Abstract
Loss of mechanosensory hair cells in the inner ear accounts for many hearing loss and balance disorders. Several beneficial pharmaceutical drugs cause hair cell death as a side effect. These include aminoglycoside antibiotics, such as neomycin, kanamycin and gentamicin, and several cancer chemotherapy drugs, such as cisplatin. Discovering new compounds that protect mammalian hair cells from toxic insults is experimentally difficult because of the inaccessibility of the inner ear. We used the zebrafish lateral line sensory system as an in vivo screening platform to survey a library of FDA-approved pharmaceuticals for compounds that protect hair cells from neomycin, gentamicin, kanamycin and cisplatin. Ten compounds were identified that provide protection from at least two of the four toxins. The resulting compounds fall into several drug classes, including serotonin and dopamine-modulating drugs, adrenergic receptor ligands, and estrogen receptor modulators. The protective compounds show different effects against the different toxins, supporting the idea that each toxin causes hair cell death by distinct, but partially overlapping, mechanisms. Furthermore, some compounds from the same drug classes had different protective properties, suggesting that they might not prevent hair cell death by their known target mechanisms. Some protective compounds blocked gentamicin uptake into hair cells, suggesting that they may block mechanotransduction or other routes of entry. The protective compounds identified in our screen will provide a starting point for studies in mammals as well as further research discovering the cellular signaling pathways that trigger hair cell death.
Keywords: zebrafish, ototoxicity, selective serotonin reuptake inhibitor (SSRI), selective estrogen receptor modulator (SERM), aminoglycoside, cisplatin
1. Introduction
There are no clinically approved and effective drugs that protect patients against the ototoxic side effects of important pharmaceutical agents such as aminoglycoside antibiotics and anti-neoplastic drugs (e.g. cisplatin and carboplatin). We seek co-treatments that diminish or eliminate the toxic side effects of these beneficial drugs. As access to mature mammalian auditory and vestibular tissue prevents large-scale screening for modulators of hair cell death, we have used the larval zebrafish lateral line system for screening (reviewed in Ou, et al., 2010; Coffin, et al., 2010). The zebrafish lateral line is a sensory system that contains mechanosensory hair cells in clusters called neuromasts located in stereotyped positions along the body and head of the larval and adult fish (Metcalfe, et al., 1985; Raible & Kruse, 2000). Lateral line hair cells share structural, functional and molecular similarities to mammalian inner ear hair cells (reviewed in Coombs, 1989; Nicolson 2005). Many zebrafish genes have been identified that function in hearing and balance with phenotypes similar to that of their mammalian counterparts including components of the hair cell transduction apparatus (Nicolson et al. 1998; Ernest et al., 2000). The hair cells in this system present several advantages for screening: they can be visualized in vivo because the hair cells are located on the outside of the body and readily take up vital dyes (Harris, et al., 2003; Santos et al. 2006). Lateral line hair cells, like their mammalian counterparts, are sensitive to ototoxins, such as aminoglycosides, cisplatin and other chemotherapeutic drugs; and cell death can be reliably induced in a dose-dependent fashion (Williams & Holder, 2000; Harris, et al., 2003; Ton & Parng, 2005; Ou, et al., 2007; Owens, et al., 2009; Hirose, et al., 2011). Furthermore, zebrafish lateral line hair cells demonstrate morphological changes similar to the inner ear hair cells of birds and mammals when exposed to aminoglycosides (Owens et al, 2007) indicating that this is a robust model for understanding mammalian ototoxicity. The larvae are small, can be produced in large numbers, and are easy to handle, allowing addition to 96-well plates and rapid visualization of many individuals.
Aminoglycoside antibiotics, including gentamicin, kanamycin and neomycin, are antibacterial agents that are used worldwide for gram-negative bacterial infections. Depending on the country, they are used regularly or are reserved for use in more severe infections, e.g. tuberculosis. Aminoglyocosides kill bacteria by inhibiting ribosome function (Davis, 1987) and may lead to production of hydroxyl radicals that contribute to bacterial cell death (Kohanski, et al., 2007). Besides their beneficial toxic effects against bacteria, aminoglycosides can cause nephrotoxicity as well as hearing loss and vestibular dysfunction due to hair cell death in humans (Hinshaw, et al., 1946), rodents (Brummett, 1983), birds (Fermin, et al., 1980), and fish (Kaus, 1992; Lombarte, et al., 1993; Williams & Holder, 2000; Harris, et al., 2003). Cisplatin is a valuable and widely used anti-cancer drug that disrupts cell division by creating DNA adducts (Rosenberg, 1985). Hearing loss and hair cell loss due to cisplatin exposure has been observed in humans (Reddel, et al., 1982, Rosenberg, 1985), rodents (Fleischman, et al., 1975) and fish (Ou, et al., 2007).
We have screened drug and small molecule libraries for compounds that protect hair cells from neomycin toxicity (Ou, et al., 2009; Owens, et al., 2008). Those screens revealed several compounds with previously unknown protective properties and two compounds have proven effective in mammalian inner ear in vitro or in vivo (Owens, et al., 2008; Ou, et al., 2009; Rubel, et al., 2011). Given this success, screening additional libraries of clinically approved drugs that might protect against a spectrum of hair cell toxins may be clinically useful and provide additional insights into the processes occurring in hair cells. Among the aminoglycosides, ototoxicity and tissue sensitivity differ (Dulon, et al., 1986; Selimoglu, et al., 2003; Smith, et al., 1977; Wanamaker et al. 1999). Furthermore, aminoglycosides can exhibit divergent kinetics and potency. For example, in zebrafish neuromasts, gentamicin employs at least two processes leading to cell death: one short-term and another longer-term, while neomycin may activate only a short-term process (Owens, et al., 2009). Cisplatin likely employs separate processes leading to cell death compared to aminoglycosides, and zebrafish mutations that protect against aminoglycosides do not protect against cisplatin (Owens, et al., 2008). However, studies of hair cell ultrastructure suggest that mitochondria are early targets of both aminoglycosides and cisplatin (Owens, et al., 2007; Giari, et al., 2011). The possibility of a co-treatment effective with all aminoglycosides or with both aminoglycosides and cisplatin is enticing.
