Abstract
Substantial evidence indicates that neuroinflammation caused by over-activation of microglial in the substantia nigra is critical in the pathogenesis of dopaminergic neurodegeneration in Parkinson’s disease (PD). Increasing data demonstrates that environmental factors such as rotenone, paraquat play pivotal roles in the death of dopaminergic neurons. Here, potential role and mechanism of neuromelanin (NM), a major endogenous component in dopaminergic neurons of the substantia nigra, on microglial activation and associated dopaminergic neurotoxicity were investigated.
Using multiple well-established primary mesencephalic cultures, we tested whether human NM (HNM) could activate microglia, thereby provoking dopaminergic neurodegeneration. The results demonstrated that in primary mesencephalic neuron-glia cultures, HNM caused dopaminergic neuronal damage characterized by the decreased dopamine uptake and reduced numbers and shorted dendrites of dopaminergic neurons. HNM-induced degeneration was relatively selective to dopaminergic neurons since the other types of neurons determined by either gamma-aminobutyric acid uptake and total neuronal numbers after staining showed smaller decrease. We demonstrated that HNM produced modest dopaminergic neurotoxicity in neuron-enriched cultures; in contrast, much greater neurotoxicity was observed in the presence of microglia. HNM-induced microglial activation was shown by morphological changes and production of proinflammatory and neurotoxic factors. These results suggest that HNM, once released from damaged dopaminergic neurons, can be an potent endogenous activator involved in the reactivation of microglia, which may mediate disease progression. Thus, inhibition of reactive microglia can be a useful strategy for PD therapy.
Keywords: Parkinson’s disease, Substantia nigra pars compacta, Human neuromelanin, Microglia, Activation, Dopaminergic neuron, Neurodegeneration
2. INTRODUCTION
Parkinson’s disease (PD) is a debilitating disorder characterized by the progressive loss of neuromelanin (NM)-containing dopaminergic neurons in the substantia nigra pars compacta (SNpc) and depletion of dopamine ( DA ) in the striatum. The pathogenesis of PD involves multiple factors, including aging, genetic predisposition and environmental exposures. Microglia are the major immune cells in the brain. In the normal condition, microglia play a critical role by exerting immune surveillance through phagocytizing foreign subjects and removing cell debris. However, in pathological conditions, microglia can become over activated and induce uncontrollable neuroinflammation through the production of a large amount of neuroinflammatory and neurotoxic factors (2). Increasing data indicates that neuroinflammation is a major driving force producing the progressive neurodegeneration (1). Dopaminergic neurons in the SNpc are most vulnerable to neuroinflammation in PD may be linked with the fact that the number of microglia in the SNpc is about 5 times higher than the other brain regions, plus the fact that nigral dopaminergic neurons are known to have lower anti-oxidation capacity (3).
NM, an insoluble mixture of different compounds, is localized in high concentration within dopaminergic neurons (4–5) and responsible for the pigmented color of the SNpc. NM accumulates in the SNpc with age (4). Functionally, it has been suggested that NM plays a protective role in neurons where it binds toxins (6–7). However, it has also been demonstrated that NM has the potential to be toxic, since excessive NM inhibits the function of proteasome in dopaminergic neuron (8). Analysis of the SNpc from postmortem PD patients indicates that NM level is significantly reduced, which is consistent with the loss of dopaminergic neurons (4).
Besides stored in the dopamineric neurons, NM has been found in the extracellular space in the SNpc of PD patients (9). We hypothesized that NM could be released from damaged or dying dopaminergic neurons into the extracellular environment to interact with the surrounding cells, such as remaining dopaminergic neurons and glial cells, including microglia and astroglia. The goal of this study was to determine the cellular and molecular mechanisms of how extracellular NM damages the remaining dopaminergic neurons by asking the following questions. Does NM damage neurons directly or through glial cells? What kinds of glia are involved in NM-elicited neurotoxicity and what are the underlying mechanisms? Using multiple well-established primary midbrain neuron-glia cultures, we investigated the role and underlying mechanism of human NM (HNM) on dopaminergic neurodegeneration.
3. MATERIAL AND METHODS
3.1. Reagent
All materials related to the cell cultures were obtained from Invitrogen (Carlsbad, CA, USA). Sources for other compounds included: cytosine arabinoside (Ara-C), leu–leu methyl ester (LME) (Sigma-Aldrich, St. Louis, MO, USA), [3H] DA (28Ci/mmol) and [3H] gamma-aminobutyric acid (GABA) (81Ci/mmol) (PerkinElmer Life Science, Boston, MA, USA), polyclonal anti-tyrosine hydroxylase (TH) antibody (a gift from Dr. John Reinhard at Glaxo-SmithKline, Research triangle Park, NC, USA), neuronal nuclei (Neu N) antibody (Pharmingen, San Diego, CA, USA), OX-42 (BD PharMingen, San Diego, CA, USA), Vectastain ABC kit and biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA), tumor necrosis factor-alpha (TNF-alpha) enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) and prostaglandin E2 (PGE2)ELISA kit (Cayman, Ann Arbor, MI, USA).
