Introduction
A recent report1 of a meeting of public officials and blood service managers has reiterated the messages embedded in the long-debated and controversial issue of self-sufficiency in haemotherapies. This issue has been part of the debate on blood supply policy for the past 40 years. As blood systems in emerging countries develop in tandem with clinical practice needs, the question of the optimal path to sufficiency is crucial. As the composition of donor populations change through demographic and other factors, and as clinical awareness of the issues around the safety and efficacy of haemotherapies influence usage, principles proposed in the 1970s may benefit from scrutiny through the modern concepts of evidence-based medicine. In particular, the established position of the patient in the centre of all blood transfusion processes may need to transcend the reflections of expert groups, however eminent.
Self-sufficiency: historical development
The philosophical origins of the concept of “self-sufficiency” date back to the 1970s, when the World Health Organization (WHO) identified an extensive trade in blood and blood products, which exploited people in poor countries and placed those receiving blood products at risk from pathogen transmission. This led a joint conference of the WHO and the International Red Cross to agree to promote voluntary blood donation systems and national self-sufficiency and a World Health Assembly resolution to that effect (WHA28.72). The context is described in the report of the Canadian Krever Commission, demonstrating the WHO’s concern that donors in developing countries were being drawn into the paid plasma system at the expense of their domestic blood supply2. Krever also encapsulates the arguments for self-sufficiency which have continued to be proposed:
“Self-sufficiency in blood products is a desirable goal for several reasons. First, although complete safety is impossible, the plasma obtained from Canadian donors will be safe in relative terms. Canada has fewer infectious diseases than many other countries, including parts of the USA, and all Canadian residents have access to good health care services without charge. Moreover, volunteer donors have no incentive to donate other than the desire to assist other persons. If plasma collection is controlled by a national authority and regulated by the Health Protection Branch, there will be domestic control over both the quality of the donor-screening procedures and the collection and processing of plasma. If good donor-screening measures are applied to altruistic donors, it is probable that the quality of the plasma will be superior to that obtained from remunerated donors in countries over which Canadian regulatory authority is diminished. The second advantage of being self-sufficient in blood products is that the supply of blood products in Canada will not be affected by shortages on the world market. The third advantage is that if a blood-borne pathogen emerges in another country, there will be time in Canada to take precautionary action”3.
These contentions are all reiterated in the recent WHO exercise1, interwoven with the concept of voluntary non-remunerated blood donation which invariably accompanies the self-sufficiency issue.
The pros … and the cons
The principles for maintaining this policy can be summarised as:
Safety from blood-borne pathogens
There is little evidence that such a policy will result in this outcome. The blood safety environment has changed dramatically since the days of the WHO resolution. The assumption that domestic donors are safer than overseas ones can be dangerous in countries in which there is a high endemic rate of a particular infection. For example, in certain parts of Africa, the prevalence of human immunodeficiency virus in the donor population makes the spread of AIDS through blood a continuing problem. In countries such as France and Australia, an official policy of plasma product self-sufficiency through voluntary blood donations did not prevent the spread of hepatitis agents into the chronically transfused population, approaching rates similar to those in other countries with a comparable health care standard in which such a policy was not imposed4,5. Similarly, in a globalised world reflected in the blood safety environment6, reliance on a domestic supply is no guarantee of safety from emerging infectious agents. Canada itself, despite Krever’s hope, was severely affected by severe acute respiratory syndrome (SARS) introduced by a traveller from the East7. While SARS has not been shown to be transmitted by blood, this shows that modern life does not permit much “time…. to take precautionary action”. The classic example is that of the UK, which had to abandon its oft-stated commitment to self-sufficiency with the emergence of variant Creutzfeld-Jacob disease and its possible penetration into the blood donor pool. The penetration of agents such as prions, the West Nile virus, Chikungunya and others into the blood supply demonstrate that a “fortress” mentality is no protection. As for the established transfusion-transmitted agents, scientific developments including screening and pathogen inactivation have proven to be the bulwark of protection from emerging agents, not reliance on a domestic blood supply. While pathogen inactivation is currently limited to plasma, plasma derivatives and platelets, systems for whole blood have been developed and are reaching clinical application8,9.
The ethical dimension
The self-sufficiency debate gets inexorably interwoven with the question of donor motivation, at least in the minds of its primary advocates, and with the controversial issue of compensating donors. Research has shown that blood donor motivation is more complex “than the desire to assist other persons”10 and that the incentives used by the voluntary blood sector frequently have an economic value, such as days off work11 and tax benefits12. These benefits act as powerful inducements, they increase the blood supply and they can be associated with certain risks13. The other ethical aspect emanating from this issue involves the possibility of depleting a local/national/regional blood supply through the “poaching” of donors by extraneous agencies such as plasma collection and fractionation companies, thus impeding access to haemotherapies by the patients in the affected area. This is the basis of WHA28.72. However, the use of plasma collected in emerging countries has been stopped through the globalisation of the industry, which markets its products in areas overseen by agencies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) which require that such collections conform with their rigorous standards for donor health and safety. There is no evidence that plasma collection for entry into the global supply chain for plasma products is affecting the concurrent collection of other haemotherapies such as blood and cellular components. Countries such as Germany, Austria and the USA, which permit a pluralistic system, are among the highest blood collectors worldwide. The Czech Republic, an emerging country which has instituted a similar system, has a high plasma collection rate with centres in areas where blood collection is similarly high14.