In the experiments presented below, we screened a library of FDA-approved drugs for compounds that protect hair cells of the zebrafish lateral line from the hair cell toxins neomycin, gentamicin, kanamycin and cisplatin. The Enzo Life Sciences FDA-approved drug library contains 640 compounds that have been or are currently used clinically. Of the 640 compounds in the library, ten compounds were found that protected hair cells treated with at least two of the four toxins. These compounds were subjected to further testing to examine the properties of their protective effects. The main classes of protective compounds were serotonin and dopamine-modulating drugs, adrenergic receptor ligands, and estrogen receptor modulators. Estrogen receptor modulators were further investigated and we identified three additional estrogen receptor modulators that protect hair cells from neomycin, though differences in their protective effects with gentamicin suggest that the compounds may be acting by multiple mechanisms.
2. Materials and Methods
2.1 Animal care
Larval zebrafish (Danio rerio) of the *AB wildtype strain were produced via group matings of adult fish. Larvae were housed at 28.5 °C and maintained at a density of 50 fish per 10 cm diameter petri dish in embryo media (EM; 994 μM MgSO4, 150 μM KH2PO4, 42 μM Na2HPO4, 986 μM CaCl2, 503 μM KCl, 14.9 mM NaCl, and 714 μM NaHCO3, pH 7.2). Beginning at 4 days post-fertilization (dpf), fish were fed live rotifers daily. Experiments were performed using 5 – 6 dpf larvae. The University of Washington Animal Care and Use Committee approved of the animal procedures described here.
2.2 Drug Library
Enzo's FDA Approved Drug Library (Enzo Life Sciences Inc., Plymouth Meeting, PA, USA, formerly BIOMOL International, L.P.) was used to screen zebrafish larvae for compounds that protect against toxin-induced lateral line hair cell death. The library consists of 640 compounds dissolved at 2 mg/ml in dimethyl sulfoxide (DMSO; Sigma-Aldrich, #D1435). The compounds were aliquoted into eight 96-well plates with one compound per well and 80 compounds per plate. The plates were stored at 4 °C during initial screening and re-testing.
2.3 Screening
Larvae were pre-labeled with 2 μM YO-PRO1 (Invitrogen, Carlsbad, CA, USA; #Y3603) in embryo medium for 30 min and then rinsed three times. YO-PRO1 is a cyanine monomer fluorescent vital dye that labels hair cell DNA (Santos et al. 2006). After pre-labeling, larvae were transferred to Nunc 96-well optical bottom plates (Thermo Fisher Scientific, #265301), with one fish per well in 147 μL of embryo medium. Library compounds were diluted 1:10 in embryo medium and then 3 μL of the diluted mixture were added to 96 well plate containing larvae (one compound per well) for a final concentration of 4 μg/ml of library compound and final DMSO concentration of 0.2% in each well. Larvae were incubated for 1 hr with library compounds, then one of the following hair cell toxins was added and fish were incubated in library compound and hair cell toxin together. The duration and concentration of the toxin was adjusted to kill most hair cells in each neuromast under control conditions without any of the library drugs but at the same DMSO concentration: 200 μM neomycin (Sigma, #N1142) for 1 hr; 50 μM gentamicin (Sigma, #G1397) for 6 hr; 400 μM kanamycin (Abraxis Pharmaceuticals Products) for 24 hr; or 50 μM cisplatin (Bedford Laboratories) for 24 hr (Harris et al, 2003; Ou et al. 2007; Owens, et al., 2009). Eight fish in each plate (in column 1) served as mock controls and received no treatment (negative controls). Eight more fish (in column 12) were treated with hair cell toxin but no library drug to serve as positive controls for toxin potency. After incubation in library compound and hair cell toxin for the times listed above, larvae were anesthetized with buffered 0.001% MS222 (3-aminobenzoic acid ethyl ester methanesulfonate; Sigma, #E10521) and immediately viewed using fluorescence microscopy on an automated stage (Marianas imaging system, Intelligent Imaging Innovations) using a Zeiss Axiovert 200M inverted microscope (Carl Zeiss). Fish were scored on a scale from 0 to 2 with 2 corresponding to mostly healthy hair cells and a 0 corresponding to mostly dead hair cells. Evaluation of one plate took approximately 40 min. Compounds that scored a 2 were retested on five larvae with the above protocol, and those that scored a mean of 1.5 or greater in retesting were considered “protective compounds” and subjected to further evaluation, as described below. All experiments were conducted at 28.5 °C.
