Abstract
Background
Requirements for allogeneic red cell transfusion after total knee arthroplasty are still high (20–50%), and salvage and reinfusion of unwashed, filtered post-operative shed blood is an established method for reducing transfusion requirements following this operation. We performed a cost analysis to ascertain whether this alternative is likely to be cost-effective.
Materials and methods
Data from 1,093 consecutive primary total knee arthroplasties, managed with (reinfusion group, n=763) or without reinfusion of unwashed salvaged blood (control group, n=330), were retrospectively reviewed. The costs of low-vacuum drains, shed blood collection canisters (Bellovac ABT®, Wellspect HealthCare and ConstaVac CBC II®, Stryker), shed blood reinfusion, acquisition and transfusion of allogeneic red cell concentrate, haemoglobin measurements, and prolonged length of hospital stay were used for the blood management cost analysis.
Results
Patients in the reinfusion group received 152±64 mL of red blood cells from postoperatively salvaged blood, without clinically relevant incidents, and showed a lower allogeneic transfusion rate (24.5% vs 8.5%, for the control and reinfusion groups, respectively; p =0.001). There were no differences in post-operative infection rates. Patients receiving allogeneic transfusions stayed in hospital longer (+1.9 days [95% CI: 1.2 to 2.6]). As reinfusion of unwashed salvaged blood reduced the allogeneic transfusion rate, both reinfusion systems may provide net savings in different cost scenarios (€ 4.6 to € 106/patient for Bellovac ABT, and € −51.9 to € 49.9/patient for ConstaVac CBCII).
Discussion
Return of unwashed salvaged blood after total knee arthroplasty seems to save costs in patients with pre-operative haemoglobin between 12 and 15 g/dL. It is not cost-saving in patients with a pre-operative haemoglobin >15 g/dL, whereas in those with a pre-operative haemoglobin <12 g/dL, although cost-saving, its efficacy could be increased by associating some other blood-saving method.
Keywords: total knee arthroplasty, allogeneic red cell transfusion, post-operative blood salvage, length of hospital stay, cost-effectiveness
Introduction
Unilateral total knee arthroplasty (TKA) can result in substantial blood loss and 20–50% of patients undergoing this operation require allogeneic red cell transfusion (ARCT)1–3. Since allogeneic blood is a scarce and increasingly expensive resource, and ARCT is not a risk-free therapy, different methods to avoid ARCT in these patients have been developed4. Among such methods, pre-operative autologous blood donation, peri-operative blood salvage, and administration of drugs either to reduce blood loss (e.g., tranexamic acid) or to increase red cell mass (e.g., iron, human recombinant erythropoietin), have been investigated and found to have different degrees of effectiveness4.
In TKA, although there may be substantial hidden blood loss due to bleeding into the tissues and residual blood in the joint following the operation, 50% of the true total loss occurs during the post-operative period2 and, consequently, salvage and return of unwashed, filtered post-operative shed blood (PSB) from drains may represent an easy-to-implement alternative to ARCT in these patients5. However, as the efficacy of cell salvage in reducing the need for ARCT in orthopaedic surgery is diminished if a transfusion protocol is applied6, the cost-effectiveness of the procedure is controversial.
The purpose of this study was, therefore, to examine blood management costs in TKA patients for whom a transfusion protocol was defined and PSB return was used, and to compare these costs with those of patients managed with no PSB reinfusion (i.e. post-operative drain and ARCT when appropriate). In addition, we evaluated in which patients this blood-sparing method is more likely to produce cost savings, according to the blood conservation device used, the patients' pre-operative haemoglobin (Hb) concentration, or the different ARCT rates in patients managed with each treatment option.
Materials and methods
Patients and surgery
Data from 1,093 patients who underwent primary TKA at the University Hospital “Virgen de la Victoria”, Málaga (Spain) between January 2004 and December 2009 were retrospectively reviewed. Data from 953 out of the 1,093 patients were previously used for a retrospective analysis of the efficacy of PSB reinfusion for reducing ARCT requirements7. The remaining 140 patients belonged to a multicentre prospective study, which was approved by our Institutional Review Board (unpublished data). Patients' informed consent was not necessary because only non-identifiable data were collected, and complete patient confidentiality was maintained.
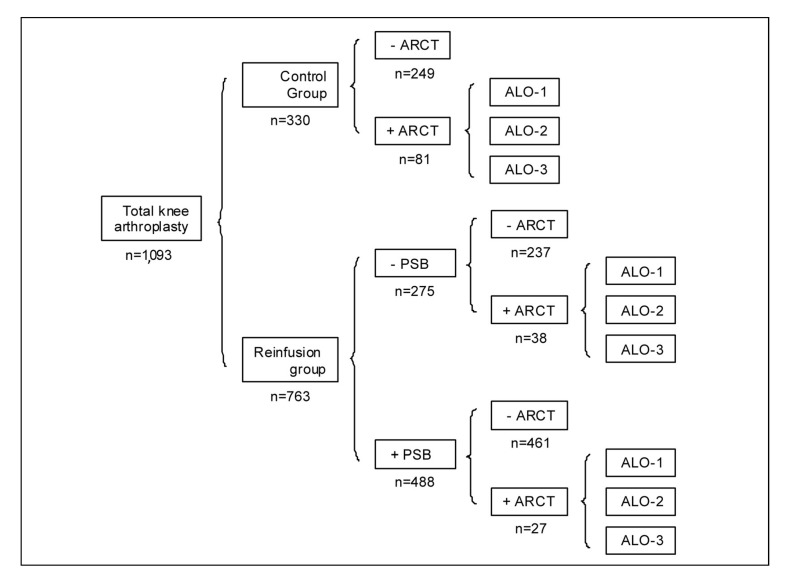
Overall, 763 patients were managed with reinfusion of PSB (reinfusion group), whereas the remaining 330 patients (control group) did not receive PBS because of the patients refusal, surgeons' choice, unavailability of a reinfusion device, expected early discharge (<3 hours) from the anaesthesia recovery unit in patients scheduled to have surgery in the afternoon, or unknown reasons. PSB return was not contraindicated in any patient (Figure 1).
Figure 1.
Distribution of patients according to group (control or reinfusion), reinfusion of postoperative shed blood (PSB), and need for allogeneic red cell transfusion (ARCT).
For patients receiving ARCT, three possible cost scenarios were considered (ALO-1, ALO-2, ALO-3) (see “Materials and methods” for further details).
(+) yes, (−) no, n = number of patients in each subgroup.
