differed from prototype A by 1 to 4 features were then considered members of category A. These animals all shared a majority of their features with prototype A, sharing 90% to 60% of their features with the prototype as distance increased from 1 to 4, and consequently, sharing 10% to 40% of their features with prototype B. In contrast, those animals at distances 6 to 9 from prototype A were considered members of category B, as they shared 90% to 60% of their features with prototype B and only 10% to 40% of their features with prototype A (see Figure 1). This established two categories along a continuum, each with an internal structure related to the percentage of features shared with each of the two prototypes.
Figure 1.
Sample animal stimuli contributed by Zeithamova et al. (2008). Animals are arranged according to the number of features with which they differ from each prototypical animal. The number of features by which an animal differs from each prototype is referred to as its distance from the prototype.
Animals were coded with a unique ten-digit string, with binary dimensions each represented as a 0 or 1. Animal 0000000000 of one stimulus set had a short neck, straight tail, pointed toes, rounded snout, pointed ears, blue color, pyramidal body, spots, downward facing head and short legs while animal 1111111111 had a long neck, curly tail, curved feet, pointed nose, rounded ears, pink color, round body, stripes, upward facing head and long legs.
2.3 Design and Procedures
We used a mixed experimental design involving two groups of participants: patients and controls. Over one to two testing days, each participant completed two category learning tasks, one with paired-associate instruction and the other with feedback-based instruction. All patient participants completed the WAB, the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983) and the Cognitive Linguistic Quick Test (CLQT; Helm-Estabrooks, 2001) in order to determine severity of aphasia and naming ability as well as to characterize patients’ cognitive strengths and weaknesses.
All testing was conducted in a quiet room located at Boston University with a speech-language pathologist present to explain tasks and answer questions. At the start of each experiment, participants were instructed that they would be learning to recognize animals as belonging to one of two categories. Instructions for the category learning tasks were provided orally by the clinician with the aid of illustrated pictures. There was no limit placed on the duration of instruction so that clinicians could provide sufficient examples for patients to demonstrate comprehension of the task. Additional directions were provided orally and in writing at the start of each computerized paradigm. Learning tasks were programmed using E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA; www.pstnet.com) and consisted of a ten minute training phase involving 60 trials followed by a ten minute 72 trial testing phase. All responses were made through a computer button press. Because many patients with aphasia have compromised use of their right hand, all participants were instructed to enter responses with the middle and index fingers of their left hand. Stimulus sets and learning tasks were counterbalanced across participants.
As previously acknowledged, stimuli for the experiment were developed by Zeithamova et al. (2008). One of the experiments implemented by Zeithamova et al. (2008) provided the framework for our feedback-based task described below. The second experimental paradigm, our paired-associate task, was adapted from Poldrack et al.’s (2001) experiment in which researchers compared neural activations during paired-associate and feedback-based versions of the weather prediction task.
2.3.2 Feedback based (FB) training
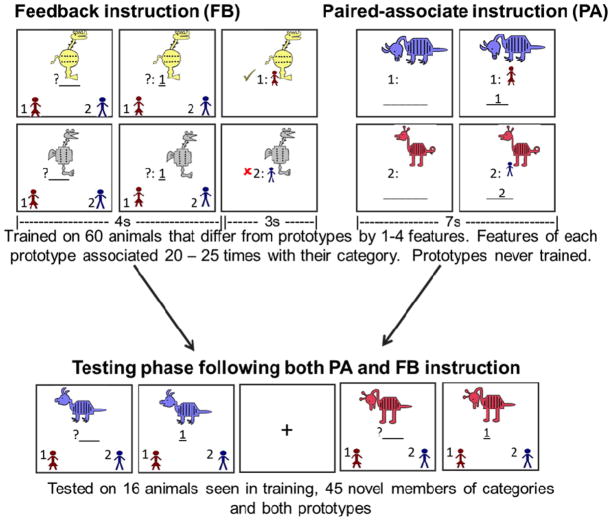
In the training phase of the FB task, category A animals and category B animals were randomly presented one at a time on a computer screen. As each animal appeared on the screen, participants were given 4000 msec to guess to which of the two categories the animal belonged. Pictures and identifiers in the lower left and right corners indicated that button presses “A” and “B” corresponded to the two different categories (see Figure 2). After responding with a button press, participants received feedback for 3000 msec telling them the correct category and whether their response was correct or incorrect.
Figure 2.
Structure of paired-associate (PA) and feedback-based (FB) instruction paradigms. Learning tasks both involved ten minute training phases followed by ten minute testing phases. During PA learning participants were provided with category labels with each stimulus presentation. During FB learning, participants had to guess each animal’s category affiliation, receiving feedback telling them whether they were correct or incorrect.
Training was comprised of 60 trials. Participants were trained on 20 animals that differed from each prototype by 1–4 features. Participants were never trained on prototypes. Trained animals were selected so that each feature appeared an equal number of times (30) during training. Features that were typical of a category (shared with the prototype) were seen 21–24 times associated with that category, in contrast to atypical features which were only associated with the category 5–9 times each. Participants were instructed to try to learn to recognize animals as belonging to one category or to another without concentrating on one particular feature. They were told that in the beginning they would be guessing entirely at random, but that through feedback and practice they would begin to learn to recognize items. A counter in the upper right-hand corner of the screen reflected the participants’ percentage of correct responses with each trial. Only participants’ first responses were recorded, scored and analyzed. Following the training phase, participants were tested on their ability to categorize trained and untrained items, this time receiving no feedback.
2.3.1 Paired associate (PA) training
Similar to the FB task, category A animals and category B animals were presented one at a time, however instead of learning through trial-by-trial feedback, in this paradigm each animal was presented along with a label denoting its category affiliation. Participants were instructed to press the button that matched the category affiliation as soon as they saw an animal and affiliation appear on the screen. They were told that the image would remain on the screen for a fixed number of seconds. Participants were instructed to study animals and their category labels with the goal of later recognizing animals as belonging to one category or to the other. Participants were instructed to pay attention to all of the characteristics of the animals without focusing in on one single feature.
Animals remained on the screen for 7000 msec, followed by a 1000 msec fixation cross, matching the total trial time of the FB task. Again, participants were trained on 60 animals that differed from each prototype by 1–4 features and were not included in the FB task, with each feature appearing an equal number of times. Prototypical animals were not shown. Features that were typical of a category were seen 20–25 times associated with that category, in contrast to atypical features, which were only associated with the category 5–10 times each. Following the training phase, participants were tested on their ability to categorize both trained and novel members of the categories.