Clearly, none of these countries is immune from the possibility that an emerging infection enters their blood and plasma supply. This possibility should be factored into policies which seek to limit the extension of plasma collection into countries other than the traditional providers specified above. Currently, the removal of, as an example, the USA plasma donor population from the global supply chain would have a catastrophic effect on product availability and patients’ care. We note that emerging agents such as variant Creutzfeld-Jacob disease and West Nile virus have infected the recipients of (voluntarily donated) blood components but not the recipients of (commercially and voluntarily donated) plasma products for which protective manufacturing steps are possible. It is difficult to understand how the routes of these particular infections, which constitute the main blood transfusion-borne emerging agents in the past decade, can be associated differentially with donor compensation status. As in many areas, in this issue, data need to supplant dogma, and we propose that the integration of sociological constructs such as Maslow’s Hierarchy of Needs15 may provide a useful tool in assessing donor motivation, separate from the current emotional tones of the debate.
There is an ethical dimension to the self-sufficiency debate which does merit further examination, and this is the effect on patients. We discuss below the constraints which are inevitable on supplying key haemotherapies through a restricted blood supply. The ethical issues around this require visibility. It seems that the extreme proponents of self-sufficiency are reluctant to consider the effects on patients and give primacy to ethical issues around donors. We propose that primacy in any area of health care delivery should be accorded to the patient, who needs access to therapies to alleviate the suffering resulting from the arbitrary misfortunes imposed by nature. It is desirable that the same visibility which is given to historical declarations of self-sufficiency and the negation of commerce involving the human body is also given to statements such as the World Medical Association’s Declaration on the Rights of the Patient16, with its emphasis on access and choice of treatment. Such access and choice are severely affected if scarcity results from a dogmatic adherence to restrictive supply policies. In countries which adhere to best practice evidence-based guidelines for the supply of key plasma products such as immunoglobulin, the levels of consumption significantly exceed the levels in countries in which restriction through self-sufficiency is imposed. We review this further in this work.
Domestic control of supply and regulation
In the global economy, the loss of domestic control in several areas appears to be an established feature. The international plasma industry has now reached a stage at which it is difficult to identify an entity such as a “national fractionator” in many of the countries, such as France, the United Kingdom and Switzerland, in which this concept applied in the past. The trade of plasma and intermediate fractions is an established feature of both “for profit” and “non-profit” blood systems, resulting from the globalised trade in plasma which all parts of the industry engage in. This merits some reflection by those ethically driven to oppose donor compensation and commercialisation of blood given that, as an example, the established sale of recovered plasma from voluntary donors by blood collection agencies is done without informing donors, at least as far as is apparent from the pre-donation consent forms. A trade in blood components also exists, albeit, because of the limitations imposed by the products’ biological properties, it occurs more on a national level. For example, in Germany and Austria red cells and platelets harvested from compensated donors are sold to hospitals by the “for profit” sector, while in the USA, the “non-profit” sector competes for the market provided by the users of voluntarily donated components using methods which are difficult to distinguish from those of the “for profit” sector17. The globalisation of the blood industry is further reflected in its regulation through standards and principles established by the two main regulatory bodies, the FDA and the EMA. While labile components are necessarily more confined within national borders, the quiet substitution of “national” by “regional” self-sufficiency by bodies such as the European Union and the WHO recognizes the possibility of a wider market for the products.
However, proponents of the self-sufficiency policy, with its natural extension into a single supplier, overlook the difficulties which national authorities face when responsible for the overviewing and assurance of such a system. In these situations, authorities in Australia18, Canada19 and France20 have drawn criticism for delaying the introduction of good manufacturing practices and current viral screening tests in blood and diagnostics agencies seen as requiring a special status. A multiplicity of approved products, all compliant with standards and all present in the market, is the best guarantor of a viable supply.
Sufficiency: self or otherwise
The recent WHO statement has identified a number of products it claims are drivers of sufficiency in haemotherapies. These products are claimed to include: (i) whole blood and red blood cells recovered either from whole blood or by apheresis; (ii) platelets recovered either from whole blood or by apheresis; (iii) plasma for transfusion either recovered from whole blood or sourced by apheresis and prepared by any production method; (iv) plasma-derived clotting factor VIII (FVIII) prepared by any production method; (v) polyvalent human immune globulin (IG); and (vi) human albumin solutions for transfusion.
Although this recognizes the increasing, and desirable, move towards apheresis as a procurement method for transfusion components, we would suggest that the primary haemotherapy drivers are red cell delivery treatments, whether through red cells or whole blood, and immunoglobulin. We propose that whole blood collection to an evidence-based target for red cell requirements, which we discuss below, should yield sufficient plasma and platelets for transfusion. The role of IG as the driver for plasma procurement is, we believe, accepted, and for plasma products, the issue therefore, resolves into “what is the evidence-based clinical demand for IG and which is the best route for procuring the raw material?” We discuss these issues in turn.
Demand for red blood cells
The optimal number of blood collections is based on poorly understood variations in clinical demand which do not necessarily translate into differences in outcomes. In Canada a variation between 45, 23 and 35 donations per 1,000 population over 1985–2005 as a result of the Krever-driven changes21 did not led to any reported changes in outcomes. Fuelled by safety-related donor deferrals, a drop in red cell issues of 26.4% over 1998–2008 in the UK’s blood services was absorbed without reported adverse clinical outcomes22. It seems that developed blood systems cope with donor loss when the circumstances dictate no other option. The blood collection rates of high income countries average 36.4 units per 1,000 population23, a level mirrored in the countries of the Council of Europe, in a report which also proposes that a level below 20 units per 1,000 population indicates shortage24. The WHO has proposed a minimum collection rate of 10 units per 1,000 population for African countries25. Social market health care providers such as Australia, Canada and the UK now average 33 units per 1,000 population and deliver similar health care outcomes according to data from the Organisation for Economic Co-operation and Development (OECD)26. Estimating red cell demand through the established indications for red cell transfusion is challenging as, while consensus on evidence is emerging through guidelines based on systematic reviews27,28, these have not been matched with epidemiological data to model estimates of demand, as has been done with some plasma protein therapies29. On the basis of the usage data cited, 35 units of whole blood donations per 1,000 population may be sufficient for the red cell needs of a developed health system. Scrutiny of the needs for the other basic components -platelets and transfusion plasma- does not provide any evidence that these cannot be met through processing of this amount of collected whole blood. Blood management programmes have been shown to decrease these collection requirements significantly30. Any collections over requirements may be expected to result in an excess and expiring level of red cells. The risk of iron deficiency in blood donors31 needs to be factored into considerations of red cell usage and potential wastage.