2.4 Dose-response matrix testing
All confirmed protective compounds were further tested against all four hair cell toxins regardless of whether a given protective compound was positive with a specific toxin in the initial screen. These experiments were designed to determine the optimal concentration of protective compound, defined as the minimum concentration of the protective compound that confers protection against the concentrations of toxins that resulted in maximum hair cell death (those noted above). Larvae at 5–6 dpf were transferred to 6-well Corning Netwell (#3480) baskets and placed in 6-well plates in EM with approximately 10 fish per basket. This allowed for easy transfer between treatment media. Protective compound and toxins were diluted in EM and wells were filled to 7 mL. Larvae were pretreated for 1 hr in the protective compound followed by co-treatment in the protective compound and one of the hair cell toxins. Incubation times in toxins were the same as those used for the 96-well drug screen format (see section 2.3 above) with the following exceptions. Protective compounds that did not show protection with the 6 hr gentamicin exposure were tested with a 1 hr gentamicin treatment in addition to the 6 hr treatment. Kanamycin (Sigma, #K0254) was tested at 400 μM with a 24 hr exposure. After protective compound and toxin treatments were complete, larvae were rinsed 4 times in EM and treated with 0.005% of DASPEI (2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide; Sigma, St. Louis MO, #D0815) in EM for 15min to label neuromasts. Then larvae were rinsed 2 times and anesthetized with MS222. Larvae were transferred to glass depression slides and viewed on a Leica epifluorescent microscope with a DASPEI filter (Chroma Technologies, Brattleboro VT). Neuromasts were scored as previously described (Owens, et al., 2009). To find optimal concentrations, each protective compound was tested at concentrations of 0, 0.5, 1, 5, 10, 50, 100 μM. For protective compounds that were toxic by themselves at high concentrations, lower concentrations were tested to determine if intermediate doses were optimal.
The next series of experiments for each protective compound were designed to determine the range of toxin concentrations at which each protective compound is effective. In these experiments, the concentration of the protective compound was held at the optimal concentration, as determined above, and the toxin concentration was varied over a broad range: aminoglycosides—0, 25, 50, 100, 200, 400 μM; cisplatin—0, 5, 10, 25, 50, 100 μM). Analytical procedures were the same as above. These two sets of experiments define what we call the 'dose-response matrix'.
2.5 Minimum inhibitory concentration and minimum bactericidal concentration testing
All protective compounds were tested at double their experimentally determined optimal protective concentration to determine whether bactericidal activity of neomycin was altered. Escherichia coli (ATCC 25922) was used to determine minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) in the presence of protective compounds and an aminoglycoside. MIC and MBC tests were performed in accordance with the National Clinical and Laboratory Standards Institute (Wayne, 2008; Wikler, 2006).
2.6 Cancer cell culture experiments
The compounds that protected hair cells from cisplatin toxicity were tested in cancer cell culture experiments to determine whether cisplatin chemotherapeutic activity was altered. Human adenocarcinoma cells from the A549 cell line (alveolar basal epithelial cell line, ATCC # CCL-185; Giard, et al., 1973) were cultured in Costar 3917 assay plates (Corning, Inc., Corning, NY) in 100 μL Dulbecco’s Modified Eagle Medium High Glucose 1X (DMEM; Gibco, Invitrogen, Grand Island, NY, #11965) with 10% Fetal Bovine Serum (FBS; heat inactivated at 56°C for 30min; Valley Biomedical, Inc., Winchester, VA, #B53033) and 1% L-glutamine (200 mM 100X, Gibco, Invitrogen, Grand Island, NY, #25030) with 5,000 cells per well. All cell incubations took place at 37°C in a humidified incubator with 5% CO2 . Cells were allowed to attach overnight (15–18 hr) and then 100 μL of protective compound (see below) was added to bring the total volume per well to 200 μL. The solutions of protective compounds were prepared by serially diluting stocks in DMEM with 10% FBS and 1% L-glutamine before being added to cell plates. A matrix of combining one concentration of cisplatin (at 0, 25, 50, or 100 μM), and one concentration of benzamil (at 0, 12.5, 25, 50, or 100 μM) or paroxetine (at 0, 3.1, 6.1, 12.5 or 25 μM) was used. After protective compound and cisplatin addition, cells were incubated for 72 hours. Following incubation, the treatment-containing media was replaced with 200 μL of DMEM with 2% FBS and 1% L-glutamine and cells were allowed to recover for 24 hr. Cell viability was evaluated using the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI, #G7573) following manufacturer’s instructions. Cell luminescence was assessed using a TopCount NXT microplate scintillation and luminescence counter (Packard Instrument Company, Meriden, CT).
2.7 Pretreatment experiments
To test whether pre-treatment in protective compound is necessary for the protective effects of the compound against aminoglycoside toxicity, 10 larvae were pretreated in each protective compound for either 60 min, 15 min, or 0 min (no pretreatment), followed by 1 hr co-treatment in the protective compound and 200 μM neomycin. Then larvae were rinsed four times, hair cells were labeled with DASPEI, rinsed two times, and anesthetized with MS222. Hair cell survival was evaluated using the method and equipment described in 2.4 Dose response matrix testing. Negative controls were treated with EM only and positive controls were treated with neomycin only.
2.8 Gentamicin-conjugated Texas Red (GTTR) Imaging
To determine whether the compounds that are protective against aminoglycoside toxins also influence aminoglycoside entry into hair cells, we studied uptake of labeled gentamicin. Gentamicin-conjugated to Texas Red (GTTR) was prepared following the protocol of Steyger et al. (2003). Larvae were pretreated with the protective compound at its optimal protective concentration against 50 μM gentamicin for 5 min, followed by co-treatment with 50 μM GTTR for 3 min. Then fish were rinsed four times, anesthetized with MS222 and mounted on bridged coverslips for imaging. Using fluorescence microscopy on an automated stage (Marianas imaging system, Intelligent Imaging Innovations) with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss), we first determined the location of 2–3 neuromasts by viewing under brightfield illumination. Then, z-stacks of fluorescent images were collected of the 2–3 neuromasts. Image collection of larvae occurred within 6 min of being rinsed out of GTTR. Each fish was imaged once and 5 fish were imaged for each treatment group. Control fish were treated with EM only or with 3 min of 50 μM GTTR only.