3.2. Isolation of NM from human SNpc
NM was prepared from human SNpc as reported (5).
3.3. Animals
Time-pregnant Fisher 344 rats were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Breeding schedules for the rats were designed to achieve accurately timed-pregnancy of 14 ± 0.5 d. Housing, breeding and experimental use of the animals were performed in strict accordance with the National Institutes of Heath guidelines.
3.4. Preparation of cell cultures
3.4.1. Primary mesencephalic neuron-glia cultures ( neuron-microglia-astroglia cultures )
Rat primary mesencephalic neuron-glia cultures were prepared from the brains of embryonic day 14 ± 0.5 d of Fisher 344 rats, following our previously described protocol (10). Briefly, the ventral mesencephalic tissues were removed and dissociated by mild mechanical trituration. Cells were seeded at 5 × 105/well to a 24-well culture plate precoated with poly-D-lysine (20 μg/mL) and maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air in 0.5 mL/well maintenance medium. The medium consisted of minimum essential medium, containing 10% heat-inactivated fetal bovine serum and 10% heat-inactivated horse serum, 1 g/L glucose, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin. Three days after the initial seeding, 0.5 mL of fresh maintenance medium was added to each well. Seven-day cultures were used for the treatment. The composition at the time of treatment was about 48% astroglia, 11% microglia, and 40% neurons, of which about 1% was TH-immunoreactive (TH-ir) neurons (dopaminergic neurons). Seven days after the treatment, [3H] DA and [3H] GABA uptake assays were performed as described previously (10).
3.4.2. Primary neuron-enriched cultures
Rat mesencephalic neuron-enriched cultures, with a purity of > 95%, were established as described previously (10). Briefly, 24 h after seeding the cells, Ara-c was added to a final concentration of 7.5 μM to suppress glial proliferation. Three days later, cultures were changed back to the maintenance medium and were used for the treatment 7 d after the initial seeding. Seven days after the treatment, [3H] DA uptake assay was performed as described previously (10).
3.4.3. Primary microglia-depleted cultures (neuron-astroglia cultures )
Rat primary microglia-depleted cultures were obtained by suppressing microglial proliferation with 1.5 mM LME 24 h after seeding the cells described previously (10). Three days later, cultures were changed back to maintenance medium and were used for the treatment 7 d after the initial seeding. The composition of the cells at the time of treatment was 54% astroglia, 1% microglia, and 45% neurons. Seven days after the treatment, [3H] DA uptake assay was performed as described previously (10).
3.4.4. Primary glial cultures (microglia-astroglia cultures )
Primary glial cultures, with a purity of > 98%, were prepared from the whole brains of 1-d-old Fisher 344 rat pups, following our previously described protocol (10). Briefly, brain tissues were triturated after removing the meninges and blood vessels. Cells (5×107) were seeded in a 150 cm2 culture flask.
3.4.5. Primary microglia-enriched cultures
Microglia-enriched cultures were obtained by shaking off microglia in primary glial cultures for 1 h at 180 rpm after a confluent monolayer of glial cells had been obtained (10).
3.4.6. Primary microglia-reconstituted cultures
Primary microglia-reconstituted cultures were established by adding 10% (5.0×104/well), 20% (1.0×105/well) and 30% (1.5×105/well) of microglia back to the neuron-enriched cultures 1 d before the treatment, and 7 d after the treatment, [3H] DA uptake assay was performed as described previously (10).
3.4.7. Primary astroglia-enriched cultures
In primary glial cultures, after at least 4 separations of microglia, cells were detached with trypsin-EDTA and seeded in the same culture medium as that used for microglia (10).
3.4.8. Primary astroglia-reconstituted cultures
Primary astroglia-reconstituted cultures were established by adding 40% (5.0×104/well), 50% (1.0×105/well) and 60% (1.5×105/well) of astroglia back to the neuron-enriched cultures 1 d before the treatment, and 7 d after the treatment, [3H] DA uptake assay was performed as described previously (10).
3.5. Treatment
HNM was freshly prepared as a stock solution (1 mM) with L-cystein as a solvent (11) and diluted to the desired final concentrations in the treatment medium. Neuron-glia cultures in a 24-well culture plate were treated with vehicle or HNM (1–5 μg/mL) in a final volume of 1 mL/well.
3.6. [3H] DA and [3H] GABA uptake assays
Neurotoxicity was measured by determining the uptake of [3H]DA and [3H]GABA (10). Cultures were incubated for 20 min at 37°C with 1 μM [3H]DA and [3H]GABA in Krebs-Ringer (KR) buffer (16 mM Na3PO4, 119 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.3 mM EDTA, and 5.6 mM glucose, pH 7.4). After washing 3 times with ice-cold KR buffer, cells were collected in 1 N NaOH. Radioactivity was determined by liquid scintillation counting. The nonspecific DA and GABA uptake observed in the presence of mazindol (10 μM) and NO711 (10 μM) with beta-alanine (1 mM), respectively, was subtracted.