2.3.3 Testing phases
Short testing phases that followed each training task were identically structured following PA and FB instruction (see Figure 2). Animals were presented one at a time on the computer screen and participants were given 4000 msec to categorize each animal as belonging to category “A” or “B”. Patients received no feedback related to accuracy. If in the initial trials of a testing participants took too long to respond or did not respond, a researcher quietly encouraged them to make a button press reflective of their best guess.
Testing phases immediately followed training and were comprised of 72 trials. Participants categorized 16 animals that were seen in training, 45 novel members of the categories and both prototypes. Participants were tested on their categorization of three repetitions of prototype A and prototype B animals (6 trials), seven instances each of animals varying from prototypes A and B by 1 to 4 stimulus features (56 trials) and five midline animals varying from prototypes by 5 features (5 trials). Animals that differed from prototypes by 5 features represent the middle of the spectrum and therefore have no accurate categorization. For data analysis purposes these animals were coded with an “A” response and participants were expected to show around 50% “A” response. Data were collected on accuracy and reaction time. For the current paper, we limit our analyses to accuracy rates.
2.4 Data Analysis
One control participant and one patient participant reported attending to only one feature during categorization. Review of their data confirmed that responses favored one feature over others and were therefore dropped.
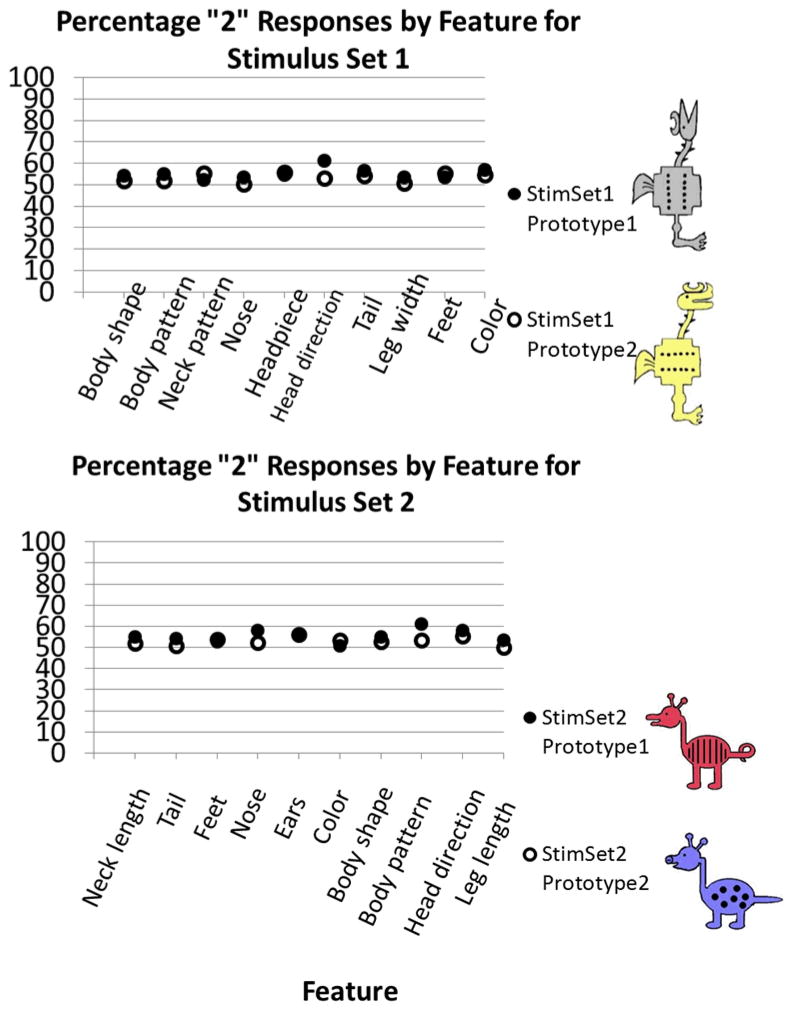
In order to ensure that no single feature had been more salient than others in its influence on responses during categorization paradigms, we completed preliminary analyses of raw data, examining the frequency with which each feature was categorized with a specific prototype. If gray color, for example, disproportionately stood out as a salient feature of category B and led participants to base their categorization on this feature alone, we would expect a greater percentage of “B” responses for those animals with the gray color feature. If all features were equivalently salient in their influence on category responses, we would expect features to be categorized with each prototype an equal number of times. Analyses confirmed that features had equal salience, the average “B” response for each feature being 54.21%, SD = 2.55. See Figure 3 for a plot of percentage of “B” responses by feature for each stimulus set.
Figure 3.
Analysis of category responses as a factor of feature dimension. Responses close to 50% represent equally salient feature dimensions. Prototypes for stimulus set 1 (upper plot) and stimulus set 2 (lower plot) shown.
Data included in further analyses were then interpreted in terms of participant ability to learn categories following the two training methods. Responses were first converted from percent accuracy score at each distance into a percent “B” response score (%BResp) at each distance. Due to the continuous, probabilistic feature structure of the two categories, we hypothesized that successful category learning would reflect internal category structure with accurate %BResp predicted to increase by a factor of 10% with each incremental distance increase from prototype A. As described in Knowlton et al. (1994), in probabilistic learning tasks, participants have a tendency to “probability match” meaning that responses will reflect the probability with which stimulus-response associations are reinforced during learning. Applied to our task, a probability match for an animal at distance 1 is hypothesized to correspond to a 10%BResp (i.e. 90% categorization with category A and 10% categorization with category B) since animals at distance 1 share 10% of their features with prototype B. Learning of our categories, therefore, is predicted to correspond to a linearly increasing %BResp with a slope of 10 (see Figure 4 for model prediction). Chance response would result in a 50%BResp at each distance, corresponding to a linear slope of zero.
Figure 4.
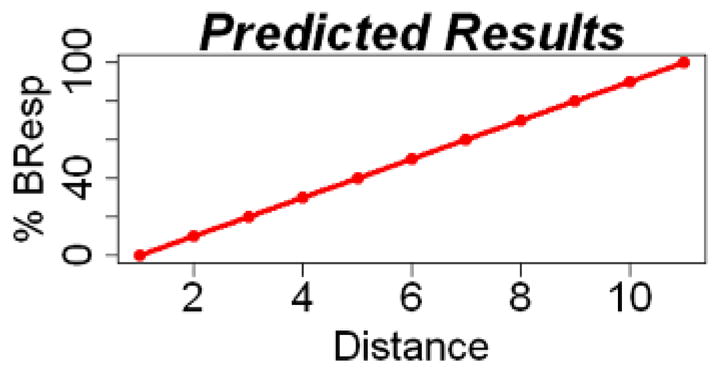
Predicted percent “B” responses (%BResp) as a function of distance. Based on the hypothesized probabilistic relationship between %BResp and distance, successful category learning is thought to correspond to %BResp that increase linearly by a factor of 10 (slope of 10).
