Introduction
Correct typing of donors’ and recipients’ blood group is of paramount importance in transfusion services. There are two distinct parts in ABO typing, forward and reverse typing, both of which are routinely carried out1. Reverse typing is obligatory, because it can help to reveal mistyping, weak A subgroups with anti-A1 and unexpected IgM antibodies. Any discrepancy between the results of the tests with serum or plasma and red cells should be investigated. Reverse grouping cells, including A, B, O and A2 type red blood cells (RBC), are important to resolve ABO discrepancies. Anti-A and/or anti-B antibodies can be easily detected by red blood cells with A and/or B blood group antigens. The presence of an anti-A1 should be confirmed by testing serum against A1, A2, and O red cells. This method necessitates A2 reverse grouping cells. Very few people in China have the A2 blood group. In the Xi’an area (north-western China) only about 0.0006% of the population have A2 RBC, while about 0.003% of the population have the A2B group are. The ratio of the prevalence of A2B:A2 is 5 (unpublished data). In the Shanghai area (eastern China), this same ratio is about 2.5. Thus, there are more people with A2B group RBC than there are with A2 RBC2,3. As it is known that α-galactosidase, a kind of exoglycosidase, can remove the reducing end α-galactose residues of group B antigen by hydrolysis and based on the characteristics of the group B epitopes and α-galactosidase hydrolysis reaction, α-galactosidase has been used in B to O RBC conversion study since the 1980s4. Our group has been researching in this area for more than 10 years5,6 and we recently obtained a novel α-galactosidase from B. fragilis (which belong to CAZy GH110) with highly specific activity, greatly restricted substrate specificity and a neutral pH optimum7,8. Our research showed that this novel α-galactosidase can convert B RBC to O RBC with high substrate specificity and at low cost7,8. We, therefore, started to study the A2B to A2 conversion using α-galactosidase in order to broaden the source of A2 RBC.
Materials and methods
Enzymatic treatment of A2B red blood cells with B. fragilis α-galactosidase
Fresh human whole blood (blood group A2B) was obtained from Shaanxi Province Blood Centre (Xi’an, China), and the buffy coat was removed. Enzymatic conversion was performed in 1 mL reaction mixtures containing 200 mmol/L glycine and 3 mmol/L NaCl, at pH 6.8 (conversion buffer), with 30% packed RBC as described by Liu et al.9,10. Briefly, RBC were prewashed 1:1 and 1:4 (v/v) in conversion buffer before addition of α-galactosidase (0.005 mg/mL packed RBC) and incubation for 60 min with gentle mixing at 26 °C, followed by four repeat washing cycles with 1:4 (v/v) phosphate-buffered saline (PBS) by centrifugation at 2,000 rpm for 5 min. The washed, enzyme-converted RBC were stored in monoammonium phosphate nutrient solution at 4 °C.
Flow cytometry
Flow cytometry analysis of native and enzyme-converted RBC was performed using an FACScan flow cytometer (Cytomics FC 500 Beckman Coulter, Brea, United States of America) with anti-B monoclonal antibodies (Changchun Brother Biotech Co., Ltd., Changchun, China) and fluorescein isothiocyanate-conjugated affinity purified goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, United States of America). Briefly, 10 μL cells were fixed overnight at room temperature under gentle agitation by the addition of 100 μL of 2% paraformaldehyde (w/v, Sigma-Aldrich, St. Louis, United States of America) in PBS to prevent agglutination of antigen-positive cells. Next, 2 μL packed RBC were prewashed with 500 μL PBS twice and resuspended in 50 μL PBS. Then 50 μL of undiluted primary antibody was added and incubated for 60 minutes in the dark at 25 °C. After two washes and resuspension in 100 μL PBS, 2 μL of undiluted secondary antibody was added and incubated for 60 minutes in the dark at 25 °C. The cells were analysed after another two washes (as above) and resuspension in 500 μL PBS.