3.7. Immunocytochemistry
TH-ir and total neurons were detected with anti-TH (1:20,000) and anti-Neu N (1:1,000) antibodies, respectively, whereas microglia were detected with OX-42 antibody (1:500) (10). Briefly, 3.7% formaldehyde-fixed cultures were treated with 1% hydrogen peroxide (15 min) followed by sequential incubation with blocking solution (20 min), primary antibody (4°C, overnight), biotinylated secondary antibody (2 h), and ABC reagents (2 h). Color was developed with 3, 3′-diaminobenzidine. For the morphological analysis, the images were recorded with an inverted microscope (Nikon, Tokyo, Japan) connected to a charge-coupled device camera (DAGE-MTI, Michigan City, IN) operated with MetaMorph software (Universal Imaging Corporation, Downingtown, PA, USA). For visual enumeration of the immunostained dopaminergic neurons in primary mesencephalic neuron-glia cultures, 9 representative areas per well of the 24-well plate were counted under the microscope at ×100 magnifications. The overall dendrite length for individual TH-ir neurons was measured by following our previously published protocol (11).
3.8. TNF-alpha assay
The release of TNF-alpha in primary mesencephalic neuron-glia cultures was measured in 50 μL supernatant 3 h, 6 h and 24 h after HNM treatment with a rat TNF-alpha ELISA kit as described previously (12).
3.9. Nitric oxide (NO) assay
The production of NO was determined by measuring the accumulated level of nitrite (an indicator of NO) in the supernatant after 24 h, 48 h and 72 h of HNM treatment using a colorimetric reaction with Griess reagent (12). Briefly, supernatant were collected and mixed with an equal volume of Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% H3PO4]. The mixture was incubated in the dark for 10 min at room temperature, and the absorbance at 540 nm was measured with a Spectra Max Plus microplate spectrophotometer (Molecular Devices, USA). The concentration of nitrite in the samples was determined from a sodium nitrite standard curve.
3.10. PGE2 assay
The production of PGE2 in the primary mesencephalic neuron-glia cultures was evaluated in 50 μL supernatant 24 h and 48 h after HNM treatment with a PGE2 EIA kit according to the manufacturer’s instructions (13).
3.11 Statistical analysis
Approximately 30 embryos pooled from pregnant rats were used for the generation of cultured cells. In each treatment, 3 wells were used. This was considered as an independent experiment. The same experiment was repeated 4–6 times and the standard errors were calculated from the means of 4–6 independent experiments. The data were expressed as the mean ± SEM. Statistical significance was assessed with an analysis of one-way ANOVA, with the freedom representing the numbers of experiments minus one, followed by Bonferroni’s t-test using the StatView program (3.0; Abacus Concepts, Berkeley, CA). A value of P < 0.05 was considered statistically significant.
4. RESULTS
4.1 HNM produced dopaminergic neurotoxicity in primary mesencephalic neuron-glia cultures
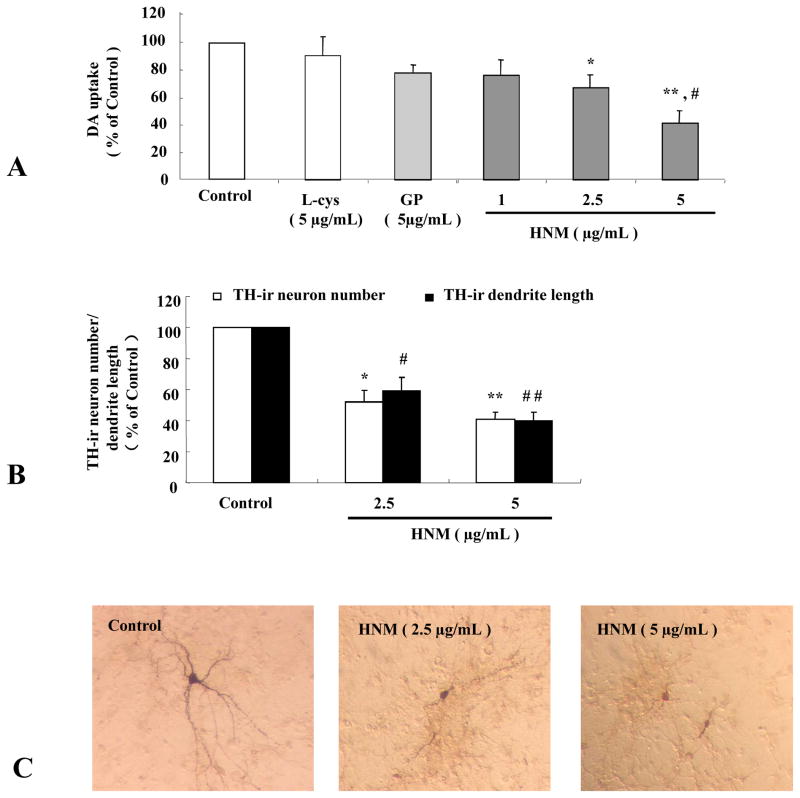
Primary mesencephalic neuron-glia cultures were treated with different concentrations of HNM (1, 2.5 and 5 μg/mL) for 7 d and the toxicity of dopaminergic neurons was evaluated by detecting [3H]DA uptake, counting TH-ir neuronal numbers, measuring neuronal dendrite length and observing morphological changes. As illustrated in Figure 1, HNM reduced the capacity of DA uptake in a dose-dependent manner. In contrast, the control solvent L-cystein (L-cys) and gold particles (GP) showed little effect on DA uptake (1A). In addition, neuronal count showed that HNM caused the loss of TH-ir neuronal numbers and neurite length, in a dose-dependent manner (1B). HNM induced apparent morphological alterations of TH-ir neurons indicated by the shrunk cell bodies, reduced cytoplasmic stainings in addition to the decreased, shorter, thinner and broken neuronal dendrites (1C).