Overall performance was analyzed using a mixed model analysis of variance (ANOVA), with %BResp at each distance (11) and task (2 – PA, FB) as within-subject factors, and Group (2-controls, patients) as the between-subject factor. In this analysis, if overall results match our predicted model, we expect to see a significant main effect of %BResp at each distance corresponding to increasing %BResp scores with increasing ordinal distance. A significant main effect of task would suggest that average results were higher following one method of instruction over another. Similarly, higher overall scores for one group over another will result in a main effect of group. Our question of interest is to examine whether the pattern of change in %BResp with increasing distance differs between groups. Different patterns of change in %BResp at each distance between patients and controls (i.e. controls show increasing %BResp with increasing distance while patients show steady %BResp with increasing distance) will result in a significant group x %BResp interaction. If there is a significant interaction between task and %BResp at each distance, it would indicate that one method of instruction, FB or PA, is superior to the other.
We also conducted polynomial trend analyses at the overall participant, group and group x task levels in order to test our model linear prediction. Finally, we investigated individual results, calculating a linear correlation coefficient for each individual’s data between %BResp at each distance and the ordinal variable distance. Individual results were tested for linearity using a method in which significance levels of regressions were compared when the independent variable was squared (quadratic) or cubed vs. non-squared. Results were considered linear when the non-squared regression reached significance with an alpha value <.05 and the significance of the squared term exceeded this level (Cox & Wermuth, 1994; Gasdal, 2012). We propose that if linear trends are maintained in the data, regression lines can be fitted to individual results and scores reduced to slopes; a slope of 10 representing ideal learning as described above.
3. Results
3.1 Grouped Results
Our 11 × 2 × 2 mixed-model ANOVA with a Huyhn-Felt correction yielded a significant main effect of %BResp at each distance, F(4.39 Huyhn-Felt corrected df, 290) = 30.39, p < .00, matching our prediction that %BResp changed as stimulus distance from prototype A increased. There was no significant main effect of task, F(1, 29) = 2.83, p = .10, proposing that overall performance was the same for both tasks. Mean accuracy following FB instruction was 51.43% (SD = 1.15) compared with a mean accuracy following PA instruction of 48.89% (SD = .98). There was also no main effect of group, F(1, 29) = .061, p = .81, with mean accuracy rates of 50.35% (SD = 1.18) for controls and 49.97% (SD = .94) for patient participants. The mixed-model ANOVA yielded a significant interaction for group x %BResp at each distance, F(4.39Huyhn-Felt corrected df,290) = 14.21, p < .00, demonstrating that patients and controls showed different patterns of learning. The interaction between task x %BResp at each distance was not significant, F(4.04 Huyhn-Felt corrected df, 290) = .97, p = .42, demonstrating that performance did not change based on method of instruction.
The polynomial trend analysis conducted over all participant results produced a statistically significant linear trend for distance, F(1, 30) = 63.17, p < .001. No higher order trends reached significance. At the group level, polynomial trend analysis confirmed a linear relationship between %BResp and distance for the control group, F(1, 11) = 154.60, p < .001. All higher order trends were non-significant. A significant linear trend was maintained in the %BResp at each distance x task comparison, F(1,11) = 5.18, p = .04, with no significant higher order trends. A linear trend was maintained for the PA task F(1, 11) = 634.17, p < .001, as well as for the FB task, F(1, 11) = 33.29, p < .001, with non-significant higher order trends. Thus, control group results support our hypothesis that learning is reflected through a linearly increasing %BResp with increasing distance. In addition, a linear increase was observed following both FB and PA instruction.
For the patient group, neither linear nor quadratic trends reached significance, F(1, 18) = 3.34, p = 0.08; F(1,18) = 1.57, p = .23, respectively. Instead, results significantly matched third and fourth order trends, F(1, 18) = 4.29, p = .05; F(1, 18) = 7.61, p = .01. Polynomial trend analysis of %BResp at each distance x task did not yield any significant first, second or third order trends. Control and patient results are summarized in Figure 5, in plots of mean accuracy as a function of distance, in which a linearly increasing trend is apparent in the control group, while absent from patient results.
Figure 5.
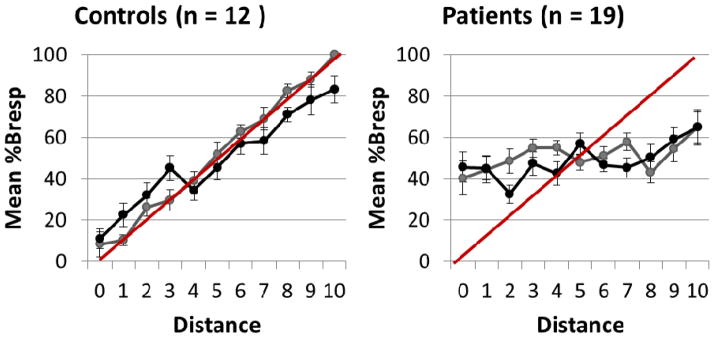
Mean %BResp as a function of distance and standard deviations for controls (left) and patients (right). Red lines represent predicted measures demonstrating successful learning of category structure.
3.2 Individual Results
Control group results matched our prediction of linearly increasing %BResp as a function of distance such that at the individual level, successful learning was defined as a significant positive correlation between %BResp and ordinal distance that also satisfied our tests of linearity.
Based on these criteria, all twelve controls demonstrated successful learning of our category tasks, with 10/12 controls showing successful learning following both methods of instruction. One additional control showed successful learning following PA instruction and FB scores that approached significance (p = .06). One control participant showed successful learning following PA instruction, but not FB instruction (see Table 3).
Table 3.
