Introduction
Primary cold agglutinin disease (CAD) is a haemolytic anaemia caused by high titres of cold antibodies, usually IgM, directed against red blood cells1,2. Cold agglutinins are present at low titres in all normal individuals, but at high concentrations, the agglutinins can have pathological consequences. In the cool peripheral circulation, cold agglutinin-coated red blood cells activate the complement cascade to the C3b/C3d stage and in the hepatic circulation C3b+ erythrocytes trigger phagocytosis by macrophages. An autoimmune disorder is responsible for the high titres of circulating cold antibodies and CAD accounts for approximately 13–15% of cases of autoimmune haemolytic anaemia3. Moreover, CAD has been found to be associated with a clonal lymphoproliferative disorder in many cases. Indeed, the cold agglutinins are monoclonal IgM in more than 90% of CAD patients and the presence of a lymphoid neoplasm, in particular B-cell non-Hodgkin’s lymphoma (NHL), has been observed in approximately 75% of patients with primary CAD4,5.
Treatments, including corticosteroids or alkylating agents, which are effective in other forms of autoimmune haemolytic anaemia are usually ineffective in CAD. On the other hand, half of the patients with CAD respond to rituximab alone, a drug that has markedly improved the prognosis of patients with B-cell lymphomas. Moreover, combining rituximab with fludarabine has further improved the outcome of CAD patients, with a 75% overall response rate being achieved in a recent, prospective trial6.
Most CAD patients are elderly and their advanced age makes the use of potentially harmful therapies questionable. It is, therefore, reasonable to search for less toxic regimens for CAD patients. Recently, bendamustine, a molecule analogous to fludarabine, has been successfully employed in the treatment of low-grade B-cell NHL7,8. Compared to fludarabine, bendamustine has fewer side effects and an excellent tolerability. Thus, bendamustine with rituximab might be an effective chemo-immunotherapy option for elderly patients with CAD. The case report here presented, gives support to the use of bendamustine in CAD.
Case report
A 74-year old Caucasian male with worsening severe anaemia was referred to our Centre in January 2009. The patient had a past medical history of poliomyelitis at the age of 4 years. He also reported a recent diagnosis of benign prostatic hypertrophy and hypertension, treated with ACE-inhibitors. On physical examination, the patient was remarkably pale with conjunctival icterus; he had bilateral lower limb oedema and mild tachycardia. There was no significant peripheral lymphadenopathy or evidence of hepatosplenomegaly.
The complete blood count revealed anaemia with a haemoglobin concentration of 7 g/dL, an increased reticulocyte count (218×109/L) and normal white blood cell and platelet counts. The suspected haemolysis was confirmed by the marked increase of serum lactate dehydrogenase (LDH) at 1,667 U/L along with an undetectable serum haptoglobin and a raised level of indirect bilirubin (2.9 mg/dL). Analysis of the urine showed an increased level of urobilinogen along with moderate haemoglobinuria. The direct Coombs’ test was strongly positive for complement C3d and a high titre (1/2,048) of anti-I cold antibody was detected. The direct Coombs’ test was negative for IgG and no monoclonal IgM was detected by serum electrophoresis.
A bone marrow biopsy showed a hypercellular marrow with erythroid hyperplasia and a slight interstitial excess of small, CD20-positive lymphocytes. Immunophenotyping of the bone marrow aspirate revealed the presence of a B-cell clonal population, which was negative for CD5 and CD10. These cells accounted for approximately 50% of bone marrow lymphocytes. Computed tomography of the chest and abdomen did not reveal any lymphadenopathies. Virological markers of hepatitis C virus and human immunodeficiency virus were negative, while antibodies to the core antigen of hepatitis B virus were detected.
Based on these findings, a diagnosis of CAD associated with a CD5-negative B-cell lymphoproliferative disorder was made.