Figure 1. HNM produced dopaminergic neurotoxicity in primary mesencephalic neuron-glia cultures.
Rat primary mesencephalic neuron-glia cultures were seeded in 24-well plate at 5 × 105/well/mL and treated with 1 μg/mL, 2.5 μg/mL and 5 μg/mL HNM for 7 d. Effect of HNM on dopaminergic neurons in neuron-glia cultures were assessed with [3H]DA uptake. *P<0.01, **P<0.001: HNM-treated group compared with vehicle-treated control group, #P<0.01: HNM-treated group compared with GP-treated control group. Typical [3H]DA uptake was 2000–4000 counts/min at basal levels without any treatment (A). TH-ir neurons and dendrite length were counted and measured directly. *P<0.05, **P<0.01: HNM-treated group compared with vehicle-treated control group, #P<0.01, ##P<0.001: HNM-treated group compared with vehicle-treated control group (B). Results were expressed as a percentage of vehicle-treated control group and were the mean ± SE from at least 3 independent experiments in triplicate. Representative microscopic images were shown for TH-ir neurons treated with vehicle, 2.5 μg/mL and 5.0 μg/mL HNM, respectively, for 7 d. (C).
4.2 HNM-induced degeneration was relatively selective to dopaminergic neurons
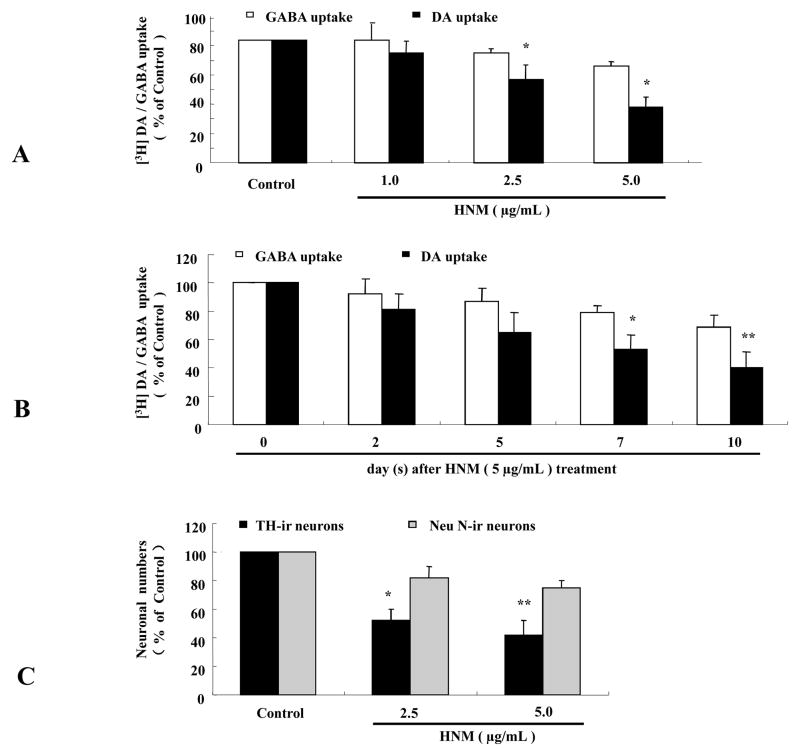
To determine the selectivity of HNM-induced neurotoxicity, both DA and GABA uptake capacities were measured in primary mesencephalic neuron-glia cultures following the treatment of HNM in different concentrations and time points. As illustrated in Figure 2, while HNM clearly reduced the capacity of DA uptake in a dose-dependent manner, the capacity of GABA uptake was little changed (2A). Similar results were obtained in a time course study (2B). Direct cell count after TH staining (as an index for dopaminergic neurons) and Neu N staining (as an index for total neurons) revealed a significant difference when cells were treated with 2.5 μg/mL and 5.0 μg/mL HNM for 7 d (2C). These results indicate that dopaminergic neurons in the primary mesencephalic neuron-glia cultures are much more vulnerable to HNM treatment than GABA neurons.