It may be anticipated that, as evidence accrues, this level may change. While confirmation that stored red cells increase morbidity and mortality may be expected to reduce usage, the ageing of the population with its attendant demands on increased health care may require more red cells/whole blood.
Clearly, many emerging countries are not achieving rates comparable to those of developed countries32 and donation rates in many countries are desperately inadequate to address problems such as post-partum haemorrhage, which is a major contributor to in-hospital maternal deaths in sub-Saharan Africa33. The WHO’s recent report seems to be denying this vulnerable population access to blood collected through routes other than those that the report endorses34. We suggest that discussions regarding ethics and principle should not take precedence over the grim clinical reality posed in environments such as sub-Saharan Africa.
Demand for immunoglobulins
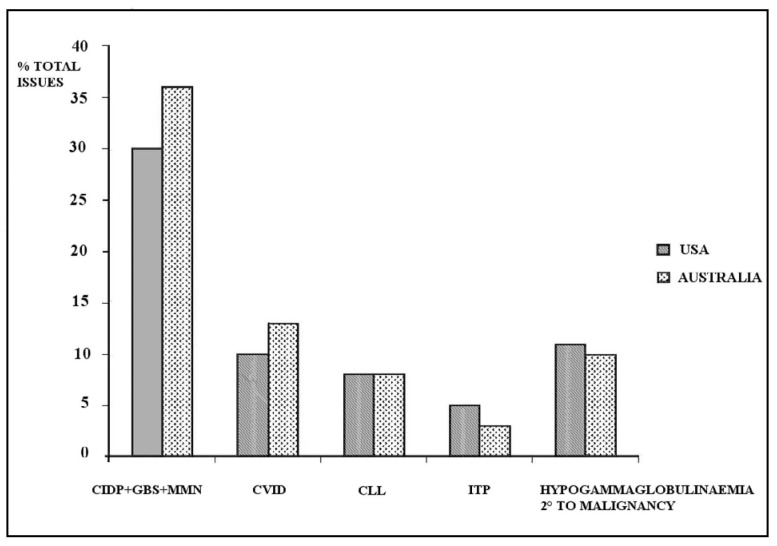
We have reviewed this topic35 and would reiterate that the clinical demand for IG is best assessed through environments in which a strict evidence base is used to ensure distribution of the product. As in the case of red cells, IG usage varies greatly between countries with a similar health care status. Unlike red cells, however, therapeutic claims for this product have to be approved by regulators through evidence before it is put on the market, and, increasingly, product reimbursement and hence, usage, is based on such approved claims. A continuing assertion that IG is used in the absence of evidence does itself lack an evidence base. The usage of IG in two high-consuming countries -the USA and Australia- is shown in Figure 1, using data from the respective payer agencies36,37. Table I lists the main indications for IG with the attendant evidence levels assigned by the American Academy of Allergy, Asthma and Immunology and the Australian National Blood authority38,39. It is difficult to understand the constant mantra regarding “off-label” use when the majority of indications are clearly supported by a high evidence status. It is important to clarify that labelling for a specific product is based on approval for a specific indication by an authority for that product. Scrutiny of the indications listed in Table I demonstrates that products specifically approved for one or the other indication cover the whole spectrum40. The evidence for IG usage is based on clinical trials to acquire approval for marketing the products, a process from which blood components have been protected. Despite this, given its economic importance to health care systems and the plasma industry, doubts continue to be raised regarding its use, given the large variation between countries41. Variations in usage also occur regionally within countries, as observed in the Australian system, and are linked to factors such as the location of specialist centres, such as immunology and neurology centres. The Australian system, while committed to self-sufficiency, reimburses both domestic and imported IG through a single payer (government), which allocates products on the basis of evidence-based clinical guidelines drawn up by an expert body39. The National Blood Service enforces a strict gate-keeping role for the issue of the product. Thus the Australian system presents the features of a single payer providing product based on evidence. The current (2009–10) annual consumption of IG in this system is 120 g/1,000 population. This level is the result of a system demonstrating commendable transparency in its processes and putting clinical need at the head of policy aims, which include Self-sufficiency.
Figure 1.
IG use in the USA and Australia, 2009–2010, reimbursed by public payers. The top indications are shown36,37.
CIPD: chronic inflammatory demyelinating polyradiculopathy; GBS: Guillain-Barré syndrome; MMN: multifocal motor neuropathy; CLL: chronic lymphocytic leukaemia; ITP: idiopathic thrombocytopenic purpura.
Table I.
| Disease | Benefit | Evidence level |
|---|---|---|
| Primary immune deficiency - common variable immune deficiency, X-linked agammaglobulinaemia | Definitely beneficial | IIb |
| Chronic lymphocytic leukaemia with reduced IgG and a history of infections | Probably beneficial | Ib |
| Idiopathic thrombocytopenic purpura | Definitely beneficial | Ia |
| Guillain-Barré syndrome | Definitely beneficial | Ia |
| Multifocal motor neuropathy | Definitely beneficial | Ia |
| Chronic inflammatory demyelinating polyradiculopathy | Definitely beneficial | Ia |
| Hypogammaglobulinaemia secondary to haematological malignancies (non-Hodgkin’s lymphoma, multiple myeloma) | Established therapeutic role | IIa |
While this indicates that the usage levels in the “high” IG consumers such as Canada, the USA and Australia reflect evidence, there are indications that clinical demand, in an idealised world in which reimbursement and plasma supply are not restricted, is potentially much higher. Similarly to the situation described for haemophilia29, disease prevalence and clinical variables such as dosage can be used to model demand for IG in the disease states treated with this product. In a decision analysis model for IG use in common variable immune deficiency, the latent clinical demand for IG for this condition alone is estimated at 44 g per 1,000 population, using inputs for disease epidemiology and management extracted from the literature42 (Figure 2). This level exceeds the total IG usage of many countries in Europe, and also exceeds the level used for common variable immune deficiency in Australia and the USA. This suggests that, while more data are needed to estimate the potential demand for all the currently approved indications for IG, the need for this product is not exaggerated through the usage data in North America and Australia. Hence, while we propose that a usage of 120 g per 1,000 population is supportable by evidence, an increased demand may be expected as disease prevalence increases through diagnosis, leading to the establishment of treatment.