Image analysis was done using Slidebook 5 (Intelligent Imaging Innovations, Denver CO) and Excel 2003 (Microsoft, Redmond, WA). To assess GTTR uptake that occurred, we performed several operations to isolate the fluorescence intensities corresponding to neuromasts. First, we subtracted the fluorescence intensities of the background images from the intensities of the experimental images to isolate GTTR-related signals. We traced the borders of the 2–3 neuromasts to delineate regions of interest. We copied the shapes defined by the traced borders of the neuromasts and applied these shapes to a nearby region of the image of the fish that did not contain neuromasts to assess a region of background with the same volume as the analyzed neuromasts. The mean background intensity and standard deviation (SD) was used to create a background threshold. We used Boolean addition to isolate signals within the neuromast regions of interest above the background threshold (“thresholded neuromasts”). The mean intensity, standard deviation of intensity, sum intensity and volume in voxels of the thresholded neuromast signal were measured. To compare between protective compounds and controls, we performed additional operations to normalize the intensity measures. To create an index of intensity, the thresholded neuromast signal was normalized to the background intensity for each fish and averaged for 5 fish. Then the average for mock-treated fish was subtracted to zero the background, and these values were divided by the average of the GTTR only group to produce the “Intensity normalized to GTTR only (%)”.
2.9 Statistical analyses
All values reported below are means and all error bars are S.E.M unless otherwise noted. Statistical analyses were performed using GraphPad Prism version 5.0a for MacOS (GraphPad Software, San Diego, CA). For single groups, unpaired t-tests or 1-way analysis of variance (ANOVA) tests followed by Dunnett’s Multiple Comparison were used. For experiments with multiple groups, 2-way ANOVA tests followed by Bonferroni post-hoc tests were used. Results were considered statistically significant if p < 0.05. Microsoft Excel was used to perform linear least squares regression.
3. Results
3.1 FDA-approved drug screen reveals compounds that protect hair cells from aminoglycosides and cisplatin
We screened each compound in the Enzo FDA-approved library for compounds that protect hair cells of the zebrafish lateral line from each of four hair cell toxins – cisplatin, neomycin, gentamicin and kanamycin. For screening, fish were labeled with a DNA dye YO-PRO1, pretreated with a library compound for 1 hour prior to co-treatment with a toxin and then examined in vivo to assess the protective capacity of the library compound. In each fish, we evaluated a set of ten neuromasts (IO1–4, SO1–2, M2, MI1–2, O2) , which have stereotyped locations (see Raible and Kruse, 2000). Ten compounds (listed in Table 1) were identified that protect hair cells from two or more of the hair cell toxins. Phenoxybenzamine was identified in a previous chemical screen for compounds that protect hair cells from neomycin (Ou et al. 2009). The compounds fall into distinct drug mechanistic classes including several compounds with known estrogen-related activities, serotonin and dopamine-related activities, or adenoreceptor-related activities. However, the compounds present different profiles of toxin protection. Some protect against only neomycin and short-term gentamicin exposure, while one compound, benzamil, protects against all four toxins that were examined. The disparate drug activities and profiles of protection suggest that the compounds protect hair cells via distinct mechanisms.
Table 1.
Drugs from an FDA-approved drug library that protect hair cells from toxin-induced cell death
| Protective Drug | CAS #a | Ototoxinb | Optimal Concentrationc (μM) | Pretreatd (min) | Uptakee | MIC/MBCf | FDA targetg | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neo 1hr | Gent 1hr | Gent 6hr | Kan 24hr | Cis 24hr | |||||||
| Estrogen-related | |||||||||||
|
| |||||||||||
| raloxifene | 82640-04-8 | P | P | P | 10 | 0 | <.001 | normal | SERM | ||
|
| |||||||||||
| tamoxifen | 10540-29-1 | P | P | 10 | 0 | n.s. | normal | SERM | |||
|
| |||||||||||
| Serotonin and dopamine-related | |||||||||||
|
| |||||||||||
| paroxetine | 110429-35-1 | P | P | P | P | 10 | 0 | n.s. | normal | SSRI | |
|
| |||||||||||
| fluoxetine | 56296-78-7 | P | P | 50 | 0 | n.s. | normal | SSRI | |||
|
| |||||||||||
| methiothepin | 74611-28-2 | P | P | P | 10 | 60 | n.s. | normal | Serotonin and dopamine agonist | ||
|
| |||||||||||
| fluspirilene | 1841-19-6 | P | P | 10 | 0 | n.s. | normal | Dopamine antagonist | |||
|
| |||||||||||
| Adenoreceptor-related | |||||||||||
|
| |||||||||||
| phenoxybenzamine | 63-92-3 | P | P | P | 50 | 15 | <.05 | normal | antagonist at alpha adrenoceptor | ||
|
| |||||||||||
| ractopamine | 90274-24-1 | P | P | P | P | 50 | 0 | <.01 | normal | agonist at beta adrenoceptor | |
|
| |||||||||||
| Other classes | |||||||||||
|
| |||||||||||
| loperamide | 34552-83-5 | P | P | P | P | 10 | 0 | n.s. | normal | μ-opioid receptor agonist | |
|
| |||||||||||
| benzamil | 161804-20-2 | P | P | P | P | P | 50 | 0 | <.05 | MBC increased | Na/Ca channel blocker |
CAS # is the Chemical Abstract Service Registry number.