Figiue 2. HNM-induced degeneration was relatively selective to dopaminergic neurons.
Rat primary mesencephalic neuron-glia cultures were seeded in 24-well plate at 5 × 105/well/mL and treated with 1 μg/mL, 2.5 μg/mL and 5 μg/mL HNM for 2 d, 5 d, 7 d and 10 d. Effect of HNM on dopaminergic and GABAnergic neurons in neuron-glia cultures were assessed with [3H]DA uptake and [3H]GABA uptake and cell counts after TH and Neu N stainings. *P<0.005: DA uptake compared with GABA uptake 7 d after 2.5 μg/mL and 5 μg/mL HNM treatment (A). *P<0.05: DA uptake compared with GABA uptake 7 d after 5 μg/mL HNM treatment,**P<0.01: DA uptake compared with GABA uptake 10 d after 5 μg/mL HNM treatment (B). *P<0.01, **P<0.001: Neu N-ir neuronal numbers compared with TH-ir neuronal numbers 7 d after treatment with 2.5 μg/mL and 5.0 μg/mL HNM, respectively (C).
4.3 Microglia, not astroglia, contributed to the neurotoxic effect of HNM
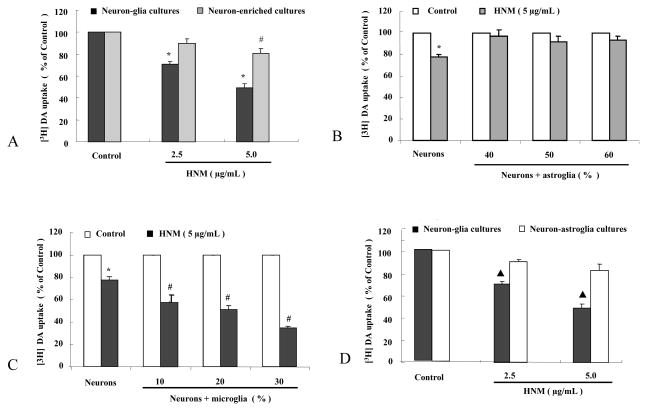
To determine the role of glia in HNM-mediated neurotoxicity, primary mesencephalic neuron-glia cultures and neuron-enriched cultures were treated with 2.5 μg/mL and 5 μg/mL HNM and dopaminergic neurotoxicity was assessed with [3H]DA uptake 7 d later. HNM induced significant dose-related dopaminergic neurotoxicity in primary mesencephalic neuron-glia cultures. In contrast, the same concentrations of HNM produced little dopaminergic neurotoxicity in neuron-enriched cultures, indicating that glial cells are involved in HNM-induced neurotoxicity (3A).
To further distinguish the type of glial cells mediating HNM-induced neurotoxic effect, we performed reconstituted experiments by adding either enriched astroglia or microglia back to the neuron-enriched cultures. Primary mesencephalic neuron-glia cultures contain about 50% astroglia. Addition of 40% (2.0×105/well), 50% (2.5×105/well) and 60% (3.0×105/well) astroglia back to neuron-enriched cultures failed to decrease the capacity of DA uptake significantly (3B).
Two different approaches were used to demonstrate the role of microglia in the degeneration of dopaminergic neurons elicited by HNM. Our primary mesencephalic neuron-glia cultures contain about 10% microglia. Addition of 10% (5×104/well), 20% (1×105/well) and 30% (1.5×105/well) microglia back to neuron-enriched cultures decreased DA uptake in HNM-treated cultures in a graded fashion (3C). In neuron-glia cultures with microglia depleted by the addition of LME (neuron-astroglia cultures), 2.5 μg/mL and 5 μg/mL HNM-produced neurotoxicity was not evident when compared with vehicle-treated control group, and was much less toxic when compared with neuron-glia cultures (3D).