Individual control and patient results on PA and FB category-learning tasks
| Participant | PA task | FB task | Learner classification | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Slope | R | p | R2 term | p | Slope | R | p | R2 term | p | ||
| Cn1 | 9.61 | 0.98 | ***<.001 | 0.98 | 0.54 | 10.52 | 0.98 | ***<.001 | 0.99 | 0.11 | PA & FB learner |
| Cn2 | 9.48 | 0.96 | ***<.001 | 0.97 | 0.08 | 11.56 | 0.95 | ***<.001 | 0.95 | 0.75 | PA & FB learner |
| Cn3 | 9.22 | 0.95 | ***<.001 | 0.98 | *0.02 | 8.70 | 0.95 | ***<.001 | 0.95 | 0.89 | PA & FB learner |
| Cn4 | 8.74 | 0.9 | ***<.001 | 0.91 | 0.36 | 6.62 | 0.8 | **<.01 | 0.82 | 0.48 | PA & FB learner |
| Cn5 | 11.75 | 0.93 | ***<.001 | 0.93 | 0.46 | 4.63 | 0.58 | 0.06 | 0.58 | 0.83 | PA learner |
| Cn6 | 8.70 | 0.93 | ***<.001 | 0.93 | 0.53 | 9.74 | 0.94 | ***<.001 | 0.94 | 0.97 | PA & FB learner |
| Cn7 | 11.49 | 0.96 | ***<.001 | 0.96 | 0.47 | 8.96 | 0.91 | ***<.001 | 0.91 | 0.83 | PA & FB learner |
| Cn8 | 8.12 | 0.87 | **<.01 | 0.87 | 0.71 | −3.63 | −0.5 | 0.12 | 0.50 | 0.90 | PA learner |
| Cn9 | 9.94 | 0.93 | ***<.001 | 0.96 | 0.05 | 3.07 | 0.62 | *0.04 | 0.66 | 0.40 | PA & FB learner |
| Cn10 | 10.56 | 0.98 | ***<.001 | 0.98 | 1.00 | 7.96 | 0.86 | **<.01 | 0.92 | *0.04 | PA & FB learner |
| Cn11 | 8.40 | 0.87 | **<.01 | 0.91 | 0.08 | 7.71 | 0.94 | ***<.001 | 0.94 | 0.89 | PA & FB learner |
| Cn12 | 7.53 | 0.8 | **<.01 | 0.863 | 0.31 | 5.89 | 0.72 | *0.01 | 0.72 | 0.96 | PA & FB learner |
| Pt 1 | −9.07 | −0.92 | **<.01 | 0.92 | 0.71 | 10.26 | 0.95 | **<.01 | 0.95 | 0.51 | FB learner |
| Pt 2 | 8.29 | 0.91 | **<.01 | 0.93 | 0.17 | 9.48 | 0.92 | **<.01 | 0.93 | 0.72 | PA & FB learner |
| Pt 3 | −9.46 | −0.97 | **<.01 | 0.97 | 0.76 | 7.66 | 0.87 | **<.01 | 0.87 | 0.84 | FB learner |
| Pt 4 | 7.96 | 0.87 | **<.01 | 0.89 | 0.30 | 9.48 | 0.94 | **<.01 | 0.95 | 0.33 | PA & FB learner |
| Pt 5 | 9.57 | 0.93 | **<.01 | 0.934 | 0.53 | −1.90 | −0.29 | 0.38 | 0.49 | 0.25 | PA learner |
| Pt 6 | 5.15 | 0.72 | *0.01 | 0.78 | 0.18 | −7.32 | −0.81 | **<.01 | 0.81 | 0.91 | PA learner |
| Pt 7 | 9.81 | 0.94 | **<.01 | 0.94 | 0.74 | −9.74 | −0.88 | **<.01 | 0.89 | 0.41 | PA learner |
| Pt 8 | 6.06 | 0.82 | **<.01 | 0.82 | 0.98 | 6.71 | 0.70 | 0.02 | 0.82 | 0.07 | PA & FB learner |
| Pt 9 | 4.87 | 0.67 | *0.02 | 0.68 | 0.77 | 1.95 | 0.29 | 0.39 | 0.53 | 0.17 | PA learner |
| Pt 10 | 7.84 | 0.82 | **<.01 | 0.83 | 0.59 | 3.94 | 0.57 | 0.07 | 0.60 | 0.48 | PA learner |
| Pt 11 | −0.17 | −0.03 | 0.92 | 0.2 | 0.59 | 8.83 | 0.87 | **<.01 | 0.87 | 0.72 | FB learner |
| Pt 12 | −2.45 | −0.31 | 0.35 | 0.32 | 0.79 | −3.33 | −0.51 | 0.11 | 0.74 | 0.05 | Non-learner |
| Pt 13 | 1.91 | 0.27 | 0.42 | 0.29 | 0.76 | −0.52 | −0.07 | 0.84 | 0.54 | 0.11 | Non-learner |
| Pt 14 | −4.37 | 0.59 | 0.06 | 0.62 | 0.50 | −0.78 | 0.12 | 0.72 | 0.21 | 0.63 | Non-learner |
| Pt 15 | −1.04 | −0.17 | 0.61 | 0.27 | 0.56 | 2.27 | 0.34 | 0.32 | 0.70 | 0.04 | Non-learner |
| Pt 16 | 3.4 | 0.51 | 0.11 | 0.57 | 0.41 | −1.17 | −0.15 | 0.65 | 0.56 | 0.10 | Non-learner |
| Pt 17 | −2.66 | −0.32 | 0.33 | 0.62 | 0.10 | 2.55 | 0.49 | 0.13 | 0.49 | 0.98 | Non-learner |
| Pt 18 | 1.93 | 0.34 | 0.31 | 0.38 | 0.62 | −1.17 | −0.21 | 0.54 | 0.30 | 0.55 | Non-learner |
| Pt 19 | −0.76 | 0.01 | 0.77 | 0.77 | 0.67 | −0.52 | −0.07 | 0.83 | 0.23 | 0.55 | Non-learner |
p < .05
p < .01
p < .001
Note. Slope values in italics indicate best slope of learning identified for each patient.
Among our patient group, only eleven out of nineteen patients had learning scores that satisfied our criteria for learning following at least one method of instruction (learners: PWA 1–11). In contrast, for the remaining eight patients, there were no significant positive correlations between %BResp and distance, and patterns of increase of %BResp did not follow linear trends, suggesting that these patients did not demonstrate category learning following either method of instruction (non-learners).