Figure 2.
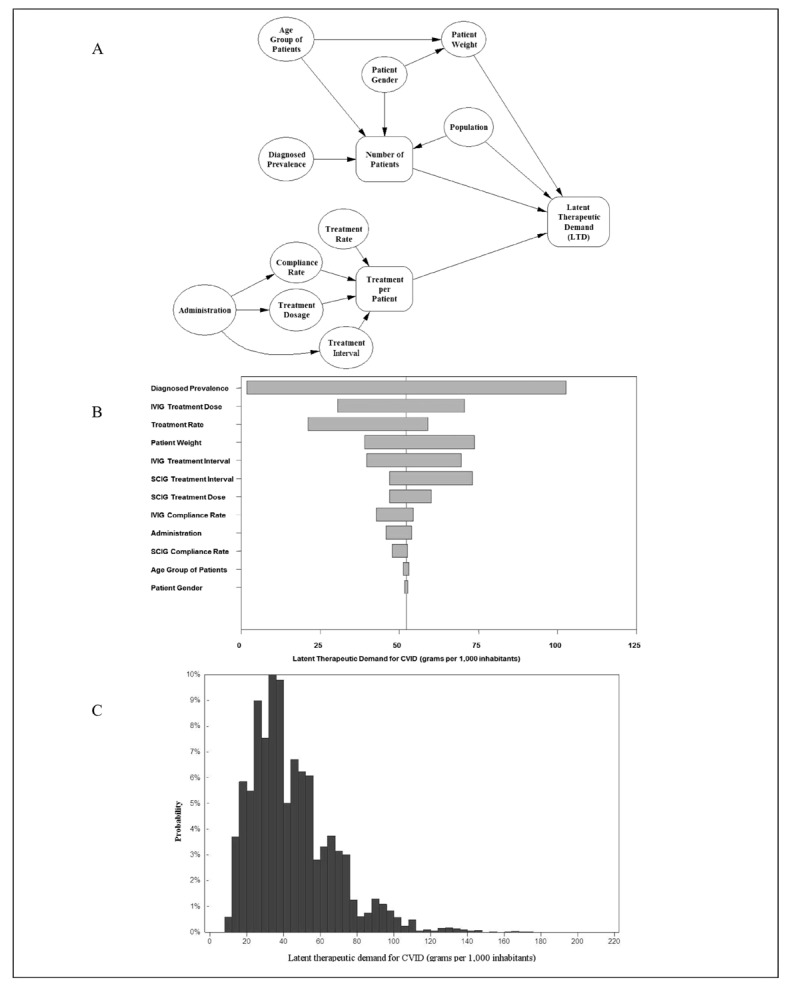
Model for estimated latent therapeutic demand (LTD) for IG use in common variable immune deficiency (CVID). A model drawing on the methodology described by Stonebraker et al.29 was constructed using peer-reviewed literature for the inputs shown in A. The model estimated the importance of each input in the tornado diagram shown in B and estimated a distribution for the usage of IG in CVID (C). The estimated mean usage was 44 g per 1,000 population.
On this basis of the need for IG, some estimates of the required plasma volume in a national environment may be attempted. Although higher yields of IG are reported43, the Cohn fractionation process in use in the majority of plants worldwide is unlikely to yield more than 4 g/L44. On this basis, simple calculation suggests that, given access to a fractionation capacity, a population can generate 120 g from 30 L of plasma collected per 1,000 population. Using current technology, this volume of plasma should be able to generate 6,000 IU of FVIII; i.e. 6 IU per capita of population, which is close to estimates of the optimal level supplied if access to product was unrestricted29. Estimates for other products will confirm that amounts of albumin, other coagulation factors, alpha 1 antitrypsin, etc. which are conformant to current clinical needs can be produced from this volume of plasma.
These estimates must be considered with caution. Assignment of “target” levels for clinical needs for plasma products needs to recognise that these are, essentially, “moving targets”. As clinical practice evolves and treatments improve, so shall the “targets” go up. Thirty years ago, the target level of FVIII for the treatment of haemophilia A was 1- 2 IU per capita45, a level then achieved by very few countries. This level is currently viewed as being inadequate, and countries with state-of-the-art treatment such as Sweden have usage levels which exceed 10 IU/per capita as treatment protocols such as continuous prophylaxis and tolerisation to inhibitors are implemented. Similar therapeutic improvements which need to be recognised include a possible role for albumin in sepsis46, which, if confirmed by further clinical trials, will extend usage of this product beyond the traditional indications. Continuing increase of IG use to the levels indicated by analysing the latent clinical demand should, however, generate enough plasma to absorb the expansion of other products. The advent of recombinant therapy has largely detached the demand for anti-haemophilic coagulation factors, particularly FVIII, from the plasma supply, but we emphasise that FVIII in very significant amounts can continue to be extracted from the plasma necessary for IG needs, and the current “wastage” of plasma-derived FVIII will be discussed below.