The patient was referred to our Centre under steroid therapy (prednisone 1 mg/kg), started in the preceding 2 weeks. This steroid therapy had been of little if any benefit, with the patient’s parameters of haemolytic anaemia remaining unchanged. Thus, following admission, the dose of prednisone was progressively tapered down and the treatment was stopped within 2 months. Taking into consideration the co-existing B-cell NHL, treatment was started with rituximab, an anti-CD20 monoclonal antibody. Rituximab was prescribed as weekly infusions of 375 mg/m2. However, after two infusions, a further drop of haemoglobin to 6.8 g/dL was recorded. Additional therapy was, therefore, required, and a combination of rituximab with cyclophosphamide, vincristine and prednisolone (R-CVP) was chosen, based on a report demonstrating the efficacy of R-CVP in indolent B-cell NHL9. Following R-CVP, due to the persistence of both severe anaemia and a high titre of cold agglutinins, the patient underwent multiple plasmapheresis procedures and two red cell transfusions. A transient reduction of haemolysis was observed while the patient was under daily plasmapheresis and his haemoglobin level reached the value of 10.7 g/dL along with a progressive decrease of serum LDH and bilirubin level. Three plasmapheresis procedures were performed. However, haemolysis rapidly recurred a few days after discontinuation of the plasmapheresis. The persistent haemolysis necessitated a modification of the chemo-immunotherapy approach and a regimen of rituximab, oxaliplatin, dexamethasone and cytarabine (R-OxDHA) was started. Recent studies showed the excellent tolerability of the OxDHA scheme, in which oxaliplatin replaces the highly nephrotoxic cisplatin that was included in the original DHAP scheme10–12. In a preliminary experience by our group, the R-OxDHA combination resulted unexpectedly effective in indolent B-cell NHL and chronic lymphocytic leukaemia13. Indeed, both the original DHAP and the modified Ox-DHA regimens are known to have potent anti-tumour activity and are particularly suitable for patients with refractory or recurrent NHL. Unfortunately our patient with CAD had a poor response to the R-OxDHA combination, with further progression of haemolysis, a serum LDH value spiking at >1,500 U/L and increasingly severe anaemia (haemoglobin value down to 6 g/dL).
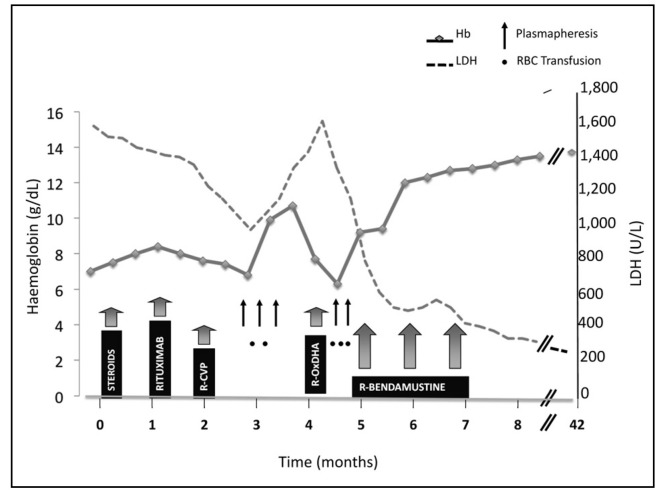
Two additional plasmapheresis procedures were performed and three transfusions of incompletely matched packed red blood cells were given with minimal benefit. The patient needed further therapy. Fludarabine was a possible option for this patient, since it has been proposed as the most suitable drug in the case of autoimmune haemolytic anaemia associated with lymphoproliferative disease refractory to conventional chemo-immunotherapy5,6,14. However, the side effects of fludarabine, alone or in combination, cannot be minimised, particularly in elderly patients previously exposed to immunosuppressive treatments. Thus, bendamustine was considered as an alternative. In the first course the dose of bendamustine was 70 mg/m2/die for 2 consecutive days every 4 weeks, whereas the dose from the second course was 90 mg/m2/die. Rituximab was given at the standard dose of 375 mg/m2 with each course of bedamustine. The patient had an unexpectedly rapid and marked response. Indeed, already after the first course of rituximab-bendamustine, the patient’s haemoglobin concentration increased progressively and no more red blood cell transfusions were necessary. The patient was discharged and his treatment was continued in a Day Hospital regimen. The changes in haemoglobin and serum LDH values in the various phases of treatment are summarised in Figure 1.
Figure 1.
Changes of haemoglobin (Hb) and serum lactate dehydrogenase (LDH) values during the different phases of treatment.
R-CVP: rituximab + cyclophosphamide, vincristine, prednisolone; R-OxDHA: rituximab + oxaliplatin, dexamethasone, cytarabine; see text for details of drug doses and schedules.