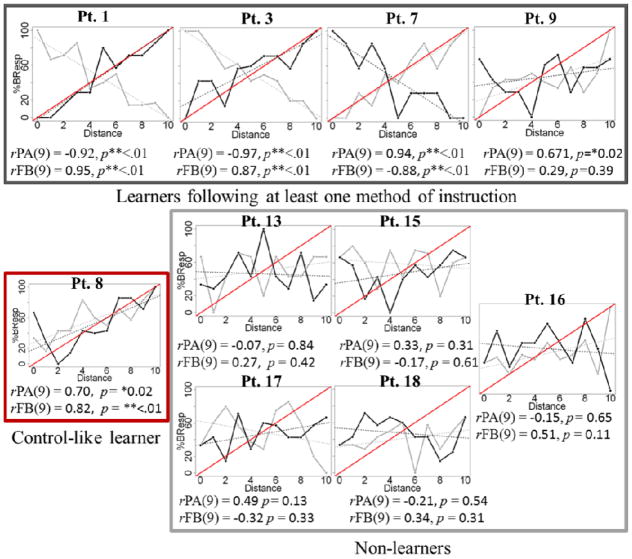
Closer examination of the eleven learners revealed that three were able to learn following FB instruction, but not PA instruction (PWA 1, PWA 3, PWA 11); four learned following PA instruction but not FB instruction (PWA 5, PWA 6, PWA 7, PWA 9), and three patients demonstrated control-like behavior, learning categories following both PA and FB instruction (PWA 2, PWA 4, PWA 8, see Table 3). One patient, PWA10, learned following PA instruction and had FB scores which approached significance (p = .07). Four patient learners, two of whom were classified as FB learners (PWA 1 and PWA 3) and two PA learners (PWA 6 and PWA 7) showed a pattern in which correlations between %BResp and distance were linear, and coefficients approached negative one (see Figure 6 for a representative sample of patient result plots). We suggest that this might reflect some learning of category structure, as %BResp increased linearly by a factor of negative ten, however that categories were reversed. For this reason we remain conservative in our conclusions regarding comparisons between instruction methods. We do, however, confidently report that learning in patients with aphasia was different from learning in healthy controls, with only 60% of our patient participants demonstrating successful nonverbal category learning. Furthermore, the patterns of learning observed in the patients characterized as learners differed from the patterns of learning observed in non-learners. While those patients classified as learners showed categorical learning following at least one method of instruction as evidence by significant positive correlations between distance and %BResp as a function of distance, the eight patients who we classified as non-learners did not show significant positive or negative correlations between %BResp and distance following either method of instruction.
Figure 6.
Representative sample of individual patient results for nine participants, grouped by learner type. Dark lines reflect FB learning and gray lines represent PA learning. Red lines reflect model measures of successful learning.
In order to interpret results relative to patient characteristics such as months post onset, aphasia type and severity to identify any predictors of learning ability, we aimed to reduce each individual patient’s results into a single score. Control results demonstrated linear trends in 22/24 tests (12 participants, two tasks) and thus confirmed that for each task, %BResp at each distance was linearly related to the dependent variable and could therefore be reduced to a single score. Supported by these findings, we reduced each patient’s data to two scores: one for the PA task and one for the FB task. A regression line was fitted to individual results, and slopes of regression lines were recorded. Slopes were assigned as learning scores, and were used to conduct further analyses considering the relationship between learning ability and patient profile, language and cognitive function as characterized by standardized tests. Scores for patients who did not show successful learning of our task and whose results therefore violated the assumption of linearity were still reduced. We confirmed that slopes for patient learners were closer to ten than the slopes of those patients who did not demonstrate successful learning (see Figure 7 for patient slope scores).
Figure 7.
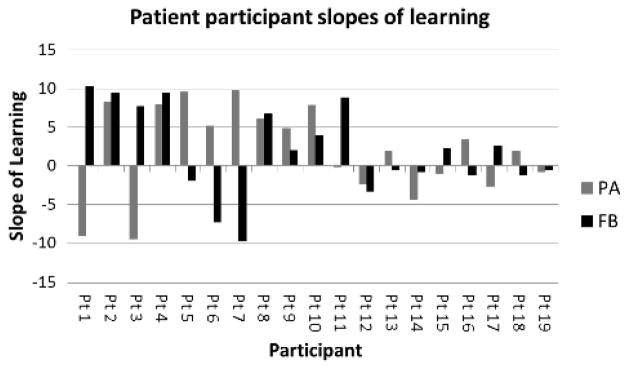
Learning slopes for each patient participant. FB scores are presented in black, PA scores in gray. Slopes closest to positive 10 represent ideal learning of categories.
For subsequent interpretations of learning and patient profile we selected a “best slope” for each patient, this slope being the slope that most closely approached positive ten whether instruction was PA or FB based (see italicized values in Table 3). Pearson correlations of learning slopes with age and years of education were completed to explore the relationship between category learning and aphasia characteristics. We conducted additional correlations of learning slopes with aphasia quotients (AQs), raw scores on the BNT and CLQT subtests of memory, attention, executive functions and visuospatial skills.
Bivariate correlations between best slope and patient age, months post onset of stroke and years of education were non-significant. In addition, correlations between best slope of learning and BNT scores, scores of attention, scores of memory, executive function, visuospatial skills and AQs were not significant (see Figure 8). Visual inspection of the data demonstrated that three clusters arose among participants with respect to AQ scores. The first cluster was made up of patients who produced high scores of learning on our task and also had the most severe aphasia as characterized by AQ scores. The second cluster was comprised of patients who produced low scores of learning and had AQ scores in the middle range of severity, while the third cluster was made up of patients with high scores of learning and the highest AQ scores.
Figure 8.
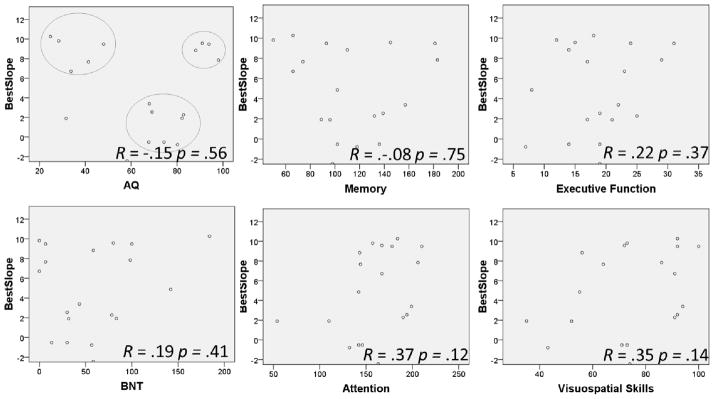
Pearson correlations between patient best slopes of learning and AQ, BNT and scores of memory, executive function, attention and visuospatial skills as measured by the CLQT. Visual inspection of the data demonstrated the presence of three clusters related to AQ scores (upper left plot).
4. Discussion
The aim of this study was to explore whether patients with aphasia learn novel non-linguistic information following PA and FB instruction. We first needed to characterize learning in healthy individuals and found, as hypothesized, that control participants were able to learn to categorize animals following both FB and PA instruction. Research exploring category learning has proposed that the process of recognizing and grouping patterns is essential in enabling our fast recognition of objects. Category learning requires individuals to process and detect commonalities across stimuli, accruing information about a series that is then organized within a framework, a process very different from single item recall or recollection (Knowlton & Squire, 1993; Seger & Miller, 2010). The current results add to the body of work that demonstrates how healthy individuals have a rapid ability to recognize and group patterns even in the absence of explicit instruction.