All surgical procedures were intended to be performed under regional anaesthesia, involved the application of a tourniquet that was deflated upon knee closure, and used a total condylar knee prosthesis (Duracon, Stryker, Kalamazoo, MI, USA), with the tibial component being cemented. All patients received standardised antibiotic and anti-thrombotic prophylaxis, according to our institutional protocols. There was a similar distribution of senior and junior surgeons between groups.
Post-operative blood salvage and reinfusion
At the end of surgery, the low-vacuum blood collection canister was connected to drainage catheters, and PSB was collected without anticoagulant, and reinfused within the first 6 hours after the operation. The canister is connected to the reinfusion bag to which PSB is transferred, scrapping the last 60–80 mL to minimise fat particles and other debris being transfused into the patient, and allowing for several returns if needed. A 40-μm screen filter was intercalated in the patient's line to eliminate micro-aggregates. Reinfusion was performed exclusively in the anaesthesia recovery unit, and only if the volume of PSB collected was ≥300 mL. There was no transfusion trigger for reinfusion of PSB. The mass of red blood cells in PSB was calculated according to the equation:
Patients in the control group received standard low-vacuum drainage, without reinfusion of PSB. No other blood-sparing techniques were used.
Allogeneic blood transfusion protocol
Although elderly patients may tolerate anaemia poorly, they were not intended to receive ARCT if their Hb level was >9 g/dL, unless they presented sign/symptoms of acute anaemia (hypotension, tachycardia, tachypnoea, dizziness, fatigue, etc.)8,9. In fact, most patients were managed with a restrictive transfusion trigger (Hb <8 g/dL). This transfusion protocol was uniformly applied by anaesthesiologists and surgeons to all patients in the operation theatre, in the anaesthesia recovery unit, and in the ward for the entire duration of hospitalisation. For calculation of requirements for ARCT, one unit of leucodepleted packed red cells was considered as one blood unit, and the mean red blood cell mass per unit (260 mL, haematocrit 65%) was assumed to be 170 mL10.
Clinical data
A set of demographic and clinical data was collected for all patients, including: age, gender, weight, volume of PSB returned, PSB transfusion index (PSBT index, units/patient), patients receiving ARCT (ARCT rate, %), number of allogeneic red cell units, both as a total and as units per patient (ARCT index), overall transfusion index (ARCT+PSB; overall index, unit/patient), peri-operative and pre-transfusion Hb levels, post-operative infections, and length of hospital stay (LOS). Circulating red blood cell mass (RBC mass) was calculated according to estimated blood volume (EBV; 65 mL/kg for females; 70 mL/kg for males) and blood haematocrit (Hct) according to the equation:
Economic data
For the purpose of this study, we considered fixed and variable costs related to patients' blood management. All costs were expressed in euros (€), updated to January 2012 according to changes in the consumer price index in Spain, and included:
-
- Allogeneic red cell acquisition costs. These costs were obtained from the METIS study, which used a time-driven activity-based costing (TDABC) methodology to develop the cost model because of its ability to capture a wide spectrum of indirect costs and the cost for unused capacity as well, by: (i) identifying the cost per process, (ii) calculating the capacity cost rates per process as the ratio of the identified costs to the practical capacity of the resources actually performing the work in the process, (iii) estimating the process time as the total practical capacity of the resources actually performing the activities to capture the cost for unused capacity, (iv) calculating the cost per process by multiplying the capacity cost rate by the time estimate of the process, and (v) allocating the cost based on the volume of transfused blood components (red blood cells, plasma and platelets)11,12.
These costs included the facilities, material, equipment and personnel costs incurred at the Regional Transfusion Centre for collecting blood in mobile units, collecting blood onsite, processing blood and leucodepletion, serological and nucleic acid amplification testing, immunohematology tests, storage and distribution, and societal cost for the donors.
- Transfusion service costs. These included the facilities, material, equipment and personnel costs incurred at the hospital blood bank for selecting the red cell unit, performing cross-matching, and releasing the unit, and at the hospital orthopaedic ward for bed-side checking of the patient's blood group, the transfusion giving set, and transfusing the unit to the patient. These costs were also obtained using the TDABC methodology. At our institution, all patients scheduled for TKA have a type and screen, irrespectively of whether they are going to be managed with a post-operative blood conservation device or not. The cost for typing and screening was not included in the cost analysis.
- Haemoglobin assessment costs. All patients have their Hb level measured within 24–48 hours after surgery to evaluate the need for ARCT. The costs for these determinations were not, therefore, included in the model. We considered only those Hb measurements requested for an indication for ARCT or for monitoring the effect of ARCT between the third post-operative day and the day of discharge. These costs included the facilities, material, equipment and personnel costs for drawing blood, measuring the Hb and interpreting the data.
- Post-operative blood salvage and reinfusion costs. Patient in the reinfusion group were managed with a post-operative blood salvage and reinfusion device. For the present cost analysis we considered the costs of the two devices most frequently used in our area: Bellovac ABT (Wellspect HealthCare, Mölndal, Sweden) and the ConstaVac CBC II (Stryker, Kalamazoo, Michigan, USA). The Bellovac ABT kit includes drain tubing with trocar, the blood collection device, which is manually operated, the reinfusion bag, and the blood-giving set with filter; additional reinfusion bags have to be purchased separately. The ConstaVac CBC II kit only includes the battery-operated blood collection device and reinfusion bag. The drain tubing with trocar and blood-giving set with filter have to be purchased separately. Usually, patients received one reinfusion when the volume of salvaged shed blood was ≤600 mL, and two reinfusions when it was >600 mL. The cost of transfusing PSB was also estimated .Patients in the control group managed without salvage and reinfusion of PSB had a no-reinfusion drain placed at the end of surgery.
- Hospitalisation costs. The cost of one day of hospitalisation at the orthopaedic ward was obtained from the Servicio Andaluz de Salud (Spain).
Blood management cost scenarios
In the basic cost scenario (ALO-1), blood management costs were calculated taking into account the costs of acquisition and transfusion of allogeneic red cells, the costs of acquisition and use (reinfusion) of the blood conservation device, the costs of a low-vacuum drain, and the cost of extra analytical measurements (Hb assessments). We considered another two possible cost scenarios by adding the cost of one (ALO-2) or two (ALO-3) extra days of hospitalisation in patients receiving ARCT. Furthermore, blood management costs per patient in the three scenarios were also analysed after stratifying patients according to their pre-operative Hb into five Hb strata (<12 g/dL, 12–12.9 g/dL, 13–13.9 g/dL, 14–15 g/dL, and >15 g/dL). Finally, a sensitivity analysis was performed for the three scenarios by varying the percentage of patients receiving ARCT in the control group (15%, 20%, 25%, 30%, and 35%) and in the reinfusion group (5% and 10%), and assuming a mean transfusion index of two red blood cell units per patient. All the cost analyses in the different scenarios were performed separately for the Bellovac ABT and ConstaVac CBC II.