The patient was re-assessed in July 2009, after three courses of rituximab-bendamustine. The bone marrow biopsy showed complete remission of the lymphoma. Minimal residual disease analysis showed a residual, very weak band in the polymerase chain reaction for Ig clonal rearrangement. No evidence of hepatosplenomegaly was found in the ultrasound examination. Cold agglutinin antibody resulted positive with a titre of 1/1,024 and the patient’s haemoglobin was 13 g/dL. Serum LDH, bilirubin and haptoglobin levels were normalised (Figure 1). In consideration of: (i) the excellent response; (ii) the advanced age of the patient; (iii) the multiple immunosuppressive treatments received; and (iv) the infantile poliomyelitis infection, the rituximab-bendamustine treatment was discontinued and the patient was placed under clinical observation without any additional therapy for the CAD.
In June 2012, 3 years after the rituximab-bendamustine treatment, the patient is still transfusion-independent and in continuous clinical remission of the previous CAD. The last complete blood count revealed a haemoglobin level of 14.4 g/dL, with a reticulocyte count of 34×109/L and persistently normal white blood cell and platelet counts. Evaluation of serum cold agglutinins showed residual positivity with a titre of 1/128. Normal values of LDH, haptoglobin, reticulocytes and bilirubin confirmed the complete remission of the haemolytic anaemia.
Discussion
We report here the favourable outcome of an elderly patient with CAD treated with rituximab and bendamustine. CAD is an autoimmune haemolytic anaemia which is frequently associated with B-cell NHL. In fact, the patient described in this report was diagnosed with a low-grade lymphoma. The peculiarity of this case was the refractoriness of the disease to most treatments known to be quite effective in autoimmune anaemia associated with B-cell NHL, such as steroids, rituximab monotherapy and rituximab combined with chemotherapy. It is known that rituximab is a key drug in the treatment of B-cell NHL and it has been shown to be frequently effective in immune cytopenias15–18. The most striking feature of this case was the good response to bendamustine given together with rituximab. The patient’s haemolysis resolved rapidly following treatment with bendamustine and three courses of the rituximab-bendamustine combination were sufficient to induce a complete clinical response, which is still maintained more than 3 years after treatment completion. Finally, the tolerability of the rituximab-bendamustine salvage treatment was surprisingly good, considering the advanced age of the patient and the multiple previous immunosuppressive treatments. This case report confirms that bendamustine is an effective anti-lymphoma drug, which is exceptionally suitable for elderly patients with low-grade NHL.
Bendamustine is an alkylating agent with cytotoxic activity and was first synthesised in the early 1960s in Jena, in the former East German Democratic Republic, by Ozegowski and Krebs19. The intention was to synthesise a nitrogen mustard compound that was less toxic than, but at least as effective as, other alkylating agents. Bendamustine has structural similarities to both alkylating agents and purine analogues and in general it is very well tolerated. In fact, the compound proved to be quite effective and it was extensively used over a 20-year period in East Germany in cancer patients. Since its licensing in Europe, bendamustine has been increasingly employed in the management of NHL, either as a single agent or in different combinations, primarily in association with rituximab. Several studies have shown that bendamustine is clinically active in patients with disease refractory to conventional chemotherapy20,21. It has distinct mechanistic features, which probably contribute to its high clinical efficacy. In fact, due to its partial cross-reactivity with other chemotherapeutic agents, bendamustine is among the drugs most extensively employed in the treatment of relapsed/refractory NHL. Moreover, there is now great interest in the potential role of bendamustine as initial therapy, given its promising efficacy and good safety profile. At present, bendamustine has been approved as first-line therapy for chronic lymphocytic leukaemia and studies are ongoing regarding its front-line use for low-grade follicular lymphoma21.