Worldwide plasma supply
Table II lists the world’s top plasma procurement countries47. It may be observed that the 30 L/1,000 population specified earlier is attained by only four countries. These are the countries which have the highest levels of plasmapheresis collection, a mode of collection which is efficient but time-consuming and arduous. It is not surprising that this level and mode of procurement requires donor compensation to collect the volumes required. The question of the maximum volume permissible through apheresis collection continues to be controversial48, but studies indicate that the levels approved by the American FDA are not harmful to donor health49. The dropout rate amongst plasmapheresis donors is considerable, but has been shown to be unrelated to donor health or safety issues49. Levels permitted in the European Union’s jurisdiction are considerably lower50 but do not limit the capacity of the Czech Republic, Germany and Austria to join the USA as important plasma producers.
Table II.
Top plasma-producing countries47.
| Country | Plasma production litres/1,000 population | Donor status |
|---|---|---|
| United States of America | 66 | Uncompensated & compensated |
| Austria | 56.6 | Uncompensated & compensated |
| Czech Republic | 33 | Uncompensated & compensated |
| Germany | 31.6 | Uncompensated & compensated |
| Australia | 21.5 | Uncompensated |
| Netherlands | 18.8 | Uncompensated |
| Denmark | 17 | Uncompensated |
| France | 16.3 | Uncompensated |
| Sweden | 16.1 | Uncompensated |
| Belgium | 15.5 | Uncompensated |
The plasma for fractionation produced by these countries is of two types. Some (domestic plasma) is used for fractionation into products for the domestic market through arrangements between the plasma collector and a single fractionator. This is the situation in Australia, Belgium, Denmark, the Netherlands and France, and is related to the “self-sufficiency” concept practiced in these and other countries. In other countries, the plasma (market plasma) collected is traded as an international commodity by the collectors and is manufactured into products for the international market. This is the case for most of the plasma collected in the top collecting countries. The world supply of plasma products is heavily dependent on these countries and in particular on the large volume of plasma collected in the USA. An expansion of the countries contributing to the world plasma supply is certainly desirable, but is self-sufficiency the answer?
Routes to plasma procurement
Plasma for fractionation is generated through two means. Plasma “recovered” as a by-product of whole blood collection was initially viewed in many blood bank systems as an ancillary product secondary to the main blood bank components needed for mainstream clinical transfusion purposes. As the amount of blood collected from most blood donors rarely exceeds two units/year51, the volume of plasma per donor using this route is seldom in excess of 500 cc/year, and never more than 1 L/donor per year based on mandatory restrictions on blood collection rates.
Apheresis collection is capable of generating considerably higher volumes of source plasma, depending on the regulatory requirements in the country of collection. The most important country, the USA, mandates a maximum of two collections/week, giving a hypothetical collection rate of 104 donations of about 800 mL/donor per year52. This implies that 83 L can be collected per year, but this rate is rarely attained, as shown by the fact that 22 million collections came from 1 million donors in 2010.
The infrastructure needed for apheresis collection is expensive and this contributes to the preference of some systems to base their plasma access policy on the collection of whole blood. This may be inadvisable. As may be seen from this review, attaining the national collection level required for the IG “driver” has only been achieved by those countries with a high level of source collection. Therefore, irrespectively of any clinical and reimbursement policies in place, it appears that source collection is a prerequisite for generating the required volumes. A policy based on plasma recovery from whole blood is fraught with problems, principally the inevitable and eventual wastage of red cells. Assuming a whole blood to red cell concentrate conversion of 95%, which is what is attained in modern and “plasma-driven” blood systems, the afore-specified plasma collection level of 30 L/1,000 population would result from collection of 126 donations/1,000 population and packing 120 of them into red cells. No blood system in the world is remotely close to this, and neither does it have to be, given that current clinical needs indicate that 35–40 red cell units/1,000 population are sufficient. Striving for levels higher than this will inevitably lead to over-usage and wastage of red cells. The iron deficiency imposed on, particularly female, whole blood donors31 adds to the ethical unacceptability of this situation.
Source collection is, therefore, pivotal to meeting the clinical needs worldwide. It is clearly desirable to put to good use any plasma, generated as a result of red cell production, for treating normovolaemic anaemia. It is likely that this indication covers not more than half of blood collection needs, which also include the treatment of hypovolaemic blood loss from trauma, surgery, etc. Emerging indications that many of these patients may be at risk from transfusion of stored red blood cells53 and may do better with the transfusion of whole blood54 should give pause for reflection to those who continue to advocate more recovered plasma collection. The examples of the Netherlands and Australia which, although still not at the hypothetical plasma sufficiency level, are generating substantial plasma from a mix of recovered and apheresis collection, are worth examining.
Sufficiency: “self” versus “global”
The European Directive 89/381 describes that Member States shall take the necessary measures to promote Community self-sufficiency in human blood or human plasma55. It is our view that national self-sufficiency in unfrozen blood and blood components for transfusion purposes is sensible, because of the logistical difficulties in ferrying these products across significant distances and because the objective clinical needs can be met within a local population. The reality of recipients of plasma proteins being dependent on plasma from the big producers means that restricting products from these sources imposes limitations on the treatment of patients, both in terms of choice and in terms of supply.
The UK followed the principle of self-sufficiency until the entire UK plasma supply was banned after the enormous development of bovine spongiform encephalopathy in that country. From that moment on, USA plasma was imported and used to manufacture plasma products in the plant of an English state-owned company. It is difficult to position this agency within the “self-sufficiency” paradigm advocated by its umbrella organisation41. In Japan, an avowed and vigorously enforced self-sufficiency policy includes the practice, assessed as originating from factors detached from patient care56, of labelling all blood products according to donor status57. This has not helped to increase plasma product usage to international norms, as demonstrated by FVIII and IG consumption of 3.16 IU per capita and 30.8 g per 1,000 population, respectively, reported in 201158. It is noteworthy that countries claiming and imposing self-sufficiency, i.e. restricting access to non-domestically sourced plasma products, are among the lowest users of therapies. This includes countries with big economies such as Japan and China. Conversely, countries which have abandoned this dogma, such as Canada and Ireland, are high users. Notably, countries such as Australia and the UK, which previously restricted access based on this policy, have seen consumption levels of plasma products rise as the policy was relaxed.