Statistics
Data are expressed as incidence (n) and percentage (%), as the mean ± standard deviation, or as the mean and 95% confidence interval (CI). Pearson's χ2 test or Fisher's exact test was used to compare qualitative variables, whereas a parametric ANOVA or non-parametric Kruskall-Wallis test was used for the comparison of quantitative variables, after consideration of distributional characteristics. As the large sample size of this study could lead to small (and irrelevant) differences being recognised as statistically significant, the effect size, as measured by Cohen's d, for each comparison of clinical data is provided. Effect sizes can be interpreted in terms of the percent of non-overlap of the treated group's scores with those of the untreated group. An effect size of 0.0 indicates that the distribution of scores for the treated group overlaps completely with the distribution of scores for the untreated group (there is 0% of non-overlap). An effect size of 0.8 indicates a non-overlap of 47.4% in the two distributions. An effect size of 1.7 indicates a non-overlap of 75.4% in the two distributions13. Net savings (+) or incremental costs (−) for patients' blood management using each cell salvage device are presented as the differences of mean costs and 95% confidence interval between the control group and the reinfusion group. All statistical computations were performed with IBM SPSS Statistics 19 (Licensed to the University of Málaga, Spain) and a p value <0.05 was considered statistically significant.
Results
Efficacy of post-operative shed blood reinfusion
There were no differences in the patients' characteristics between groups, except for gender distribution, and weight, although the effect size, as measured by Cohen's d, was small (Table I). In the reinfusion group, 488 out of 763 patients (64%) received a mean of 506±212 mL of PSB (152±64 mL RBC, equivalent to 0.89±0.38 units of packed red cells), without clinically relevant incidents (Figure 1). Of the 488 reinfused patients, 139 received one or more PSB units (1.39±0.32 units/patient), and 349 received less than one PSB units (0.70±0.14 units/ patient).
Table I.
Demographic and clinical data of two series of patients undergoing surgery for total knee arthroplasty, managed with (reinfusion group) or without (control group) post-operative reinfusion of unwashed shed blood.
| Control group | Reinfusion group | p | Effect size Cohen's d | |
|---|---|---|---|---|
| Patients (n.) | 330 | 763 | ---- | ---- |
| Gender (male/female) | 82/248 | 145/618 | 0.035 | ---- |
| Age (years) | 71±5 | 70±7 | 0.074 | 0.16 |
| Weight (kg) | 74±19 | 76±17 | 0.036 | 0.11 |
| EBV (mL) | 4,895±1,289 | 5,058±1,126 | 0.084 | 0.13 |
| Pre-operative Hb (g/dL) | 13.6±1.2 | 13.5±1.2 | 0.233 | 0.08 |
| RBC mass (mL) | 2,284±640 | 2,294±566 | 0.331 | 0.02 |
| 48 h post-operative Hb (g/dL) | 9.9±2.1 | 10.1±1.6 | 0.128 | 0.11 |
| ARCT units, n (%) | ||||
| 0 | 249 (75.5) | 698 (91.5) | ||
| 1 | 8 (2.4) | 4 (0.5) | 0.001 | ---- |
| 2 | 56 (17.0) | 54 (7.1) | ||
| 3 | 11 (3.3) | 4 (0.5) | ||
| 4 | 6 (1.8) | 3 (0.4) | ||
| Pre-ARCT Hb (g/dL) | 7.6±1.4 | 7.7±1.1 | 0.905 | 0.08 |
| Post-operative infection, n. (%) | 13 (3.9) | 16 (2.1) | ||
| Surgical wound | 5 | 5 | ||
| Pneumonia | 1 | 2 | 0.148 | ---- |
| Urinary tract | 5 | 9 | ||
| Sepsis | 1 | 0 | ||
| Cellulitis | 1 | 0 | ||
| Length of hospital stay (days) | 12.1±4.4 | 9.5±3.6 | 0.001 | 0.65 |
Legend ARCT: allogeneic red cell transfusion; EBV: estimated blood volume; Hb: haemoglobin; Pre-ARCT Hb: haemoglobin level prior to ARCT; RBC mass: circulating red cell mass.
Data are expressed as mean ± SD, or incidence and %.
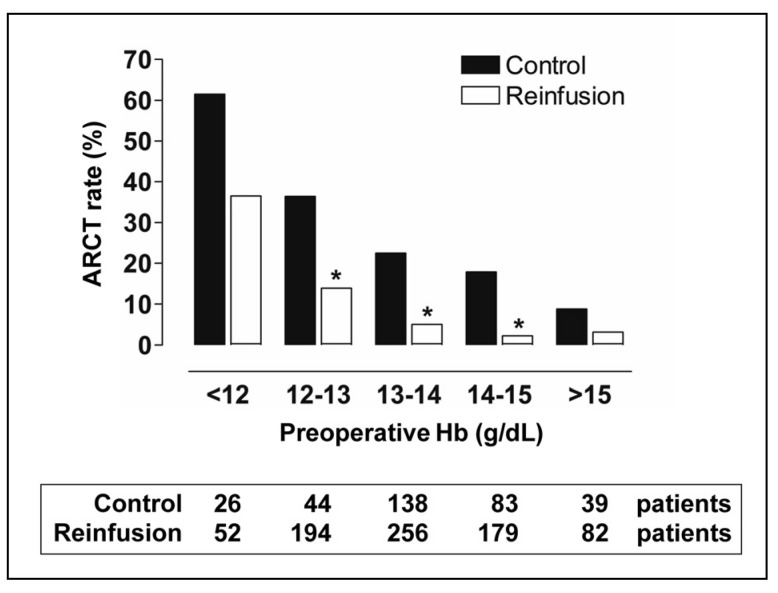
Only 65 patients from the reinfusion group needed additional ARCT (27 among those receiving PSB, and 38 among those not receiving PSB), whereas ARCT was given to 81 patients in the control group (Figure 1). Thus, with respect to the control group, the percentage of patients with ARCT (24.5% vs 8.5%; p <0.001) and the number of transfused allogeneic red cell units (Table I) and ARCT index (Table II) were lower in the reinfusion group. In contrast, the overall transfusion index was higher in the reinfusion group (+0.21 units/patient; Table II). These differences remained statistically significant for the subgroup of patients without ARCT (0.59 units/patient), although patients in the reinfusion group had lower pre-operative Hb levels (−0.3 g/dL; Table II). For the subgroup of patients with ARCT, there were no differences in ARCT or overall index, but again patients in the reinfusion group had lower pre-operative Hb levels (−0.5 g/dL; Table II). In addition, after stratifying patients according to their pre-operative Hb, the differences in ARCT rate between groups remained significant for Hb levels between 12 g/dL and 15 g/dL (Figure 2). Most ARCT were given within 48 hours after surgery.