Blood group typing
The B status was detected by classical serological techniques and FACS analysis. Agglutination reactions were carried out with three commercial anti-B monoclonal reagents (from Changchun Brother Biotech Co., Ltd., Changchun, China, Shanghai Hemo-pharmaceutical & Biological Co., Ltd. Shanghai, China and Beijing Kinghawk Pharmaceutical Co., Ltd, Beijing, China), and anti-B sera from ten group A donors. A2 RBC were confirmed by commercial anti-A1 monoclonal blend reagents (Shanghai Hemo-pharmaceutical & Biological Co., Ltd.) and anti-A1 sera from two donors who had been previously identified. The contrast control of A2 RBC (native A2 RBCs) was obtained from Medson Technology Co., Ltd., Atlanta, United States of America. The results were confirmed by direct observation under the microscope after centrifugation immediately and after 30 minutes at 4 °C.
Results
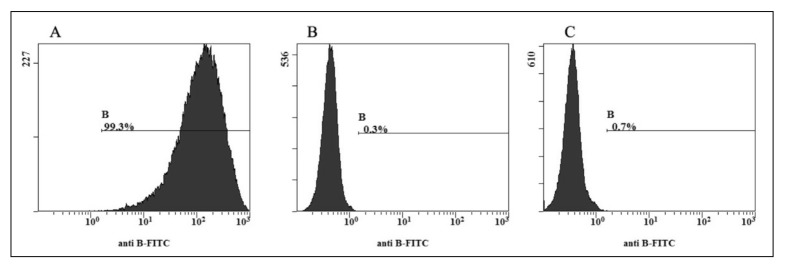
The enzyme-treated RBC did not agglutinate with the three kinds of commercial anti-B monoclonal antibodies or with the anti-B serum from ten group A donors in multiple observations of visual microscope fields in different conditions. All the results were rechecked using anti-B antibody: native A2B showed a higher positive rate, 99.3%, but native A2 and enzymatically converted A2 RBC gave low positive rates of 0.3% and 0.7%, respectively (Figure 1). Moreover the enzymatically produced A2 RBC, just like the A2 control RBC, did not agglutinate with anti-A1 monoclonal antibodies or anti-A1 serum (Table I). These results indicate that we successfully obtained A2 RBC from A2B RBC through treatment with α-galactosidase. These enzymatically type panel produced RBC could be used to prepare A2 cells.
Figure 1.
FACS analysis of A2 RBC converted from A2B cells. The FACS histograms show the B antigen site density as measured by anti-B fluorescein isothiocyanate-labelled monoclonal antibody on A2B (A), native (B) and enzymatically converted A2 RBC (C). The x-axis represents the fluorescence intensity on a logarithmic scale, whereas the y-axis shows the number of RBC evaluated. The antibody diluting concentration was 1:128. The native A2B showed a higher positive rate, 99.3%, but native A2 and enzymatically converted A2 RBC gave low positive rates of 0.3% and 0.7%, respectively.
Table I.
Comparison of the antigenic properties of A2B, A2 and converted A2 RBC.
| Typing results* | |||||
|---|---|---|---|---|---|
|
|
|||||
| Monoclonal antibody | Human serum | ||||
|
|
|||||
| RBC | Anti-A | Anti-B | Anti-A1 | Anti-A1 | Anti-B |
| A1 | 4+ | 0 | 4+ | 2+~3+ | 0 |
| A2B control | 4+ | 4+ | 0 | 0 | 2+~3+S |
| A2 | 4+ | 0 | 0 | 0 | 0 |
| Converted A2 from A2B | 4+ | 0 | 0 | 0 | 0 |
Legend
Typing with three kinds of licensed ABO typing reagents and methods as indicated. The agglutination score ranges from 0 to 4+. s: strongly
Discussion
This study confirmed that the method of preparing A2 reverse grouping cells from group A2B RBC by α-galactosidase was feasible and some distinguishing features of converted A2 could be detected. We had previously established the method of B antigen epitope cleaning by α-galactosidase and demonstrated that B epitopes could be removed completely7,8. In addition, we have confirmed that other antigenic characteristics of converted cells were not changed by the enzymatic hydrolysis11. In this study, we treated A2B cells with α-galactosidase in accordance with our previous enzymatic hydrolysis technology, and detected B and A epitopes with anti-B/A/A1 monoclonal antibodies and FACS. In order to ascertain that A1 epitopes did not exist, human anti-A1 sera was used for further confirmation. Our results showed that A2B RBC can be converted to A2 cells successfully and that the converted A2 RBC can have A2 antigen traits.