Shaded box with letter P indicates that a protective effect was observed with that protective compound-toxin combination. Neo=neomycin 1 hr exposure, Gent 1=gentamicin 1 hr exposure, Gent 6=gentamicin 6 hr exposure, Kan=kanamycin 24 hr exposure, Cis=cisplatin 24 hr exposure.
The lowest concentration of protective compound tested that confers the maximum protection against toxins.
The pretreatment time in minutes before toxin exposure that is needed to observe effective protection.
Whether gentamicin-Texas Red is able to enter cells in the presence of protective drug based on our fluorescence assay. The level of significance is indicated for drugs with altered uptake relative to GTTR only; n.s. = gentamicin entry not significantly different from GTTR only control. All drugs had uptake that was significantly different from embryo media only (EM) except for ractopamine and raloxifene.
Shows whether protective drug affected minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC), where an increase in MIC or MBC indicates that neomycin is less effective at killing bacteria in presence of hair cell protective drug.
The accepted target of the drug in the literature, which may or may not describe the mode of action when protecting hair cells. SERM = selective estrogen receptor modulator; SSRI = selective serotonin reuptake inhibitor.
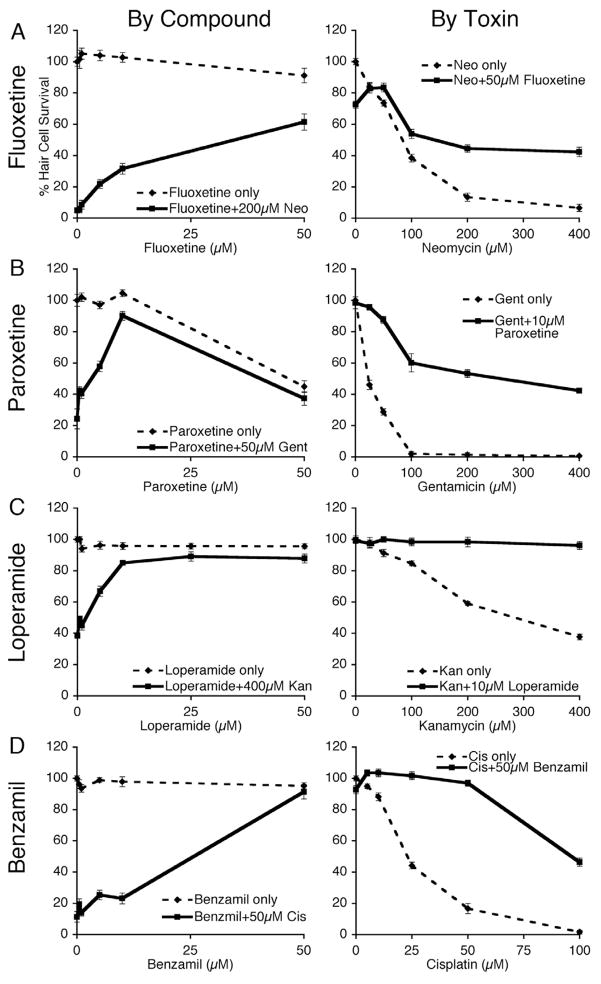
We performed dose-response matrix testing to determine the magnitude of hair cell protection across dose ranges of each protective compound and each toxin. Figure 1 shows examples of the dose-response relationships between protective compounds and each of the four toxins tested in the screen. Fluoxetine (Fig. 1A), paroxetine (Fig. 1B), and loperamide (Fig. 1C) are widely prescribed drugs. Their protective abilities are notable as they represent previously unknown interactions with hair cell toxins. These results reveal that the compounds’ protective abilities increase with concentration (left column, 1-way ANOVA, p < 0.05 for all groups) and that an optimal protective compound concentration protects across a wide range of toxin concentrations (right column, 2-way ANOVA, p < 0.05 for treatment, concentration and interaction for all groups). The supplementary data (Supplementary Table A.1) shows the mean, standard deviations and sample sizes for all protective compounds examined in this study.
Figure 1.
Dose response testing reveals broad ranges of protective effects against neomycin, gentamicin, kanamycin and cisplatin. Graphs show mean % hair cell survival ± 1 SEM of zebrafish treated with varying concentrations of protective compound (“By Compound”) or by varying concentration of toxin (“By Toxin”) for four protective compounds: fluoxetine (A), paroxetine (B), loperamide (C) and benzamil (D). Zebrafish were assayed following 1 hr pretreatment in putative protective compound followed by co-exposure to a toxin: neomycin (neo) for 1 hr, gentamicin (gent) for 6 hr, kanamycin (kan) for 24 hr or cisplatin (cis) for 24 hr with protective compound. Larvae were stained in vivo with DASPEI to evaluate neuromasts. Each group consists of >9 fish. In the left column, treating with protective compound had a significant effect (1-way ANOVA, p<0.05) for all groups. In the right column, adding protective compound to a wide range of toxin concentrations had a significant effect (2-way ANOVA, p < 0.05 for treatment, concentration and interaction) for all groups.