For the patient group, we predicted that patients with aphasia would demonstrate one of two outcomes. We hypothesized that if language deficits arise within the context of a preserved architecture to support learning, patients would demonstrate preserved non-linguistic learning. On the other hand, if language deficits in aphasia are accompanied by deficits in general cognition subsequent to brain damage, we hypothesized that patients would show impaired learning of categories. In our experiment, only eleven out of nineteen patients produced category learning results that were similar to controls following at least one method of task instruction. For 60% of the patients with aphasia who were tested, therefore, results suggest that general learning is supported, results further implying preservation of the conceptual knowledge that provides the basis for categorization (Chertkow et al., 1997) and of categorization ability (Koenig et al., 2006; Koenig et al., 2007). However, of the eleven patients who showed successful category learning, eight showed learning following one method of instruction but not the other, a pattern not observed in healthy age-matched controls. For the remaining 40% of patient participants, impairment of general learning mechanisms or of general categorization cannot be ruled out. Together, these results show for the first time that the nature of learning new category information is impaired in stroke-related aphasia.
Concerning different methods of instruction, each posing different demands, PA and FB tasks may have presented distinct cognitive challenges for each individual patient. As noted in the introduction, the demands of feedback-based and paired-associate learning are different, feedback-based learning requiring active hypothesis generation and feedback monitoring, and typically engages corticostriatal loops; while paired-associate learning depends on the formation of associations between stimuli and outcomes through observation, and likely has a high dependence on medial temporal lobe memory systems (Poldrack et al., 2001). Differences likely impacted learning strategies, attention, monitoring and motivation of patients with aphasia while completing tasks. In spite of this, results do not suggest that one method of instruction over another provided a significant advantage for patients. Previous studies in patients with amnesia, Parkinson’s Disease, Alzheimer’s disease and frontotemporal dementia identified methods of instruction that significantly benefited the population tested (Filoteo et al., 2005; Knowlton et al., 1994; Koenig et al., 2006; Koenig et al., 2007; Shohamy et al., 2004) a result that was not produced in our patients with aphasia with these particular tasks and instruction methods.
For those patients who produced results with significant, but negatively correlated %Bresp with distance, we conceive that impairments at the level of response selection and execution may have played a role. Seger & Miller (2010) draw attention to the demands posed on response selection and execution during category learning, pointing to the required coordination of cognitive and motor control. We speculate that for patients who showed significant negative correlations in their results, pattern abstraction systems may be intact with deficits arising at the level of response encoding and execution. Research has confirmed that task variables such as stimulus familiarity, complexity, modality, task demands, learning situation and response mechanism contribute to distinct neural recruitment (Poldrack et al., 2001; Seger & Miller, 2010; Squire, Stark & Clark, 2004; Zeithamova et al., 2008). Task demands have behavioral and neural implications and likely elicited damaged neural structures in our patient participants to varying degrees. Even when some learning is observed, as it was in eleven of our patients with aphasia, patients showed less consistency of learning under contrasting instruction methods, meriting further study.
Eight patients with aphasia included in the current study showed no learning of categorical structure following either method of instruction. In our experiment, we deemed these eight patients to be non-learners since they were unable to learn categories relative to controls, as well as relative to other patients with aphasia. We hypothesize that for these patient non-learners, learning ability is present but reduced. The current stimuli contained ten variable features and posed high processing demands. Furthermore, category boundaries were based on probabilistic associations of features with prototypes that are continuous, a design which can pose additional challenges. Previous research has suggested that categorization of discrete stimuli can rely on automatic recognition, while continuous or complex stimuli require pattern abstraction, rule-use and feature mapping in addition to hypothesis testing (Davis, Love, & Maddox, 2009; Love & Markman, 2003; Maddox et al., 2008; Schyns, Goldstone, & Thibaut, 1998). The pace of learning and limited trials may have provided insufficient opportunities to develop appropriate hypotheses and strategies such that some participants might have benefitted from additional training trials. While patient learners were able to overcome these complexities within the constraints of the current methods, patient non-learners may have learning systems that require additional trials, simplified stimuli, or alternate instruction methods. Previous studies have pointed to attention deficits in stroke (Marshall, Grinnell, Heisel, Newall, & Hunt, 1997; McDown, Filion, Pohl, Richards, & Stiers, 2003) and many non-learners may have experienced difficulty selectively attending to appropriate stimulus features, particularly faced with complex stimuli with multiple dimensions. It should, however, be noted that learning ability was unpredictable by standardized scores of attention; three of eight non-learners scoring within normal limits on the CLQT subtests of attention. We propose that the divergence of learning ability observed in the group of patients tested relative to controls further emphasizes the need to accurately characterize learning. Many patients likely have deficits that extend beyond language and accordingly require additional support and strategies in the setting of learning. These patients may either lack some of the cognitive support systems necessary for learning, or have compromised neural systems that require additional reinforcement and focus to optimally engage neural systems during learning.
With respect to patient characteristics, language profile and learning ability, results suggest that learning ability is unrelated to demographic variables such as age, months post onset of stroke and years of education. We had predicted that learning ability might be predicted by scores of executive function. Instead, learning scores did not correlate with any of the standardized measures obtained (AQ, BNT, or CLQT scores of memory, executive function, attention and visuospatial skills). These findings are consistent with previous studies that have failed to find a predictable relationship between verbal impairments or demographic variables and skills in nonverbal domains (Basso et al., 1973; Chertkow et al., 1997; Helm-Estabrooks, 2002). Findings further suggest that category learning ability is distinct from skills measured by the CLQT. In the present study we aimed to explore systems that are distinct from those described through existing cognitive and linguistic tests, such that experimental results which are not fully explained by standardized assessments is not surprising.