Table II.
Transfusion data of two series of patients undergoing surgery for total knee arthroplasty, managed with (reinfusion group) or without (control group) postoperative reinfusion of unwashed shed blood.
| Control group | Reinfusion group | p | Effect size Cohen's d | |
|---|---|---|---|---|
| All patients (n, %) | 330 (100) | 763 (100) | ||
| Pre-operative Hb (g/dL) | 13.6±1.2 | 13.5±1.2 | 0.233 | 0.08 |
| ARCT index (units/patient) | 0.54±1.01 | 0.18±0.61 | 0.001 | 0.43 |
| PSBT index (units/patient) | 0 | 0.57±0.52 | 0.001 | 1.55 |
| Overall index (units/patient) | 0.54±1.01 | 0.75±0.75 | 0.001 | 0.24 |
| Difference (units/patient) | 0.21 (95% CI, 0.11–0.32) | |||
|
| ||||
| Patients with ARCT (n, %) | 81 (24.5) | 65 (8.5) | 0.001 | |
| Pre-operative Hb (g/dL) | 13.0±1.4 | 12.5±1.2 | 0.042 | 0.38 |
| ARCT index (units/patient) | 2.19±0.71 | 2.06±0.61 | 0.260 | 0.20 |
| PSBT index (units/patient) | 0 | 0.35±0.51 | 0.001 | 0.97 |
| Overall index (units/patient) | 2.19±0.71 | 2.41±0.77 | 0.062 | 0.30 |
| Difference (units/patient) | 0.23 (95% CI, −0.01–0.47) | |||
|
| ||||
| Patients without ARCT n (%) | 249 (75.5) | 698 (91.5) | 0.001 | |
| Pre-operative Hb (g/dL) | 13.9±1.1 | 13.6±1.4 | 0.008 | 0.24 |
| PSBT index (units/patient) | 0 | 0.59±0.52 | 0.001 | 1.60 |
| Overall index (units/patient) | 0 | 0.59±0.52 | 0.001 | 1.60 |
| Difference (units/patient) | 0.59 (95% CI, 0.53–0.66) | |||
Legend ARCT: allogeneic red cell transfusion; Hb: haemoglobin; PSBT: post-operative salvaged blood transfusion. Data are expressed as mean ± SD or n. and % of total patients. p for reinfusion group vs control group.
Figure 2.
Allogeneic red cell transfusion (ARCT) rate (%) in two series of patients undergoing surgery for total knee replacement, managed with (reinfusion group) or without (control group) post-operative salvage and return of unwashed shed blood, according to pre-operative haemoglobin level.
*p <0.05, reinfusion group vs control group.
There was no significant difference in post-operative infection rates between groups (3.9% vs 2.1%, in the control and reinfusion groups, respectively; p =0.148) (Table I). In contrast, patients in the reinfusion group spent less time in hospital than did those in the control group (Table I). For the whole series, patients who needed ARCT did not show a higher post-operative infection rate than those who did not need ARCT (no transfusion or autologous-only transfusion) (4.1% vs 2.3%, respectively; p =0.254), but ARCT was associated with a longer stay in hospital (+1.9 days [95% CI: 1.2–2.6]; p <0.001).
Cost analysis of post-operative shed blood reinfusion
As stated in the “Material and methods” section, blood management costs were calculated taking into account the costs of acquisition and transfusion of allogeneic blood units, the costs of acquisition and use (reinfusion) of the blood conservation devices, the cost of low vacuum drains, and the cost of extra Hb measurements (ALO-1), plus the costs of prolonging the stay in hospital by 1 day (ALO-2) or 2 days (ALO-3) in patients receiving ARCT (Table III). In the control group, mean blood management costs per patient were € 146.3, € 224.8, and € 303.4, for ALO-1, ALO-2, and ALO-3, respectively (Table IV). The corresponding figures for patients managed with the Bellovac ABT were € 141.7, € 167.9, and € 197.1, respectively, thus providing no incremental cost for ALO-1 and significant savings for ALO-2 and ALO-3 when compared with the control group (Table IV). In contrast, for those patients managed with the ConstaVac CBC II, mean blood management costs per patient were € 198.2, € 225.9, and € 253.6, respectively, thus incurring an incremental cost for ALO-1, being cost neutral for ALO-2, and providing net savings for ALO-3 (Table IV).
Table III.
Direct supply, operating and complication costs associated with leucodepleted allogeneic red cell concentrate (ARC) and post-operative salvaged blood (PSB) transfusion.
| Supply cost | |
| ARC acquisition cost (per unit) | € 155 |
| Bellovac ABT device plus extra reinfusion bag (per patient) | € 80 |
| ConstaVac CBC II device plus drain tubing with trocar and blood-giving set with filter (per patient) | € 136.5 |
| Exudrain low vacuum no reinfusion drain with trocar (per device) | € 26 |
|
| |
| Operating cost | |
| ARC transfusion cost (per unit) | € 52 |
| PSB transfusion (per reinfusion) | € 16 |
| Haemoglobin assessment (per measurement) | € 36 |
|
| |
| Hospitalisation cost | |
| Hospitalisation in the orthopaedic ward (per day) | € 320 |
Table IV.
Estimation of blood management costs and cost savings in patients undergoing surgery for total knee arthroplasty, managed with two different blood conservation devices (Bellovac ABT, AstraTech, or ConstaVac CBC II, Stryker; reinfusion group), compared to those without post-operative reinfusion of unwashed shed blood (control group).