4.4 HNM increased the release of proinflammatory and neurotoxic factors in microglia
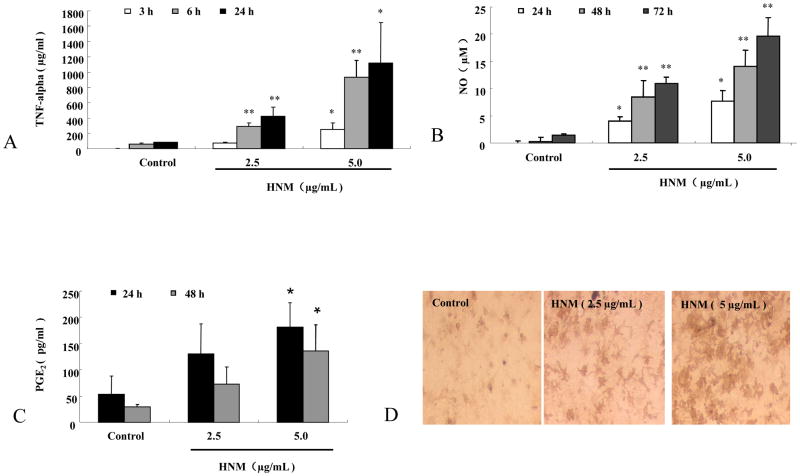
We investigated the functional changes of microglia after HNM treatment by detecting the production of neurotoxic and neuroinflammatory factors. We measured the levels of NO, TNF-alpha, and PGE2 at the time when these mediators are typically peaked in microglia treated with lipopolysaccharide, a widely used inflammagen for studying the neuroinflammatory mechanism of PD. Primary mesencephalic neuron-glia cultures were treated with 2.5 μg/mL and 5 μg/mL HNM, 50 μL supernatant was collected at different time points for the different above-mentioned assays.
TNF-alpha levels were assayed at earlier time points, 3 h, 6 h and 24 h, after HNM treatment. 2.5 μg/mL and 5 μg/mL HNM significantly induced microglial production of TNF-alpha after the treatment, in both dose- and time-dependent manners (4A).
NO levels were measured at later time points, 24 h, 48 h and 72 h, after HNM treatment. The production of NO was determined by measuring the accumulated level of nitrite (a metabolite of NO) in the supernatant. 2.5 μg/mL and 5 μg/mL HNM clearly enhanced microglial production of NO after the treatment, in both dose- and time-dependent manners (4B).
Similar results were obtained with the production of PGE2. 2.5 μg/mL and 5 μg/mL HNM produced microglial production of PGE2 24 h and 48 h after the treatment, in a dose-dependent manner (4C).
Morphological changes of microglia were also observed. In mesencephalic neuron-glia cultures, HNM-induced morphological activations of microglial were examined by OX-42 staining. HNM changed the morphology of microglia indicated by enlarged cell body, irregular shape such as rod and/or ameboid-like shape and intensified staining (4D).
5. DISCUSSION
NM is a highly insoluble mixture of compounds composed of oxidized catecholamines, lipids, peptides and metals that is resident within autophagic/lysosomal organelles of dopaminergic neurons in the substantia nigra. In physiological condition, NM can be neuroprotective since this process removes excess cytosolic catechols and its derivatives and can chelate toxic metals (14). Substances like free iron inside the cells may become one of the major free radicals causing damage to neurons when it can not be neutralized by NM (15). Investigation found that NM existed in the extracellular space (16), thus we hypothesized that NM can be released by damaged or dying neurons into the extracellular space (16). How does the extracellular NM interact with the surrounding cells, including microglia and astroglia? Is the interaction between NM and glial cells associated with the subsequent progressive neurodegeneration of the remaining dopaminergic neurons and what is the underlying mechanism? Answers of these questions will help to further elucidate the role of HNM in the pathogenesis of PD and explain why dopaminergic neurons in the SNpc are particularly vulnerable in PD patients
Here, we demonstrated that HNM caused relatively selective and progressive dopaminergic neurodegeneration in primary mesencephalic cell cultures. The major toxic effects of HNM were mediated through the activation of microglia by producing a variety of neurotoxic and proinflammatory factors. This study suggests that HNM can be considered as an endogenous activator of microglia. Once it was released from the dying dopaminergic neurons, HNM caused the activation of microglia and generated further neuroinflammation, leading to the progressive degeneration of dopaminergic neurons in the substantia nigra.
HNM exerted its neurotoxic effect on dopaminergic neurons in mesencephalic neuron-glia culture studies through both direct and indirect mechanisms. The direct toxic effect of HNM was observed by the decrease of DA uptake capacity in neuron-enriched cultures. However, the neurotoxic effect of HNM was greatly enhanced in the presence of glial cells, suggesting an indirect mechanism was operative in mediating the toxic effect of HNM. Further studies using reconstituted cultures revealed that microglia were essential for potentiating the neurotoxic effect of HNM. These results are consistent with the thesis that microglia-based neuroinflammation plays important roles in dopaminergic neurodegeneration in PD (17).
Strong evidence indicates that in the disease states, over-activated microglia produce and secret a myriad of factors, including reactive oxygen species (ROS), nitrogen species, cytokines, prostaglandins and chemokines (18–19), which may cause neuronal damage. In this study, we provided clear evidence showing that microglia were activated both functionally and morphologically when they were exposed to HNM. We found that microglia produced a large amount of extracellular superoxide (11), intracellular ROS (iROS) (11), NO, TNF-alpha and PGE2 after exposing to HNM.