Essentially, wherever self-sufficiency, i.e. restricted importation, is in place, it has resulted in a lack of patients’ access to treatments. Defining self-sufficiency as the attainment of clinical needs which are, on the basis of evidence cited in this review, artificially low, results in inadequate clinical care for chronically dependent patients, as recognised by the patients themselves59, and simply hides the problem. Access to the global market of plasma products is essential to meet these needs. Currently, this market is heavily dependent on the collection and economic policies of a handful of countries. Increasing plasma collection for the purpose of manufacture in other countries will address this imbalance. Regrettably, such an expansion in capacity frequently gets entangled in the “self-sufficiency” argument, which rapidly transitions to the “selfishness” insufficiency situation in many countries. All must contribute to global needs. Excess of products in certain countries, such as plasma-derived FVIII in the established economies, should be channelled into the supply chain to meet the needs of countries which currently lack the economic capacity to convert to more expensive recombinant therapies. Continued wastage of FVIII, such as Australia’s systematic discard of approximately 80×106 IU yearly, demands as much discussion in the ethics based debate as the issue of self-sufficiency.
Where to get the donors?
As outlined, for plasma products, source collection is required for a sufficient supply. The restriction of the donor base to a pool of committed, well characterised, repeat donors allows a high level of control and quality which contributes to safety. The levels required have only been attained in systems permitting compensation of the donors for the arduous and time-consuming process of frequent plasmapheresis. Reflecting again on the “mixed” blood economies of Australia and the Netherlands, it is possible to generate a substantial capacity from uncompensated donors, but the figures for plasmapheresis collection −4.5, 7 and 22 donations/donor in Australia, the Netherlands and the USA, respectively- speak for themselves, and support our contention that donor compensation is reasonable for the arduous and time-consuming process of plasmapheresis. In the established economies, sufficiency of fresh haemotherapies appears well covered through the established donor paradigm drawing on individuals embedded in social groups which mould their willingness to donate for reasons characterised as “voluntary”. In the emerging economies, with social and economic structures reflected in other groups, excluding donors in other parts of Maslow’s pyramid15, such as family and replacement donors, will lead to insufficiency in badly needed blood. Even in paid donor systems, the key safety issues have been shown to be reflective of donation frequency rather than donor status60,61, and the possibility of creating cadres of professional blood donors should not be discarded out of ideological grounds. For global sufficiency of all blood-derived medicines, all donor groups are required and should be welcomed. As the ageing demographics of western countries bites into the blood supply62, efforts to restrict, rather than expand, the donor base for reasons other than those based on evidence must be seriously questioned.
Conclusions
As more health care systems attain the sophistication of treating conditions with haemotherapies to the levels supported by clinical evidence, the supply of blood needs to be geared towards this task. As Lenin once remarked “Everything is linked to everything else”, while CS Lewis expanded the concept, stating that “Everything is connected with everything else; but not all things are connected by the short and straight roads we expected”. The contention that haemotherapies can only be supplied through a construct which restricts the base of contributing donors for reasons unrelated to the patient is untenable. A balanced and optimal route to collecting the raw material, avoiding wastage and harm to both donor and patient is necessary. Recognition of the reality and the potential risks and benefits of a globalised world will allow blood supply policy to adjust to real needs, unfettered by evidence-lacking dogma. Above all, the field of transfusion medicine, which started as a modest technology assisting acute illness and has evolved into a massive industry irrespective of the “for profit” vs “non-profit” divide, needs to centre on the core aim of all health interventions - the care of the sick.
Footnotes
Presented in part at the 40° Convegno Nazionale di Studi di Medicina Trasfusionale (Rimini, Italy, 23-26 May 2012).
Conflict of interest disclosure
The Authors provide contractual services to blood industry agencies described in the paper.
References
- 1.WHO Expert Group. Expert Consensus Statement on achieving self-sufficiency in safe blood and blood products, based on voluntary non-remunerated blood donation (VNRBD) Vox Sang. 2012;103:337–42. doi: 10.1111/j.1423-0410.2012.01630.x. [DOI] [PubMed] [Google Scholar]
- 2.Royal Commission of Inquiry on the Blood System in Canada. [Accessed on 02/07/2012];Final Report. 1996 1:63–4. Available at: http://epe.lac-bac.gc.ca/100/200/301/hcan-scan/commission_blood_final_rep-e/vol1-e.pdf. [Google Scholar]
- 3.Royal Commission of Inquiry on the Blood System in Canada. [Accessed on 02/07/2012];Final Report. 1996 3:1048. Available at: http://epe.lac-bac.gc.ca/100/200/301/hcan-scan/commission_blood_final_rep-e/vol3-e.pdf. [Google Scholar]
- 4.Rickard KA, Batey RG, Dority P, et al. Hepatitis and haemophilia therapy in Australia. Lancet. 1982;2:146–8. doi: 10.1016/s0140-6736(82)91105-9. [DOI] [PubMed] [Google Scholar]
- 5.de Montalembert M, Costagliola DG, Lefrère JJ, et al. Prevalence of markers for human immunodeficiency virus types 1 and 2, human T-lymphotropic virus type I, cytomegalovirus, and hepatitis B and C virus in multiply transfused thalassemia patients. The French Study Group On Thalassaemia. Transfusion. 1992;32:509–12. doi: 10.1046/j.1537-2995.1992.32692367192.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrugia A. Globalisation and blood safety. Blood Rev. 2009;23:123–8. doi: 10.1016/j.blre.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 8.Goodrich RP, Doane S, Reddy HL. Design and development of a method for the reduction of infectious pathogen load and inactivation of white blood cells in whole blood products. Biologicals. 2010;38:20–30. doi: 10.1016/j.biologicals.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Henschler R, Seifried E, Mufti N. Development of the S-303 pathogen inactivation technology for red blood cell concentrates. Transfus Med Hemother. 2011;38:33–42. doi: 10.1159/000324458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia A, Penrod J, Bult J. Payment, compensation and replacement - the ethics and motivation of blood and plasma donation. Vox Sang. 2010;99:202–11. doi: 10.1111/j.1423-0410.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldi G. Italy: national policy. Pharmaceuticals Policy and Law. 7:243–53. 2005, 2006. [Google Scholar]
- 12.Commission of the European Communities. Report on the promotion by Member States of voluntary unpaid blood donations. [Accessed on 06/07/2012]. Available at http://ec.europa.eu/health/ph_threats/human_substance/documents/blood_com_0217_en.pdf.