We did note the interesting finding that upon inspection of the data, three clusters surfaced among participants based on AQs. Those patients with the lowest and with the highest AQs were most successful performing our task, while patients with AQs in the middle range were not successful at learning categories. In other words, patients with the greatest level of language impairment performed better on our learning tasks than many patients with milder deficits. Germane to this finding is the fact that standardized measures provided by the WAB and CLQT are highly language dependent. The WAB AQ is derived from measures of spontaneous speech, verbal comprehension, repetition, naming and word finding, all measures that are highly verbal. Based on our results, we posit that some patients with severely impaired language may actually have cognitive learning systems that are largely intact yet often undervalued since so many cognitive scores are dependent on language ability. The CLQT does include measures that are nonverbal such as symbol cancellation, clock drawing, symbol trails, design memory, mazes and design generation; however verbal tests requiring patients to express personal facts, retell stories, and generate names weigh heavily on composite scores of memory and attention. Currently accepted standardized tests capture many factors that are critical to the assessment of aphasia, however it is likely that they do not fully encompass the affected systems in stroke. We suggest that an additional metric of nonverbal learning ability is missing in the characterization of aphasia. As applied to a clinical setting, we propose that those patients who appear to have higher-level language skills do not necessarily present with the most intact cognitive or pattern abstraction systems. These skills are likely affected to different degrees within individuals with aphasia, contributing to our current inefficiency at predicting outcomes.
The current study does have many limitations. We tested a small number of participants coming from heterogeneous pre and post-stroke contexts. The heterogeneity of patient participants allowed us to observe learning patterns in a varied population; however limited our conclusions as they relate to patient profile and language characteristics. In addition, our task was a complex task with a high reliance on visual processing. We propose that future studies of non-verbal learning should include stimuli with even less verbalizeable characteristics than those implemented here.
The current study provided preliminary evidence for the nature of non-linguistic learning in patients with aphasia. We propose that future studies should further examine learning ability in patients with aphasia and that learning ability should be compared with therapy outcomes. As hypothesized by previous researchers (Goldenberg & Spatt, 1994; Meinzer et al., 2010; Menke et al., 2009; Rijntjes, 2006) we suspect that progress with therapy may depend on learning and memory systems. We suggest that those patients who demonstrate the ability to learn information in general will transfer these skills to the treatment setting and show greater responsiveness to therapy relative to patients who demonstrate impaired category learning. If such a predictive relationship arises, future studies aimed at exploring the effect of stimulus characteristics, training factors and strategy use on learning in aphasia can provide insights into modifications that might translate to successful, individualized therapy. We suspect that supporting cognitive systems are differentially affected in patients with aphasia such that true predictability of outcomes will depend on a better characterization of non-linguistic deficits as well as the manner in which different cognitive systems are recruited during therapy.
In the long term, we posit that nonverbal learning ability is non-negligible in aphasia rehabilitation and is key towards developing individualized, predictable treatments for aphasia. We suggest that identifying a metric of learning and optimal instruction methods for patients may be the gateway to developing effective, individualized treatments.
Acknowledgments
We would like to thank all of our participants, caregivers and relatives for contributing to this study. We also thank David Caplan, Earl Miller and Alex Johnson for their intellectual input and Kathleen Wirth for her statistical consulting.
References
- Basso A, De Renzi E, Fagolioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligent tasks. Brain. 1973;96:715–728. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- Best W, Nickels L. From theory to therapy in aphasia: Where are we now and where to next? Neuropsychological Rehabilitation. 2000;10(3):231–247. [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster A, Sommer J, Wolbers T, Knecht S. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Kamping S, Jansen A, Schomacher M, Knecht S. Word learning can be achieved without feedback: Implications to aphasia therapy. Restorative Neurology and Neuroscience. 2004;22:445–458. [PubMed] [Google Scholar]
- Chertkow H, Bub D, Deaudon C, Whitehead V. On the status of object concepts in aphasia. Brain and language. 1997;58:203–232. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- Cox DR, Wermuth N. Tests of linearity, multivariate normality and the adequacy of linear scores. Applied Statistics. 1994;43(2):347–355. [Google Scholar]
- Davis T, Love B, Maddox T. Two pathways to stimulus encoding in category learning? Memory and Cognition. 2009;37(4):394–413. doi: 10.3758/MC.37.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen The Hippocampus – what does it do? Behavioral and Neural Biology. 1992;57(1):2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Erickson RJ, Goldinger SD, LaPointe LL. Auditory vigilance in aphasic individuals: Detecting nonlinguistic stimuli with full or divided attention. Brain and Cognition. 1996;30:244–253. doi: 10.1006/brcg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Ferguson A. Learning in aphasia therapy: It’s not what you do but how you do it! Aphasiology. 1999;13(2):125–150. [Google Scholar]
- Fillingham JK, Sage K, Lambon Ralph M. The application of errorless learning to aphasic disorders: A review of theory and practice. Neuropsychological Rehabilitation. 2005a;13:337–363. doi: 10.1080/09602010343000020. [DOI] [PubMed] [Google Scholar]
- Fillingham JK, Sage K, Lambon Ralph M. Treatment of anomia using errorless versus errorful learning: Are frontal executive skills and feedback important? International Journal of Communication Disorders. 2005b;40(4):505–523. doi: 10.1080/13682820500138572. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Zizak V, Song DD. Information-integration category learning in patients with striatal dysfunction. Journal of International Neuropsychological Society. 2005;11(5):230–241. doi: 10.1017/S1355617705050617. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18:193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Nettles C, Davis M, Morrow L, Montgomery A. Functional communication and executive function in aphasia. Clinical Linguistics and Phonetics. 2006;20:401–410. doi: 10.1080/02699200500075781. [DOI] [PubMed] [Google Scholar]
- Gasdal O. Analyzing cross sectional survey data using linear regression method: x ‘hands’ on introduction using ESS data. Bergen, Norway: Norwegian Social Science Data Services & European Social Survey Education Net; 2012. Retrieved from http://essedunet.nsd.uib.no/cms/topics/regression/4/3.html. [Google Scholar]