Benzamil’s protection against hair cell loss induced by cisplatin and the aminoglycosides is promisingly robust (Fig. 1D, Supplementary Table A.1). We previously showed that amiloride, another member of the pyrazine carboxamide class, protects hair cells from neomycin and gentamicin (Coffin, et al., 2009). We were interested in whether amiloride would also protect zebrafish lateral line hair cells from cisplatin toxicity. When tested a wide range of amiloride doses, we found no protection from cisplatin toxicity in our dose response assay (Supplementary Table A.1).
3.2 Do protective compounds alter the efficacy of aminoglycosides or cisplatin?
A requirement of any protection against ototoxicity is that it must not inhibit the primary clinical role of the toxin, namely the bactericidal action of aminoglycosides or chemotherapeutic action of cisplatin. We performed minimum inhibitory concentration (MIC) tests and minimum bactericidal concentration (MBC) tests with Escherichia coli (ATCC 25922) and neomycin in the presence of each of the protective compounds found in the screen. The MIC test gives the minimum concentration of neomycin necessary to halt bacterial cell division and the MBC test gives the minimum concentration necessary to kill the majority of bacterial cells. Only benzamil altered the ability of neomycin to kill bacteria. The MBC of neomycin increased from 8μg/ml without benzamil to 16μg/ml in the presence of 100 μM benzamil. None of the other compounds altered the MIC or MBC of neomycin (Table 1).
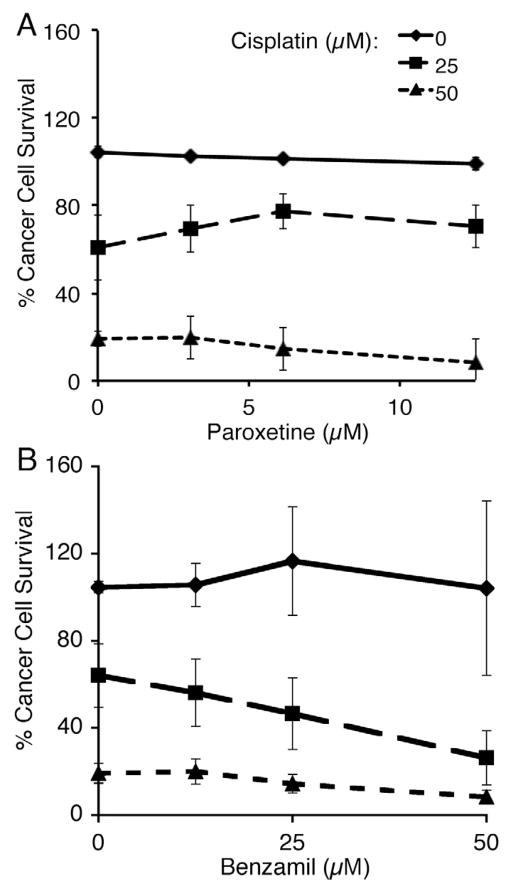
We also tested whether benzamil or paroxetine, the compounds that provided protection against cisplatin-induced hair cell loss, altered the ability of cisplatin to kill cancer cells. We cultured a human adenocarcinoma cell line derived from alveolar basal epithelia (A549) with varying concentrations of cisplatin and either benzamil or paroxetine for 72 hrs followed by 24 hr recovery period. As shown in Figure 2, neither paroxetine nor benzamil significantly increased the amount of cell survival beyond cisplatin controls. Paroxetine (Fig. 2A) did not significantly alter the toxic effects of cisplatin at all (2-way ANOVA, Interaction: p = 0.91, paroxetine concentration: p = 0.60, cisplatin concentration: p < 0.0001), while adding increasing doses of benzamil to the cisplatin treatment augments the ability of cisplatin to kill cancer cells (Fig. 2B, 2-way ANOVA, Interaction: p = 0.21, benzamil concentration: p < 0.001, cisplatin concentration: p < 0.0001). This suggests that both paroxetine and benzamil might be worth exploring as effective hair cell defenses to a cisplatin treatment regime.
Figure 2.
Cisplatin-induced cancer cell death is not inhibited by addition of benzamil or paroxetine. Graphs show the mean percentage ± 1 SEM of A549 cells surviving 72 hr treatment with cisplatin and paroxetine (A) or benzamil (B). Mean % cancer cell survival was determined using an ATP luminescence assay. Each point shows the mean of three replicate experiments. Paroxetine did not significantly alter toxic effects of cisplatin (2-way ANOVA, interaction: p = 0.91, paroxetine concentration: p = 0.60, cisplatin concentration: p < 0.0001). Benzamil significantly enhanced cisplatin toxicity (2-way ANOVA, interaction: p = 0.21, benzamil concentration: p < 0.001, cisplatin concentration: p < 0.0001).