The case reported here suggests a possible role for bendamustine in CAD and, in general, in autoimmune haemolytic anaemias associated with NHL. Significant advances have been made in the treatment of CAD22. However, there are still several unresolved issues, including the problem of treatment-related toxicity, the chances of achieving long-lasting responses and the need to find efficient second-line therapy. The excellent outcome of the CAD patient described here suggests that prospective trials should be considered to investigate the safety and efficacy of the combination of bendamustine-rituximab as a novel therapeutic option, suitable as both first- and second-line treatment for elderly patients with autoimmune haemolytic anaemia associated with a B-cell lymphoproliferative disease.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Dacie JV, Crookston JH, Christenson WN. Incomplete cold antibodies role of complement in sensitization to antiglobulin serum by potentially haemolytic antibodies. Br J Haematol. 1957;3:77–87. doi: 10.1111/j.1365-2141.1957.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA. Cold agglutinin disease. Haematol J. 2006;91:439–41. [PubMed] [Google Scholar]
- 3.Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. Br Med J. 1981;282:2023–7. doi: 10.1136/bmj.282.6281.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103:2925–8. doi: 10.1182/blood-2003-10-3597. [DOI] [PubMed] [Google Scholar]
- 5.Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematol J. 2006;91:460–6. [PubMed] [Google Scholar]
- 6.Berentsen S, Randen U, Vagan AM, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010;116:3180–4. doi: 10.1182/blood-2010-06-288647. [DOI] [PubMed] [Google Scholar]
- 7.Rummel MJ, Mitrou PS, Hoelzer D. Bendamustine in the treatment of non-Hodgkin’s lymphoma: results and future perspectives. Semin Oncol. 2002;29(4 Suppl 13):27–32. doi: 10.1053/sonc.2002.34877. [DOI] [PubMed] [Google Scholar]
- 8.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–9. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 9.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–23. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 10.Machover D, Delmas-Marsalet B, Misra SC, et al. Dexamethasone, high-dose cytarabine and oxaliplatin (DHAOX) as salvage tratment for patients with initially refractory or relapsed non-Hodgkin’s lymphoma. Ann Oncol. 2001;12:1439–43. doi: 10.1023/a:1012501305214. [DOI] [PubMed] [Google Scholar]
- 11.Chau I, Webb A, Cunningham D, et al. An oxaliplatin-based chemotherapy in patients with relapsed or refractory intermediate and high-grade non-Hodgkin’s lymphoma. Br J Haematol. 2001;115:786–92. doi: 10.1046/j.1365-2141.2001.03181.x. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Geyer SM, Kurtin PJ, et al. Salvage chemotherapy with rituximab DHAP for relapsed non-Hodgkin lymphoma: a phase II trial in the North Central Cancer Treatment Group. Leuk Lymph. 2008;49:1074–80. doi: 10.1080/10428190801993470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruella M, Ricca I, Gueli A, et al. Dexamethasone, cytarabine and cisplatin or oxaliplatin schedule (DHAP or OxDHA) is effective and widely applicable in chronic lymphocytic leukaemia and Waldenstrom macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2009;114:3434. [Google Scholar]
- 14.Jacobs A. Cold agglutinin hemolysis responding to fludarabine therapy. Am J Hematol. 1996;53:279–80. doi: 10.1002/(SICI)1096-8652(199612)53:4<279::AID-AJH17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26:3603–13. doi: 10.1038/sj.onc.1210376. [DOI] [PubMed] [Google Scholar]
- 16.Tarella C, Zanni M, Magni M, et al. Rituximab improves the therapeutic efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multicenter GITIL survey of 745 patients. J Clin Oncol. 2008;26:3166–75. doi: 10.1200/JCO.2007.14.4204. [DOI] [PubMed] [Google Scholar]
- 17.Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;141:149–69. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- 18.Barcellini W, Zanella A. Rituximab therapy for autoimmune haematological diseases. Eur J Intern Med. 2011;22:220–9. doi: 10.1016/j.ejim.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Forero-Torres A, Saleh MN. Bendamustine in non-Hodgkin lymphoma: the double-agent that came from the Cold War. Clin Lymphoma Myeloma. 2007;8(Suppl 1):S13–7. doi: 10.3816/clm.2007.s.028. [DOI] [PubMed] [Google Scholar]
- 20.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 21.Rummel MJ, Gregory SA. Bendamustine’s emerging role in the management of lymphoid malignancies. Semin Hematol. 2011;48(Suppl 1):S24–36. doi: 10.1053/j.seminhematol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309–17. doi: 10.1111/j.1365-2141.2011.08643.x. [DOI] [PubMed] [Google Scholar]