As α-galactosidase and α-acetyl-galactosaminidase from bacteria with high activity were cloned and expressed, research was focused on their roles in increasing the number of red cell samples suitable for antibody investigations12. Here we describe a new method for preparing A2 cells for reverse grouping, which can broaden the availability of such cells. As the treatment process is not complicated and the cost is low, the method could readily become widespread.
Besides α-galactosidase, there is also α-aectyl-galactosaminidase, a kind of exo-glycosidases, which can remove A epitopes13: we believe this strategy could be useful for preparing special B or O reverse ABO typing panel cells.
Acknowledgements
The Authors would like to thank the National Science Foundation of China for its financial support (Grant No. 30801063).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 2.Dashkova NG, Ragimov AA, Asoskova TK. Significance of the isoantigen A2 and immune anti-A1 antibodies in transfusiology. Anesteziol Reanimatol. 2009;6:62–5. [PubMed] [Google Scholar]
- 3.Xiang D, Liu X, Guo ZH, et al. The study of ABO subgroup in Shanghai. Chin J Blood Transfus. 2006;19:25–6. [Google Scholar]
- 4.Goldstein J, Siviglia G, Hurst R, et al. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215:168–70. doi: 10.1126/science.6274021. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YP, Yang J, Gao X, et al. B→O blood conversion. Chinese Science Bulletin. 2003;48:269–73. [Google Scholar]
- 6.Zhang YP, Gong F, Bao GQ, et al. B to O erythrocyte conversion by the recombinant α-galactosidase. Chin Med J. 2007;120:1145–50. [PubMed] [Google Scholar]
- 7.Gao HW, Li SB, Bao GQ, et al. [A reconstructed B. Fragilis-derived recombinant α-galactosidase developed for human blood type B→O conversion]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:503–7. [PubMed] [Google Scholar]
- 8.Gao HW, Li SB, Bao GQ, et al. Properties of a novel α-galactosidase from B. Fragilis and its potential for human blood-type B to O conversion. Scientia Sinica Vitae. 2011;41:1030–6. [Google Scholar]
- 9.Liu QP, Sulzenbacher G, Yuan H, et al. Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol. 2007;25:454–64. doi: 10.1038/nbt1298. [DOI] [PubMed] [Google Scholar]
- 10.Liu QP, Yuan H, Bennett EP, et al. Identification of a GH110 subfamily of alpha 1,3-galactosidases: novel enzymes for removal of the alpha 3Gal xenotransplantation antigen. J Biol Chem. 2008;283:8545–54. doi: 10.1074/jbc.M709020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Yu CY, Zhang YP. Human RBCs blood group conversion from AB to O by the combination of α-galactosidase and α-N-acetylgalactosaminidase. Chin J Blood Transfusion. 2008;21:917–20. [Google Scholar]
- 12.Daniels G, Withers SG. Towards universal red blood cells. Nat Biotechnol. 2007;25:427–8. doi: 10.1038/nbt0407-427. [DOI] [PubMed] [Google Scholar]
- 13.Yu CY, Xu H, Wang LS, et al. Human RBCs blood group conversion from A to O using a novel α-N-acetylgalactosaminidase of high specific activity. Chinese Science Bulletin. 2008;53:2008–16. [Google Scholar]