3.3 Is pretreatment necessary for protection?
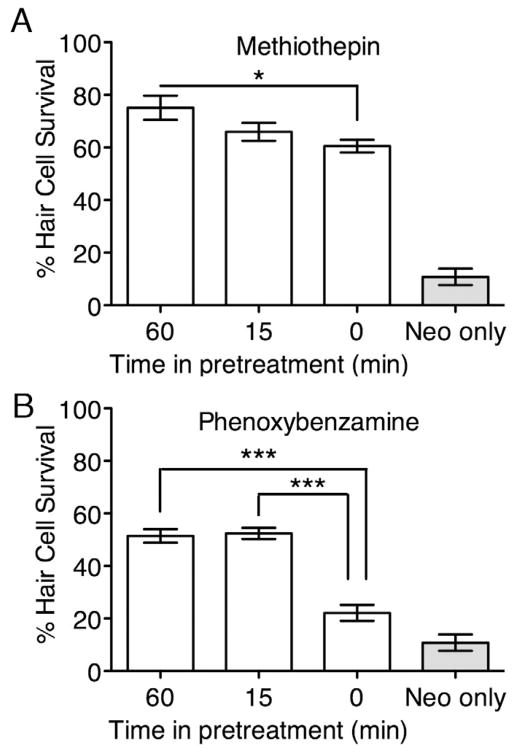
To begin analyses of the mechanisms by which compounds act to protect hair cells, we asked whether the one hour pretreatment used in the screen and initial dose-response testing was necessary for hair cell protection. Knowing the time of action of the protective compound may indicate at what point in the process the compound prevents hair cell death. We tested each protective compound at its optimal concentration as determined in initial dose-response testing (see Table 1) for its ability to protect hair cells against 200 μM neomycin following 60 min, 15 min or no pretreatment. All but two of the compounds showed no requirement for a pretreatment; similar protection was observed following all pretreatment paradigms. Methiothepin showed decreasing protection with decreasing pretreatment time (Fig. 3A, 1-way ANOVA, p < 0.05). Phenoxybenzamine provided similar protection following 60 min and 15 min pretreatment, but did not protect as well when there was no pretreatment (Fig. 3B, 1-way ANOVA, p < 0.0001). This shows that phenoxybenzamine requires at least a 15 min pretreatment before neomycin exposure. That phenoxybenzamine and methiothepin require pretreatment suggests that these compounds might prime cells for resilience by interacting with an intracellular target. Compounds that do not require pretreatment may also interact with an intracellular target or alternatively could interact directly with toxins, enhance efflux, or prevent toxin entry. We investigate the last below.
Figure 3.
Pretreatment is necessary to confer protection against neomycin with methiothepin and phenoxybenzamine. Zebrafish hair cells exposed to methiothepin (A) or phenoxybenzamine (B) along with 1 hr exposure to neomycin showed significantly decreased protection as “time in pretreatment” decreased (1-way ANOVA, p < 0.05 for both compounds; by Bonferroni’s Multiple Comparison test: * = p < 0.05; *** = p < 0.001.). Treatment groups were tested along with control group treated with 1 hr exposure to neomycin only (“neo only”). Y-axes show mean % hair cell survival ± 1 SEM normalized to untreated controls. X-axis categories show minutes of time in pretreatment in protective compound before toxin exposure.
3.4 Protective compounds differentially affect uptake of gentamicin
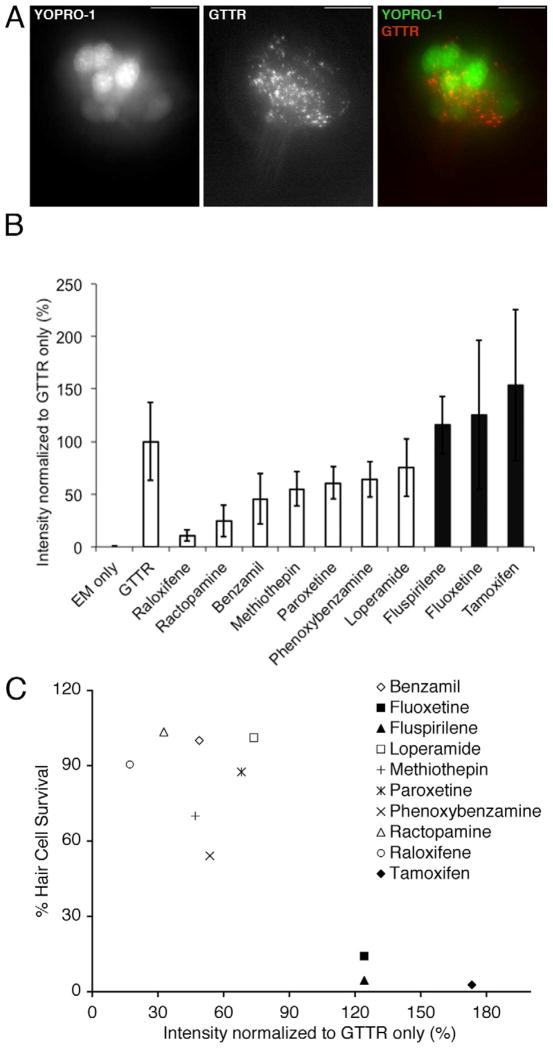
The observation that cells do not need to be primed with most of these protective compounds before toxin exposure suggested that protective compounds might act by blocking toxin entry. To explore this idea, we utilized gentamicin conjugated to the Texas Red fluorophore (GTTR; Steyger, et al., 2003). We exposed zebrafish lateral line hair cells to a 5 min pretreatment with each protective compound at its optimal concentration for protection against 50 μM gentamicin followed by 3 min of co-exposure to GTTR and protective compound. Zebrafish were then rinsed in fresh EM and mounted on slides to image their neuromasts. As shown in Figure 4A, three minutes of GTTR exposure results in observable fluorescence in neuromasts. Normalized fluorescence intensity to compare GTTR uptake levels between treatment groups is shown in Figure 4B. A priori t-tests were used to assess the significance of uptake inhibition of each protective compound. The t-test results are shown in Table 1. The results shown in Figure 4B suggest that there is a gradation of early uptake inhibition that may be responsible or may play a role in the protection shown by many compounds. This is represented by significant inhibition by raloxifene, ractopamine, benzamil and phenoxybenzamine. On the other hand, there are several compounds that protect against neomycin and short-term gentamicin exposure which do not inhibit GTTR uptake (fluoxetine and fluspirilene) or which may even enhance uptake (tamoxifen).