- Grossman M, Carey S. Selective word-learning deficits in aphasia. Brain and Language. 1987;32:306–324. doi: 10.1016/0093-934x(87)90130-1. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J. Influence of size and site of cerebral lesions on spontaneous recovery of aphasia and on success of language therapy. Brain and Language. 1994;47:684–698. doi: 10.1006/brln.1994.1063. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carey S. Selective word-learning deficits in aphasia. Brain and Language. 1987;32:306–324. doi: 10.1016/0093-934x(87)90130-1. [DOI] [PubMed] [Google Scholar]
- Gupta P, Martin N, Abbs B, Schwartz M, Lipinski J. New word learning in aphasic patients: Dissociating phonological and semantic components. Brain and Language. 2006;99:8–9. [Google Scholar]
- Helm-Estabrooks N. Cognitive linguistic quick test. New York: The Psychological Corporation; 2001. [Google Scholar]
- Helm-Estabrooks N. Cognition and aphasia: A discussion and a study. Journal of Communication Disorders. 2002;35:171–186. doi: 10.1016/s0021-9924(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Holland A, Fromm D, DeRuyter F, Stein M. Treatment efficacy: Aphasia. Journal of Speech and Hearing Research. 1996;39(5):S27–S36. doi: 10.1044/jshr.3905.s27. [DOI] [PubMed] [Google Scholar]
- Howard D, Hatfield MH. Aphasia therapy. Hove, UK: Lawrence Erlbaum Associates Ltd; 1987. [Google Scholar]
- Hula WD, McNeil MR. Models of attention and dual-task performance as explanatory constructs in aphasia. Seminars in Speech and Language. 2008;29(3):169–187. doi: 10.1055/s-0028-1082882. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph M. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Keil K, Kaszniak A. Examining executive function in individuals with brain injury: A review. Aphasiology. 2002;16(3):305–335. [Google Scholar]
- Kelly H, Armstrong L. New word learning in people with aphasia. Aphasiology. 2009;23(12):1398–1417. [Google Scholar]
- Kertesz A. The Western Aphasia Battery. Philadelphia: Gruvne & Stratton; 1982. [Google Scholar]
- Kiran S, Sandberg C. Treating communication problems in individuals with disordered language. In: Peach R, Shapiro L, editors. Cognition and acquired language disorders: An information processing approach. Saint Louis: Elsevier Mosby; 2011. pp. 298–325. [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: parallel brain systems for item memory and category knowledge. Science. 1993;262(5140):1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Koenig P, Smith E, Grossman M. Semantic categorization of novel objects in frontotemporal dementia. Cognitive Neuropsychology. 2006;23(4):541–562. doi: 10.1080/02643290542000094. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith E, Moore P, Glosser G, Grossman M. Categorization of novel animals by patients with Alzheimer’s disease and corticobasal degeneration. Neuropsychology. 2007;21(2):193–206. doi: 10.1037/0894-4105.21.2.193. [DOI] [PubMed] [Google Scholar]
- LaPointe LL, Erickson RJ. Auditory vigilance during divided task attention in aphasic individuals. Aphasiology. 1991;5(6):511–520. [Google Scholar]
- Lesniak M, Bak T, Czepiel W, Seniow J, Czlonkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dementia and Geriatric Cognitive Disorders. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- Love BC, Markman AB. The nonindependence of stimulus properties in human category learning. Memory & Cognition. 2003;31:790–799. doi: 10.3758/bf03196117. [DOI] [PubMed] [Google Scholar]
- Maddox T, Love B, Glass B, Filoteo V. When more is less: Feedback effects in perceptual category learning. Cognition. 2008;108:578–589. doi: 10.1016/j.cognition.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E, Frith C. The brain network associated with acquiring semantic knowledge. NeuroImage. 2004;22:171–178. doi: 10.1016/j.neuroimage.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Marshall SC, Grinnell D, Heisel B, Newall W, Hunt L. Attentional deficits in stroke patients: A visual dual task experiment. Archives of Physical Medicine and Rehabilitation. 1997;78:7–12. doi: 10.1016/s0003-9993(97)90002-2. [DOI] [PubMed] [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Effects of facilitation and cueing on labeling of ‘novel’ stimuli by aphasic subjects. Aphasiology. 1992;6:567–583. [Google Scholar]
- McDown JM, Filion DL, Pohl PS, Richards LG, Stiers W. Attentional abilities and functional outcomes following stroke. The Journals of Gerontology. 2003;58B:45–53. doi: 10.1093/geronb/58.1.p45. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Mohhamed S, Kugel H, Schiffbauer H, Floel A, Albers J, Deppe M. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage. 2010;53:283–290. doi: 10.1016/j.neuroimage.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, Breitenstein C. Imaging short- and long-term training success in chronic aphasia. BMC Neuroscience. 2009;10(1):118. doi: 10.1186/1471-2202-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. Attention and other cognitive deficits in aphasia: Present and relation to language and communication measures. American Journal of Speech-Language Pathology. 2012;21:S51–S64. doi: 10.1044/1058-0360(2012/11-0067). [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies A, Corbett F, Lambon Ralph M. Elucidating the nature of deregulated semantic cognition in semantic aphasia: Evidence of the roles of prefrontal and temporo-parietal cortices. Journal of Cognitive Neuroscience. 2009;22(7):1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Opitz B, Friederici A. Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. NeuroImage. 2003;19:1730–1737. doi: 10.1016/s1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Peach R, Rubin S, Newhoff M. A topographic event-related potential analysis of the attention deficit for auditory processing in aphasia. Clinical Aphasiology. 1994;22:81–96. [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack R, Packard M. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–51. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Ramsberger G. Achieving conversational success in aphasia by focusing on non-linguistic cognitive skills: A potentially promising new approach. Aphasiology. 2005;19(10/11):1066–1073. [Google Scholar]
- Rijntjes M. Mechanisms of recovery in stroke patients with hemiparesis or aphasia: New insights, old questions and the meaning of therapies. Current Opinion in Neurology. 2006;19:76–83. doi: 10.1097/01.wco.0000203886.28068.38. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Goldstone RL, Thibaut J. Development of features in object concepts. Behavioral and Brain Sciences. 1998;21:1–54. doi: 10.1017/s0140525x98000107. [DOI] [PubMed] [Google Scholar]
- Seger C, Miller E. Category learning in the brain. Annual Review of Neuroscience. 2010;33:203–19. doi: 10.1146/annurev.neuro.051508.135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127(4):851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Spreen O, Risser AH, editors. Assessment of Aphasia. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire L, Stark C, Clark R. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry. 2012 doi: 10.1038/mp.2011.177. in press. Epub ahead of print retrieved June, 2012, from http://www.ncbi.nlm.nih.gov/pubmed/22212597. [DOI] [PMC free article] [PubMed]
- Tuomiranta L, Grönholm-Nyman P, Kohen F, Rautakoski P, Laine M, Martin N. Learning and maintaining new vocabulary in persons with aphasia: Two controlled case studies. Aphasiology. 2011;25(9):1030–1052. [Google Scholar]
- Turken A, Dronkers N. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Maddox WT, Schnyer DM. Dissociable prototype learning systems: evidence from brain imaging and behavior. Journal of Neuroscience. 2008;28(49):13194–201. doi: 10.1523/JNEUROSCI.2915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn S, Bosworth HB, Hoenig HM, Swartwelder HS. Executive function deficits in acute stroke. Archives of Physical Medicine and Rehabilitation. 2007;88:173–180. doi: 10.1016/j.apmr.2006.11.015. [DOI] [PubMed] [Google Scholar]