| Cost scenarios | N | ALO-1 | ALO-2 | ALO-3 |
|---|---|---|---|---|
| Blood management costs | ||||
|
| ||||
| Control | ||||
| Without ARCT (€/patient) | 249 | 34.9 | 34.9 | 34.9 |
| With ARCT (€/patient) | 81 | 488.6 | 808.6 | 1,128.6 |
| Mean cost (€/patient) | 146.3 | 224.8 | 303.4 | |
| Range | 26–926 | 26–1,246 | 26–1,566 | |
| Reinfusion with Bellovac ABT | ||||
| Without ARCT (€/patient) | 698 | 102.5 | 102.5 | 102.5 |
| With ARCT (€/patient) | 65 | 555.8 | 862.8 | 1,195.8 |
| Mean cost (€/patient) | 141.7 | 167.9 | 197.1 | |
| Range | 80–980 | 80–1,300 | 80–1,620 | |
| Reinfusion with ConstaVac CBC II | ||||
| Without ARCT (€/patient) | 698 | 158.9 | 158.9 | 158.9 |
| With ARCT (€/patient) | 65 | 612.3 | 932.3 | 1,252.3 |
| Mean cost (€/patient) | 198.2 | 225.9 | 253.6 | |
| Range | 136.5–1,036.5 | 136.5–13,563.5 | 136.5–1,676.5 | |
|
| ||||
| Costs savings | ||||
|
| ||||
| Bellovac ABT vs control | ||||
| Mean (€/patient) | +4.6 | +56.8 | +106 | |
| 95% CI | (−16.3–25.4) | (22.8–90.9) | (58.6–154.0) | |
| p | 0.666 | 0.001 | 0.001 | |
| ConstaVac CBC II vs control | ||||
| Mean (€/patient) | −51.9 | −1.1 | +49.9 | |
| 95% CI | (−72.8–31.1) | (−35.2–33.1) | (2.1–97.5) | |
| p | 0.001 | 0.952 | 0.041 | |
Data calculation takes into account both autologous and allogeneic blood management costs (ALO-1), plus a prolongation of hospital stay by 1 day (ALO-2) or 2 days (ALO-3) in patients receiving allogeneic red cell transfusion (ARCT).
Savings: control costs – reinfusion costs expressed as mean and 95% confidence interval (CI), (+) cost savings, (−) incremental cost.
This analysis was repeated after stratification of patients according to their pre-operative Hb level. For the ALO-1 cost scenario, the use of the Bellovac ABT was consistently cost neutral for patients in all pre-operative Hb strata, whereas it provided significant cost-saving for ALO-2 and ALO-3 at all Hb strata, except for patients presenting with a pre-operative Hb >15 g/dL (Figure 3A). In contrast, the use of the ConstaVac CBC II did not seem to offer significant cost-savings in any Hb strata, but led to significant incremental cost in patients with a pre-operative Hb ≥ 13 g/dL for ALO-1 or >15 g/dL for ALO-2 (Figure 3B).
Figure 3.
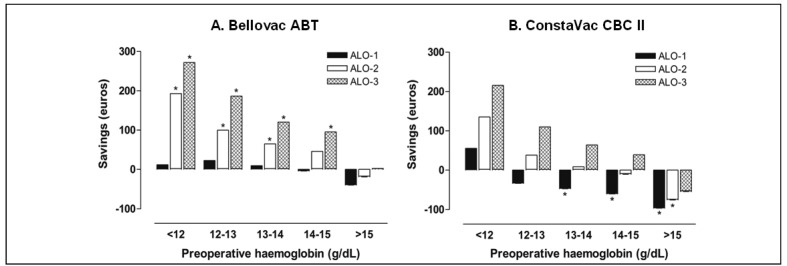
Estimation of mean blood management cost savings (€ +) or incremental cost (€−) per patient in patients undergoing surgery for total knee arthroplasty, managed with a blood conservation device (A. Bellovac ABT, Wellspect HealthCare; B. ConstaVac CBC II, Stryker), or with no reinfusion (control), for different pre-operative haemoglobin levels and cost scenarios.
Data calculation takes into account the costs per patient for both autologous and allogeneic blood management (cost scenario ALO-1), plus a hospital stay prolonged for 1 day (cost scenario ALO-2) or 2 days (cost scenario ALO-3) in patients receiving allogeneic red cell transfusion (see Table IV).
*p<0.05, reinfusion group vs control group.
The sensitivity analysis gave different results depending on the cost scenario (ALO-1, ALO-2, or ALO-3), the ARCT rate in the control group (15%-35%), the ARCT rate in the reinfusion group (5%-10%), and the post-operative blood salvage device used (Bellovac ABT vs ConstaVac CBC II). Overall, costs savings increased from cost scenario ALO-1 through to cost scenario ALO-3 (Table V). Conversely, as the ARCT rate in the control group decreased, the mean blood management costs per patient in the reinfusion group approached (reduced savings) and then exceeded that of the control group (net incremental costs). For all ARCT rates and cost scenarios, savings were higher or incremental costs were lower for the Bellovac ABT than for the ConstaVac CBC II (Table V).
Table V.
Estimation of blood management cost savings (€ +) or incremental costs (€−) per patient in patients undergoing surgery for total knee arthroplasty, managed with a blood conservation device (Bellovac ABT, Wellspect HealthCare, or ConstaVac CBC II, Stryker; reinfusion group), or with no shed blood reinfusion (control group), at different allogeneic transfusion rates.
| Control | Bellovac ABT | ConstaVac CBC II | ||
|---|---|---|---|---|
|
|
||||
| 5% ALO-1 | 10% ALO-1 | 5% ALO-1 | 10% ALO-1 | |
| 35% ALO-1 | € +68.5 | € +45.9 | € +12.1 | € −10.6 |
| 30% ALO-1 | € +45.8 | € +23.2 | € −10.6 | € −33.2 |
| 25% ALO-1 | € +23.2 | € +0.5 | € −33.3 | € −55.9 |
| 20% ALO-1 | € +0.5 | € −22.2 | € −55.9 | € −78.6 |
| 15% ALO-1 | € −22.2 | € −44.9 | € −78.6 | € −101.3 |
|
| ||||
| 5% ALO-2 | 10% ALO-2 | 5% ALO-2 | 10% ALO-2 | |
|
| ||||
| 35% ALO-2 | € +165.2 | € +127.2 | € +108.1 | € +69.4 |
| 30% ALO-2 | € +127.2 | € +87.8 | € +69.4 | € +30.7 |
| 25% ALO-2 | € +87.8 | € +49.1 | € +30.7 | € −7.9 |
| 20% ALO-2 | € +49.1 | € +10.4 | € −7.9 | € −46.6 |
| 15% ALO-2 | € +10.4 | € −27.5 | € −46.6 | € −85.3 |
|
| ||||
| 5% ALO-3 | 10% ALO-3 | 5% ALO-3 | 10% ALO-3 | |
|
| ||||
| 35% ALO-3 | € +260.5 | € +205.9 | € +204.1 | € +149.4 |
| 30% ALO-3 | € +205.8 | € +151.1 | € +149.4 | € +94.8 |
| 25% ALO-3 | € +151.1 | € +96.5 | € +94.8 | € +40.1 |
| 20% ALO-3 | € +96.5 | € +41.8 | € +40.1 | € −14.6 |
| 15% ALO-3 | € +41.8 | € −12.8 | € −14.6 | € −69.3 |
Data calculation takes into account the mean cost per patient for both autologous and allogeneic blood management (cost scenario ALO-1) and prolongation of hospital stay for 1 day (cost scenario ALO-2) or 2 days (cost scenario ALO-3) in patients receiving allogeneic red cell transfusion (see Table IV).