Among the a wide range of factors released by activated microglia after exposure to HNM, extracellular superoxide was the earliest factor produced through the activation of β-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (is also called phagocyte oxidase, PHOX), the key superoxide-producing enzyme (20). Mesencephalic neuron-glia cultures derived from PHOX−/− mice were much more resistant to HNM-induced DA uptake reduction as well as dopaminergic neuronal number loss (data not shown). These results indicated that generation of ROS by activated PHOX was a critical step in HNM-mediated dopaminergic neurotoxicity. One of the mechanisms mediating the toxic effect of superoxide is through the formation a strong toxic intermediate like peroxinitrite by reacting with NO (21). Our data showed that HNM indeed increased the production of NO. Our previous reports indicate that superoxide released from microglia displays autocrine effect to further enhance the expression of a variety of proinflammatory factors, such as TNF-alpha (22) and PGE2 (13), through the formation of hydrogen peroxide which is membrane permeable (1–2). Our study also indicates an increase in the production of these two factors by the treatment with HNM in mesencephalic neuron-glia cultures.
We have shown that a spectrum of noxious endogenous compounds in extracellular milieu, generated following neuronal injury, can activate microglia, leading to the reactive microgliosis. These compounds include membrane breakdown products, abnormally processed, modified or aggregated proteins, such as. α-synuclein, metalloproteinase-3 and laminin (23–26), and leaked cytosolic compounds such as NM (27–28). It appears that the microglial response to these endogenous toxic signals resembles their response to the invading microbes or other foreign substances (7, 10–11, 13, 15–16). Although NM has been associated with PD, its role in the pathogenesis of PD is not entirely clear. Our results indicated that potent toxic substances after HNM treatment was released to the extracellular space. We speculate that HNM may serve as endogenous substance mediating the reactive microgliosis. Reactive microgliosis occurs when neurons are damaged and has been generally considered to play a passive role on cleaning the dead or damaged neurons or debris by phagocytosis (12). Our recent studies provided convincing evidence indicating that reactive microgliosis play a critical and active role in the formation of a self-propelling vicious cycle and the sustained dopaminergic neurodegeneration by HNM.
In conclusion, this study suggests that HNM can serve as an endogenous activator of microglia. Once it is released from the dying or dead dopaminergic neurons, HNM induces microglial activation and generates uncontrollble neuroinflammation, propelling progressive degeneration of dopaminergic neurons in the substantia nigra. Accordingly, inhibition of reactive microgliosis may be a useful target for PD therapy.
Figure 3. Microglia, not astroglia, contributed to the neurotoxic effect of HNM.
Primary mesencephalic neuron-glia cultures and neuron-enriched cultures were treated with 2.5 μg/mL and 5 μg/mL HNM for 7 d, [3H] DA uptake was performed. *P<0.01: Neuron-glia cultures compared with neuron-enriched cultures; #P<0.01: 5 μg/mL HNM-treated group compared with control group (A). Astroglia-reconstituted and neuron-enriched cultures were treated with 5 μg/mL HNM for 7 d, [3H] DA uptake was conducted. *P<0.01: 5 μg/mL HNM-treated group compared with vehicle-treated control group (B). Microglia-reconstituted cultures were treated with 5 μg/mL HNM for 7 d, [3H] DA uptake was assessed. *P<0.01: 5 μg/mL HNM-treated group compared with control group, #P<0.05: 5 μg/mL HNM-treated group in microglia-reconstituted cultures compared with neuron-enriched cultures (C). Primary mesencephalic neuron-glia cultures and neuron-astroglia cultures were treated with 2.5 μg/mL and 5 μg/mL HNM for 7 d, [3H]DA uptake was explored ▲P<0.01: HNM-treated groups in neuron-astroglia cultures compared with that in neuron-glia cultures (D).
Figure 4. HNM increased the release of proinflammatory and neurotoxic factors in microglia.
Primary mesencephalic neuron-glia cultures were treated with 2.5 μg/mL and 5 μg/mL HNM, 50 μL supernatant was collected for the assays of TNF-alpha, NO and PGE2. TNF-alpha was measured 3 h, 6 h and 24 h after HNM treatment. *P<0.05, **P<0.01: HNM-treated group compared with vehicle-treated control group (A). NO was determined by measuring the accumulated level of nitrite (an indicator of NO) 24 h, 48 h and 72 h after the treatment. *P<0.05, **P<0.01: HNM-treated group compared with vehicle-treated control group (B). PGE2 was measured 24 h and 48 h after HNM treatment. *P<0.05: HNM-treated group compared with vehicle-treated control group (C). Representative microscopic images were shown for microglia treated with vehicle, 2.5 μg/mL and 5.0 μg/mL HNM, respectively, for 7 d (D).
Figure 5.