- 13.Sanchez AM, Ameti DI, Schreiber GB, et al. The potential impact of incentives on future blood donation behaviour. Transfusion. 2001;41:172–8. doi: 10.1046/j.1537-2995.2001.41020172.x. [DOI] [PubMed] [Google Scholar]
- 14.Beck S. Blood centers and plasma centers - mutual benefit. [Accessed on 02/07/2012]. Available at: http://www.pptaglobal.org/UserFiles/file/TheSource/2011/summer/201106_12_BLOOD_CENTERS_AND_PLASMA_CENTERS_MUTUAL_BENEFIT.pdf.
- 15.Farrugia A. More blood, more life? Reflections on World Blood Donor Day - 2011. Indian J Med Res. 2011;133:573–6. [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association. Lisbon Declaration of the Rights of the patient 1981. [Accessed on 02/07/2012]. Available at: http://www.wma.net/en/30publications/10policies/l4/index.html.pdf?print-media-type&footer-right=[page]/[toPage]
- 17.Bruce D. Erie blood bank battles economy, competition. [Accessed on 06/09/2012]. Available at: http://www.goerie.com/apps/pbcs.dll/article?AID=/20100722/NEWS02/307229932.
- 18.Beauchamp K. Red alert! Is regulation working for imported and CSL blood products? Australian Institute of Criminology; 1995. [Accessed on 07/12/2012]. Available at: http://www.criminologyresearchcouncil.gov.au/reports/37-91-a.pdf. [Google Scholar]
- 19.Royal Commission of Inquiry on the Blood System in Canada. The Bureau of Biologics and the Regulation of Blood and Blood Products. [Accessed on 06/09/2012];Report of the Royal Commission of Inquiry on the Blood System in Canada. 1997 1:111–147. Available at: http://publications.gc.ca/collections/Collection/CP32-62-3-1997-1E.pdf. [Google Scholar]
- 20.Steffen M. The nation’s blood: medicine, justice, and the state in France. In: Feldman E, Bayer R, editors. Blood Feuds: AIDS, Blood, and the Politics of Medical Disaster. Oxford University Press; 1999. pp. 95–126. [Google Scholar]
- 21.Saberton PJ, Paez A, Newbold KB, Heddle NM. Geographical variations in the correlates of blood donor turnout rates: an investigation of Canadian metropolitan areas. Int J Health Geogr. 2009;8:56. doi: 10.1186/1476-072X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serious Hazards of Transfusion. Annual Report 2008. [Accessed on 02/07/2012]. Available at: http://www.shotuk.org/wp-content/uploads/2010/03/SHOT-Report-2008.pdf.
- 23.World Health Organization. Global Data Base for Blood Safety - Summary Report 2011. [Accessed on 06/09/2012]. Available at: http://www.who.int/bloodsafety/global_database/GDBS_Summary_Report_2011.pdf.
- 24.Council of Europe. The Collection, Testing and Use of Blood and Blood Components in Europe. 2008. [Accessed on 06/09/2012]. Available at: http://www.edqm.eu/medias/fichiers/The_Collection_Testing_and_Use_of_Blood_and_Blood_3.pdf.
- 25.World Health Organization. Status of blood safety in the WHO African Region - Report of the 2006 Survey, page X. [Accessed on 06/09/2012]. Available at: http://www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=3835.
- 26.Organisation for Economic Co-operation and Development. Key indicators of Health Data. 2012. [Accessed on 06/09/2012]. Available at: http://www.oecd.org/els/healthpoliciesanddata/OECDHealthData2012FrequentlyRequestedData_Updated.xls.
- 27.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 29.Stonebraker JS, Amand RE, Bauman MV, et al. Modelling haemophilia epidemiology and treatment modalities to estimate the unconstrained factor VIII demand. Haemophilia. 2004;10:18–26. doi: 10.1046/j.1365-2516.2003.00841.x. [DOI] [PubMed] [Google Scholar]
- 30.Strategic Blood Management. Case studies. [Accessed on 06/09/2012]. Available at: http://www.bloodmanagement.com/case-studies/case-studies.
- 31.Farrugia A. Iron and blood donation - an under-recognised safety issue. Dev Biol (Basel) 2007;127:137–46. [PubMed] [Google Scholar]
- 32.World Health Organization. Global blood safety and availability. Facts and figures from the 2007 Blood Safety Survey. 2009. [Accessed on 02/07/2012]. Available at: http://www.who.int/mediacentre/factsheets/fs279/en/print.html.
- 33.Rogo KO, Oucho J, Mwalali P. Maternal Mortality. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd edition. Washington (DC): World Bank; 2006. pp. 223–36. [PubMed] [Google Scholar]
- 34.Allain JP. Moving on from voluntary non-remunerated donors: who is the best blood donor? Br J Haematol. 2011;154:763–9. doi: 10.1111/j.1365-2141.2011.08708.x. [DOI] [PubMed] [Google Scholar]
- 35.Farrugia A, Cassar J. Plasma-derived medicines: access and usage issues. Blood Transfus. 2012;10:273–8. doi: 10.2450/2011.0118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services USA. Data extracted for IG use by physicians. Extracted through application in 2010. [Accessed on 07/12/2012]. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/CMS-Information-Technology/AccesstoDataApplication/index.html.