Discussion
In previous studies, we found that PSB return decreased the absolute risk of receiving ARCT. The reduction was greater when a transfusion protocol was not established (48% vs 11%, for control and reinfusion, respectively; p <0.01)14 than after the introduction of a transfusion protocol (30.6% vs 8.4%, respectively; p <0.001)15. The results obtained in this study (24.5% vs 8.5%, respectively; p <0.001) (Table II) are in agreement with those previously reported by us and others using a similar transfusion protocol7,16, and also with those reported for washed shed blood return after TKA17. Despite the observed differences in gender and weight between groups, as there were no significant differences in estimated blood volume and circulating RBC mass, an adjustment of the probability of receiving ARCT by demographic and clinical parameters was not deemed necessary. The available data do, therefore, seem to support the efficacy of PSB retrieval in reducing the need for ARCT in TKA patients.
In the reinfusion group, 488 out of 763 patients (64%) received PSB without clinically relevant incidents (Figure 1), but only 139 received one or more PSB units whereas the remaining 349 received less than one PSB units. Although these figures are similar to those reported for washed, post-operative salvaged blood, they may question the value of reinfusing sub-therapeutic volumes of RBC18. In addition, the overall transfusion index was higher in the reinfusion group (Table II). However, for the sub-group of patients with ARCT, there were no differences in ARCT or overall transfusion index, despite patients from the reinfusion group presenting with lower pre-operative Hb (−0.5 g/dL; Table II), thus supporting a beneficial effect of PSB reinfusion. For patients without ARCT, those from the reinfusion group had lower pre-operative Hb levels than those from the control group (−0.3 g/dL; Table II), but received a mean of 0.59 PSB units per patient, thus suggesting that some patients might have been reinfused unnecessarily and indicating the need for better selection of patients (e.g., by excluding patients presenting with Hb >15 g/dL).
PSB is relatively diluted, has a very variable red blood cell content and may be contaminated by tissue and chemical debris (fat particles, free Hb, activated coagulation factors, fibrin degradation products, activated white blood cells or inflammatory mediators)5, and some authors have questioned the quality and safety of this transfusion product. These authors have suggested that PSB should be washed prior to being returned to the patient, even though the washing procedure does not ensure complete removal of contaminants (e.g., plasma free Hb) and may sub-lethally damage salvaged RBC19–21.
However, in an evaluation of 1,819 patients receiving unwashed PSB after elective lower limb arthroplasty in 38 Dutch hospitals, the frequency of serious adverse events (0.1%) and minor adverse events (3.5%, mostly chills and low-grade fever) was similar to that in other smaller clinical studies22. In our study, we observed no clinically relevant adverse effects of PSB return, although these might be under-reported as PSB reinfusion is not included in the haemovigilance programme. Based on the low incidence of side effects in these two large cohorts of orthopaedic patients, post-operative PSB after elective arthroplasty can be considered to be clinically safe. Nevertheless, most adverse events could be prevented by limiting the volume returned (maximum 1,000–1,500 mL), using a slow infusion rate, discarding the last 50–80 mL of PSB, using a leucocyte-reduction filter, or reducing the plasma content in the PSB5,22. In this regard, it is worth noting that a simple, colloid-based sedimentation method for improving and standardising PSB collected after TKA has been evaluated recently. After colloid treatment, the mean Hb of PSB was 19 g/dL, 90% of RBC were recovered, plasma content was reduced by 80%, and the plasma free Hb was decreased by 53%. It does, therefore, seem that sedimentation of PSB with colloid solutions may provide a low-cost alternative to blood washing, although its clinical implications need to be further evaluated22. Additionally, we did not observe an increase in the post-operative infection rate (Table I). Again, these results are in agreement with those previously published14,15,24–27, except for those of Rosencher et al.1 who found an increased postoperative infection rate in patients receiving PSB.
Given that the observed efficacy of PSB at reducing exposure to ARCT after TKA diminishes if a transfusion protocol is applied, the question regarding which patients are most likely to benefit from this blood-saving strategy, without increasing total blood management costs, is still open. We, therefore, compared blood management costs for the control and reinfusion groups in three possible cost scenarios. For the basic cost scenario ALO-1, the three main blood management cost-drivers in our study population were the ARCT rate (discussed above), the costs of acquisition and transfusion of allogeneic red cells and the costs of acquisition and use of blood salvage devices.
We used the TDABC methodology, instead of activity-based cost (ABC) methodology, because the former is simpler since it requires, for each group of resources, estimates of only two parameters: how much it costs per time unit to supply resources to the business's activities (the total overhead expenditure of a department divided by the total number of minutes of employee time available) and how much time it takes to carry out one unit of each kind of activity (as estimated or observed by the manager). Thus, TDABC has the ability to capture a wide spectrum of the indirect costs and the cost for unused capacity as well12.
Using the TDABC method, the cost of red cell acquisition was estimated to be € 155 per unit12, which is similar to that recently reported in the USA (€ 150–€ 190 per unit), Switzerland (€ 145 per unit) and Austria (€ 115 per unit) using an ABC methodology28. However, there are major differences when transfusion costs are considered. In our study, the cost incurred for transfusing one red cell unit, including the costs in the blood bank and orthopaedic ward and Hb assessment, were estimated to be € 88, which is considerably less that the costs reported in Austria (€ 300), Switzerland (€ 315) and the USA (€ 390–€ 700)28. In contrast, in a recent survey in over 200 hospitals in the USA, the mean acquisition cost for one unit of red cells purchased from a supplier was € 158 and the mean charge to the patient was € 25829; these figures seem to be in agreement with those in our study (€ 155 and € 243, respectively). The different methodologies used for capturing costs probably underlie the observed differences in red cell transfusion costs, thus indicating that estimated local costs must be applied when reproducing this study in a particular hospital.