Possible mechanism of reactive microgliosis by HNM. When neurons are damaged by neurotoxic factors, such as 1-methyl- 4-phenyl-1, 2,3, 6-tetrahydropyridine (MPTP), rotenone and paraquat, etc, signals are sent out to alert microglia so that neurons can be phagocytized. The mechanism whereby neurons signal to microglia remains unclear. Studies from our laboratory and others suggest that signals may come from two components: 1) proteins from dopaminergic neurons, such as NM, α-synuclein; 2) extracellular matrix proteins, such as metalloproteinase-3 (MMP-3), laminin, etc. These components leaked out from damaged neurons activate microglia via the activation of microglia antigen-1 (Mac-1, a receptor responsible for phagocytosis and oxidative burst) followed by the activation of PHOX (a superoxide-generating enzyme), leading to the increased production of reactive oxygen species (ROS), causing the continued death of dopaminergic neurons and formation of a self-propelling vicious cycle.
Acknowledgments
We appreciate the efforts provided by Dr. Liu, Zhuo and Dr. Huang, Xi-yan for editing the paper. This work was supported in part by the Intramural Research Program of the NIH/NIEHS.
Abbreviations
- PD
Parkinson’s disease
- NM
neuromelanin
- HNM
human NM
- SNpc
substantia nigra pars compacta
- DA
dopamine
- Ara-C
cytosine arabinoside
- LME
leu–leu methyl ester
- GABA
gamma-aminobutyric acid
- TH
tyrosine hydroxylase
- Neu N
neuronal nuclei
- TNF-alpha
tumor necrosis factor-alpha
- PGE2
prostaglandin E2
- KR
Krebs-Ringer
- NO
nitric oxide
- L-cys
L-cystein
- GP
gold particles
- ROS
reactive oxygen species
- iROS
intracellular ROS
- PHOX
phagocyte oxidase
References
- 1.Block ML, ZL, Hong JS. Microglia-mediated neurotoxicity: recognizing the key pattern of neurodegenerative diseases. Nature Re Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 2.Block ML, HJS Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, ME Glial reactions in Parkinson’s disease. Mov Disor. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 4.Zecca LFR, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002;510 doi: 10.1016/s0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- 5.Zecca L, SA, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci U S A. 2004;101 doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zecca L, CL, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem. 2008;106 doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- 7.Zecca L, ZF, Albertini A, Rizzio E, Fariello RG. A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology. 2006;67:S8–11. doi: 10.1212/wnl.67.7_suppl_2.s8. [DOI] [PubMed] [Google Scholar]
- 8.Shamoto-Nagai M, MW, Akao Y, Osawa T, Tribl F, Gerlach M, Zucca FA, Zecca L, Riederer P, Naoi M. Neuromelanin inhibits enzymatic activity of 26S proteasome in human dopaminergic SH-SY5Y cells. J Neural Transm. 2004;111:1253–1265. doi: 10.1007/s00702-004-0211-2. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese VP, HMG Parkinsonism and extraocular motor abnormalities with unusual neuropathological findings. Mov Disord. 1991;6:257–260. doi: 10.1002/mds.870060311. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WDS, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, Hong JS, Zhang J. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, PK, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D, Zecca L. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson’s Disease. Neurotox Res. 2009;3 doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, QL, Wang T, Wei SJ, Gao HM, Liu J, Wilson B, Liu B, Zhang W, Kim HC, Hong JS. 3-hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity. FASEB J. 2005;19:395–397. doi: 10.1096/fj.04-1586fje. [DOI] [PubMed] [Google Scholar]
- 13.Zhang WWT, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 14.Sulzer DBJ, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucca FA, GG, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L. The Neuromelanin of Human Substantia Nigra: Physiological and Pathogenic Aspects. Pigment Cell Res. 2004;17:610–617. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 16.Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–580. doi: 10.1016/j.tins.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi Y, YS, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 18.Dutta GZPaLB. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MSTMaG Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levesque S, WB, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133:808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SJSVaH Nitric-induced mitochondrial dysfunction: implication for neurodegeneration. Free Radic Biol Med. 2003;34:287–303. doi: 10.1016/s0891-5849(02)01327-8. [DOI] [PubMed] [Google Scholar]
- 22.Qin L, WX, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, ZW, Pei Z, Block M, Wilson B, Reece JM, Miller DS, Hong JS. Reactive microgliosis participates in MPP+-induced dopaminergic neurodegeneration: role of 67 kDa laminin receptor. FASEB J. 2006:20906–20915. doi: 10.1096/fj.05-5053com. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, CD, Block ML, Lorenzl S, Yang L, Kim YJ, Sugama S, Cho BP, Hwang O, Browne SE, Kim SY, Hong JS, Beal MF, Joh TH. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Guajardo VFF, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;20(5):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EJ, WM, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. alpha-Synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 27.Beach TG, SL, Walker DG, Lue LF, Connor DJ, Caviness JN, Sabbagh MN, Adler CH. Marked microglial reaction in normal aging human substantia nigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114:419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- 28.Zecca L, WH, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]