- 37.Australian National Blood Authority. Top ten uses for IVIG 2004-11. [Accessed on 07/09/2012]. Available at: http://www.nba.gov.au/publications/1011report/images/content/figure_3.19.gif.
- 38.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(Suppl 4):S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Australian National Blood Authority. Criteria for the clinical use of intravenous immunoglobulin. 2nd edition. 2012. [Accessed on 06/09/2012]. Available at: http://www.nba.gov.au/ivig/pdf/criteria.pdf.
- 40.Mark SM. Comparison of intravenous immunoglobulin formulations: product, formulary, and cost considerations. Hosp Pharm. 2011;46:668–76. [Google Scholar]
- 41.Rossi F, Perry R, de Wit J, et al. How expanding voluntary non-remunerated blood donations would benefit patients, donors and healthcare systems? Vox Sang. 2011;101:176–7. doi: 10.1111/j.1423-0410.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 42.Stonebraker J, Farrugia A. Using decision analysis to model latent therapeutic demand for immunoglobulin in treating CVID. J Clin Immunol. 2012;32:399. [Google Scholar]
- 43.National Blood Authority of Australia. Annual Report. 2009–10. [Accessed on 07/12/2012]. p. 55. Available at: http://www.nba.gov.au/publications/0910report/prelims/contents.html.
- 44.Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang. 2010;98:12–28. doi: 10.1111/j.1423-0410.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 45.Smit Sibinga C Th, Das PC. Use and abuses of single donor plasma. In: Kollins J, Britten AFH, Silvergleid, editors. Plasma Products: Use and Management. American Association of Blood Banks; Arlington VA USA: 1982. pp. 23–32. [Google Scholar]
- 46.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–91. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 47.Robert P. Worldwide supply and demand of plasma and plasma-derived medicines. Iran J Blood Cancer. 2011;3:111–20. [Google Scholar]
- 48.Laub R, Baurin S, Timmerman D, et al. Specific protein content of pools of plasma for fractionation from different sources: impact of frequency of donations. Vox Sang. 2010;99:220–31. doi: 10.1111/j.1423-0410.2010.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulzki T, Seidel K, Storch H, et al. SIPLA study group. A prospective multicentre study on the safety of long-term intensive plasmapheresis in donors (SIPLA) Vox Sang. 2006;91:162–73. doi: 10.1111/j.1423-0410.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 50.Council of Europe. Recommendation No. R (85) 15. Council of Europe Publishing; Strasbourg: Guide to the preparation, use and quality assurance of blood components. [Google Scholar]
- 51.Council of Europe. Trends and Observations on the Collection, Testing and Use of Blood and Blood components in Europe 2001–2005. Council of Europe; Feb, 2011. [Google Scholar]
- 52.Food and Drug Administration. Code of Federal Regulations - PART 640-Additional Standards for human bloo and blood products. [Accessed on 07/12/2012]. Available at: http://law.justia.com/cfr/title21/21-7.0.1.1.7.html.
- 53.Andreasen JJ, Dethlefsen C, Modrau IS, et al. NorthWest Denmark Transfusion Study Group. Storage time of allogeneic red blood cells is associated with risk of severe postoperative infection after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2011;39:329–34. doi: 10.1016/j.ejcts.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Bowling F, Pennardt A. The use of fresh whole blood transfusions by the SOF medic for hemostatic resuscitation in the austere environment. J Spec Oper Med. 2010;10:25–35. doi: 10.55460/0370-FW6J. [DOI] [PubMed] [Google Scholar]
- 55.European Commission. Council Directive 89/381/EEC of 14 June 1989 extending the scope of Directives 65/65/EEC and 75/319/EEC on the approximation of provisions laid down by Law, Regulation or Administrative Action relating to proprietary medicinal products and laying down special provisions for medicinal products derived from human blood or human plasma. [Accessed on 07/12/2012]. Available at: http://www.ikev.org/docs/eu/389L0381.htm.
- 56.Robertson J. Hemato-nationalism: the past, present, and future of “Japanese blood”. Med Anthropol. 2012;31:93–112. doi: 10.1080/01459740.2011.624957. [DOI] [PubMed] [Google Scholar]
- 57.Bult JM. In my view, continued challenges of doing business in Japan. The Source Spring. 2011. [Accessed on 07/12/2012]. Available at: http://www.pptaglobal.org/UserFiles/file/TheSource/2011/spring/201103_2_IN_MY_VIEW.pdf.
- 58.Blood Products Research Organization. Blood Program in Japan 2011. [Accessed on 07/09/2012]. Available at: http://www.bpro.or.jp/english/pdf_annual/BPRP2011.pdf.
- 59.O’Mahony B. EU self sufficiency - the patients perspective. [Accessed on 07/09/2012]. Available at: http://www.haemophilia.ie/PDF/BOMahony%20lecture%204%20IPPC%20Berlin.pdf.
- 60.Buciuniene I, Stonienë L, Blazeviciene A, et al. Blood donors’ motivation and attitude to non-remunerated blood donation in Lithuania. BMC Public Health. 2006;6:166. doi: 10.1186/1471-2458-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalibatas V. Payment for whole blood donations in Lithuania: the risk for infectious disease markers. Vox Sang. 2008;94:209–15. doi: 10.1111/j.1423-0410.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 62.Greinacher A, Fendrich K, Brzenska R, et al. Implications of demographics on future blood supply: a population-based cross-sectional study. Transfusion. 2011;51:702–9. doi: 10.1111/j.1537-2995.2010.02882.x. [DOI] [PubMed] [Google Scholar]