There are various devices for salvaging and reinfusing filtered shed blood and their component and acquisition costs differ. These devices include theAutoVac (Boehringer Laboratories Inc, Norristown, PA, USA), Orthofuser (Gish Biomedical Inc, Rancho Santa Margarita, CA, USA), Hemovac (Zimmer Corporate, Warsaw, IN, USA), Suretrans (Davol Inc, a subsidiary of C.R. Bard Inc, Murray Hill, NJ, USA), DONOR Autologous Blood Reinfusion System (Van Straten Medical, Nieuwegein, The Netherlands), Bellovac ABT (Wellspect HealthCare, Mölndal, Sweden) and ConstaVac CBC II (Stryker Corp, Kalamazoo, MI, USA). As they seem to be alike in terms of ARCT avoidance30 for this cost study we chose the two devices used in our area: the Bellovac ABT and ConstaVac CBC II. The acquisition costs of Bellovac ABT are uniform across Spain, whereas those of ConstaVac CBC II may vary from one region to another (€ 80–€ 150). For this study the official price for ConstaVac CBC II at the Servicio Andaluz de Salud was used (€ 100). However, as drain tubing with a trocar and the blood-giving set with filter must be purchased separately and again there is a variety of prices depending on the manufacturer, we estimated a mean overall acquisition cost for ConstaVac CBC II of € 136.5. Finally, as neither Hb measurements nor compatibility tests are needed, the costs for reinfusing PSB are mostly derived from the nursing time cost and are, therefore, lower than those for transfusion of an allogeneic red cell unit (€ 16 vs € 88, respectively).
At the rates of ARCT observed in the control and reinfusion groups in our series, for the ALO-1 cost scenario the use of the ConstaVac CBC II resulted in incremental costs when compared with those in the control group (€ 51.9 per patient; P=0.001), whereas the use of the Bellovac ABT was cost neutral (Table II). When this analysis was repeated after stratification of patients according to their pre-operative Hb level, use of the Bellovac ABT was consistently cost neutral for patients in all pre-operative Hb strata (Figure 3A). In contrast, use of the ConstaVac CBC II led to significant incremental costs in patients with pre-operative Hb ≥ 13 g/dL (Figure 3B). Very recently, Rao et al.18 estimated the costs of salvaging and reinfusing one ARCT equivalent unit of washed PSB after 317 TKA (€ 605 per unit), and compared them to those of unwashed shed blood (€ 380 per unit). Overall, 301 ARCT equivalent units of PSB were transfused. There was no control group managed without PSB salvage and reinfusion. However, using a rationale based on the premise that a PSB red cell volume of less than one ARCT equivalent unit would amount to a subtherapeutic intervention, they estimated that 195 ARCT units would have been needed at a cost of € 612 per unit. This analysis suggests that transfusing unwashed PSB is less costly than using washed PSB or ARCT in TKA.
Allogeneic blood transfusion is not a risk-free therapy and may result in patients having a poorer clinical outcome. In our study, although no adverse events were reported, ARCT was associated with a longer stay in hospital (+1.9 days [95% CI: 1.2–2.6]; p <0.001), a finding that has been previously documented7,31–33. In contrast, we found that patients receiving PSB stayed less time in hospital, which might reflect faster post-operative recovery. As patients receiving ARCT may have a prolonged stay in hospital, we considered two other possible scenarios by adding the cost of one (ALO-2) or two (ALO-3) days of hospitalisation in patients receiving at least one ARCT. Interestingly, in the ALO-2 and ALO-3 cost scenarios, the costs for two units of ARCT were € 721 and € 1,041, respectively, which approach that recently estimated from six studies in Western Europe (€ 878)34. Use of the Bellovac ABT resulted in significant savings for ALO-2 (€ 56.8; p =0.001) and ALO-3 (€ 106; p =0.001), which were consistent for patients in all Hb strata, except for patients with pre-operative levels of Hb >15 g/dL (Figure 3A). In contrast, for the whole series, use of the ConstaVac CBC II was cost neutral for ALO-2, and provided savings only in the ALO-3 scenario (€ 49.9; p=0.041) (Table IV); it did not seem to offer significant cost-savings in patients in any of the Hb strata, but led to a significant incremental cost in patients with a pre-operative Hb >15 g/dL in the ALO-2 cost scenario (Figure 3B).
As expected, the sensitivity analysis yielded different results depending on the cost scenario, the ARCT rate in the control and reinfusion groups, and the blood salvage device used. Thus, cost savings increased from cost scenario ALO-1 through to cost scenario ALO-3 (Table V). Conversely, as the ARCT rate in the control group decreased, the mean blood management costs per patient in the reinfusion group approached (reduced savings) and then exceeded that of the control group (net incremental costs). As the acquisition cost of the ConstaVac CBC II is considerably higher than that of the Bellovac ABT (Table III), savings were lower or incremental costs were higher for the ConstaVac CBC II for all ARCT rates and cost scenarios (Table V).
According to our previous publications7,15, data from this study clearly indicate that the use of a blood collection and reinfusion system is beneficial for patients with a pre-operative Hb between 12 g/dL and 15 g/dL, as it may reduce both the ARCT rate and blood management costs (Figures 2 and 3). On the other hand, for patients with a pre-operative Hb of more than 15 g/dL, PSB reinfusion led to incremental costs with apparently no benefit on reduction of ARCT rate, whereas patients with a pre-operative Hb of less than 12 g/dL would probably benefit from the combination of PSB with some other blood-saving method, despite reinfused PSB being efficacious and cost-effective35–37 (Figures 2 and 3).
Finally, a definite conclusion cannot be drawn from this study as observational and retrospective studies do not provide unbiased results, and there are several issues that need to be stressed. Firstly, the transfusion rate might have been affected by the anaesthetists' and/or surgeons' knowledge of PSB reinfusion, resulting in ARCT being delayed (in the anaesthesia recovery unit, while waiting for PSB return) or not prescribed (in the ward) for patients whose Hb actually met transfusion criteria. However, we tried to address this possible bias by performing a sensitivity analysis, which still showed cost-saving for a wide range of ARCT rates, especially when the Bellovac was used. Secondly, the shorter time spent in hospital observed in the reinfusion group, as well as the prolonged stay in hospital in patients receiving ARCT, must be evaluated with caution, as without rigid criteria for discharge, it may be that standards changes slightly during the time period. Thirdly, no cost was estimated for complications associated with the transfusion of allogeneic blood and those of unwashed salvaged blood. All these suggest the need for a large randomised clinical trial comparing standard treatment (ARCT, if needed) with PSB reinfusion in TKA patients to ascertain definitively for which patients PSB return is more likely to be cost-effective.
Acknowledgements
The Authors acknowledge the advice of Mr. Emilio Alfonso from HICY Optimising Health Care, Bruges (Belgium) regarding the use of TDABC methodology for cost calculations.
Preliminary data were presented at the 13th Annual NATA Symposium, Copenhagen, Denmark, 2012.
Footnotes
Conflict of interest disclosure
Manuel Muñoz has received honoraria for consultancy or lectures and/or travel support from Stryker Ibérica (Spain), Wellspect HealthCare (Sweden) and Ferrer Pharma (Spain), the Spanish dealer for Haemonetics (USA), but not for this study.
Daniel Ariza, Arturo Campos, Elisa Martín-Montañez and José Pavía declare no conflicts of interest.
References
- 1.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86-B:561–5. [PubMed] [Google Scholar]
- 3.Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–80. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz M, García-Erce JA, Villar I, Thomas D. Blood conservation strategies in major orthopaedic surgery: efficacy, safety and European regulations. Vox Sang. 2009;96:1–13. doi: 10.1111/j.1423-0410.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz M, Slappendel R, Thomas D. Laboratory characteristics and clinical utility of post-operative cell salvage: washed or unwashed blood transfusion? Blood Transfus. 2011;9:248–61. doi: 10.2450/2010.0063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carless P, Moxey A, O'Connell D, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transf Med. 2004;14:123–44. doi: 10.1111/j.0958-7578.2004.0489.x. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz M, Ariza D, Florez A, Campos A. Reinfusion drains reduce postoperative transfusion requirements after primary total knee replacement surgery. Transfus Med. 2008;18:269–71. doi: 10.1111/j.1365-3148.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 8.British Committee for Standards in Haematology. Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusion. Br J Haematol. 2001;113:24–32. [Google Scholar]
- 9.Sociedad Espanola de Transfusión Sanguínea y Terapia Celular. Guía sobre la transfusión de componentes sanguíneos y derivados plasmáticos. 4th ed. Madrid: SETS; 2010. pp. 43–54. [Google Scholar]
- 10.Comité de Acreditación en Transfusión. Estándares de acreditación en transfusión sanguínea. 3rd ed. Madrid: Acción Médica; 2006. pp. 59–63. [Google Scholar]
- 11.Garcia-Erce JA, Bisbe E, Alfonso E, Muñoz M. The 'true' costs of blood: the average unit production cost of an erythrocyte concentrate in Spain from a societal perspective. Transf Altern Transf Med. 2010;11(suppl 1):16. [Google Scholar]
- 12.Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82:131–8. [PubMed] [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. pp. 21–3. [Google Scholar]
- 14.Muñoz M, Ariza D, Garcerán MJ, et al. Benefits of postoperative shed blood reinfusion in patients undergoing unilateral total knee replacement. Arch Orthop Trauma Surg. 2005;125:385–9. doi: 10.1007/s00402-005-0817-3. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M, Kühlmorgen B, Ariza D, et al. Which patients are more likely to benefit from postoperative shed blood salvage after unilateral total knee replacement? An analysis of 581 consecutive procedures. Vox Sang. 2007;92:136–41. doi: 10.1111/j.1423-0410.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 16.Strümper D, Weber EWG, Gielen-Wijffels S, et al. Clinical efficacy of postoperative autologous transfusion of filtered shed blood in hip and knee arthroplasty. Transfusion. 2004;44:1567–71. doi: 10.1111/j.1537-2995.2004.03233.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas D, Wareham K, Cohen D, Hutching H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86:669–73. doi: 10.1093/bja/86.5.669. [DOI] [PubMed] [Google Scholar]
- 18.Rao VK, Dyga R, Bartels C, Waters JH. A cost study of postoperative cell salvage in the setting of elective primary hip and knee arthroplasty. Transfusion. 2012;52:1750–60. doi: 10.1111/j.1537-2995.2011.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen E, Pawlik M. Reasons against the retransfusion of unwashed wound blood. Transfusion. 2004;44(Suppl 1):45S–53S. doi: 10.1111/j.0041-1132.2004.04179.x. [DOI] [PubMed] [Google Scholar]
- 20.Waters JH, Dyga RM. Postoperative blood salvage: outside the controlled world of the blood bank. Transfusion. 2007;47:362–5. doi: 10.1111/j.1537-2995.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- 21.Ley JT, Yazer MH, Waters JH. Hemolysis and red blood cell mechanical fragility in shed blood after total knee arthroplasty. Transfusion. 2012;52:34–8. doi: 10.1111/j.1537-2995.2011.03217.x. [DOI] [PubMed] [Google Scholar]
- 22.Horstmann WG, Slappendel R, Van Hellemondt GG, et al. Safety of retransfusion of filtered shed blood in 1819 patients after total hip or knee arthroplasty. Transf Altern Transf Med. 2010;11:57–64. [Google Scholar]
- 23.Muñoz M, García-Segovia S, Ariza D, et al. Sedimentation method for preparation of post-operatively salvaged unwashed shed blood in orthopaedic surgery. Br J Anaesth. 2010;105:457–65. doi: 10.1093/bja/aeq174. [DOI] [PubMed] [Google Scholar]
- 24.Newman JH, Bowers M, Murphy J. The clinical advantage of autologous transfusion. A randomised, controlled study after knee replacement. J Bone Joint Surg Br. 1997;79B:630–2. doi: 10.1302/0301-620x.79b4.7272. [DOI] [PubMed] [Google Scholar]
- 25.Peter VK, Radford M, Matthews MG. Re-transfusion of autologous blood from wound drains: the means for reducing transfusion requirements in total knee arthroplasty. Knee. 2001;8:321–3. doi: 10.1016/s0968-0160(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz M, Cobos A, Campos A, et al. Impact of postoperative shed blood transfusion, with or without leucocyte reduction, on acute-phase response to surgery for total knee replacement. Acta Anaesthesiol Scand. 2005;49:1182–90. doi: 10.1111/j.1399-6576.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz M, Cobos A, Campos A, Ariza D, et al. Postoperative unwashed shed blood transfusion does not modify cellular immune response to surgery for total knee replacement. Acta Anaesthesiol Scand. 2006;50:443–50. doi: 10.1111/j.1399-6576.2006.00977.x. [DOI] [PubMed] [Google Scholar]
- 28.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 29.Toner RW, Pizzi L, Leas B, et al. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.So-Osman C, Nelissen RG, Eikenboom HC, Brand A. Efficacy, safety and user-friendliness of two devices for postoperative autologous shed red blood cell re-infusion in elective orthopaedic surgery patients: a randomized pilot study. Transfus Med. 2006;16:321–8. doi: 10.1111/j.1365-3148.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 31.Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81A:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Llewelyn CA, Taylor RS, Todd AA, et al. The effect of universal leukoreduction on postoperative infections and length of hospital stay in elective orthopedic and cardiac surgery. Transfusion. 2004;44:489–500. doi: 10.1111/j.1537-2995.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- 33.Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 34.Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. 2012;52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 35.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz M, García-Erce JA, Cuenca J, et al. On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10:8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]