Figure 4.
Gentamicin-Texas Red (GTTR) entry into hair cells is altered by exposure to protective compounds. A) Images of a zebrafish neuromast pre-labeled with YOPRO-1 after a 3 min exposure to GTTR. YO-PRO1 labels hair cell nuclei (left, green channel). GTTR localized to puncta the apical region of hair cells, down and to the left in this image and a lower levels throughout the cytoplasm (middle, red channel). An image merging the red and green channels is shown to the right. The scale bar is 10 μM. B) Mean fluorescence intensity ± 1 s.d. of neuromasts exposed to protective compound and GTTR, embryo media only (EM only), or GTTR only (GTTR) for 3 min. Intensity is normalized and shown as a mean percentage of the GTTR only case. GTTR fluorescence intensity is significantly altered by addition of the protective compounds raloxifene (p<.001 ), ractopamine (p< .01), phenoxybenzamine (p<.05) and benzamil (p<.05). Shaded bars represent compounds that did not protect hair cells from gentamicin toxicity after 6 hr exposure. C) Plot of GTTR intensity following 3 min exposure to 50 μM GTTR (% intensity normalized to GTTR only) vs. hair cell survival following 6 hr exposure to 50 μM gentamicin (% hair cell staining) for each protective compound.
If compounds provide hair cell protection by altering toxin uptake, we might predict that there will be a negative correlation between the uptake of GTTR and the degree of protection. To examine this relationship across compounds, we plotted the fluorescence intensity measure observed in Figure 4B versus the amount of hair cell protection provided against exposure to 50 μM gentamicin for 6 hr (see Figure 1 and Supplementary Table A.1). Figure 4C shows that the compounds that did not protect hair cells from 50 μM, 6 hr gentamicin ototoxicity had either enhanced or normal uptake, while the compounds that had strong protection against 6 hr gentamicin ototoxicity had either normal or reduced uptake. Within each group of protective compounds there was little to no correlation between GTTR uptake inhibition and the degree of protection. This result suggests that while inhibition of gentamicin uptake may help explain the ability of some drugs to protect hair cells from gentamicin exposure (e.g., of ractopamine and raloxifene), this relationship may not be robust across all hair cell protectants.
3.5 Estrogen receptor modulators protect hair cells from aminoglycoside toxicity
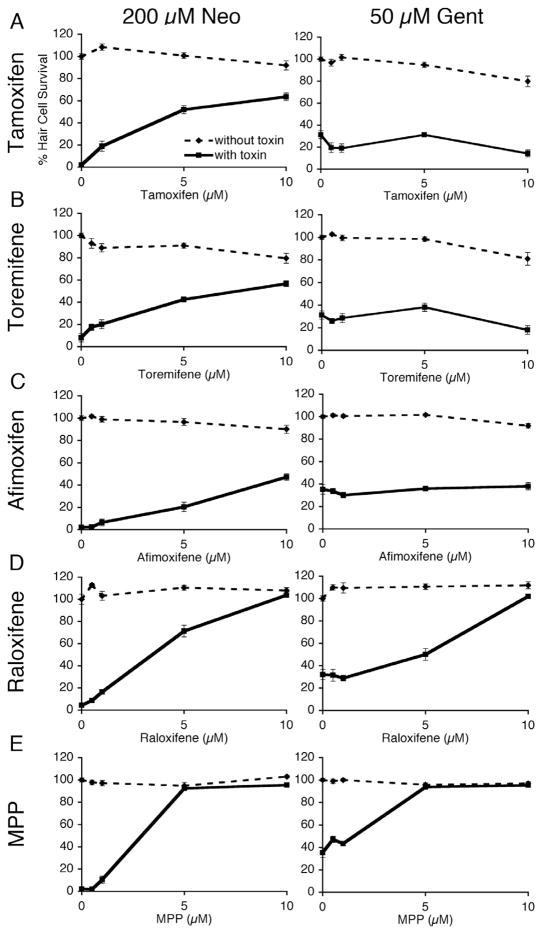
We found several compounds in our screen that belong to the same drug classes (Table 1). We hypothesized that these compounds might protect hair cells via the previously known mechanisms of action attributed to each drug class. The selective estrogen receptor modulators, tamoxifen and raloxifene, were of particular interest because estrogen receptors have been found in the mammalian organ of Corti (Stenberg, et al., 1999) and in zebrafish lateral line hair cells (Tingaud-Sequeira et al. 2004; Froehlicher, et al., 2009). We therefore tested whether other estrogen receptor ligands also protect hair cells from aminoglycoside toxicity. The compounds tested included estrogen receptor modulators that are active at multiple estrogen receptors as well as ligands to specific estrogen receptors (Table 2). Of those tested, toremifene, afimoxifene, and MPP protected hair cells from neomycin toxicity (Fig. 5). Only MPP also protected hair cells from long-term gentamicin toxicity (Fig. 5E). Five other compounds that interact with estrogen receptors had no effect (Table 2).
Table 2.
Estrogen receptor modulators tested for ability to protect hair cells from aminoglycosides
| Estrogen Receptor Modulators | CAS #a | Ototoxinb | Structure resemblesc | Estrogen receptor and modulationd | ||
|---|---|---|---|---|---|---|
| Neo 1hr | Gent 1hr | Gent 6hr | ||||
| raloxifene | 82640-04-8 | P | P | P | self | SERM |
|
| ||||||
| MPP | 289726-02-9 | P | P | P | Raloxifene | Selective ER alpha antagonist |
|
| ||||||
| tamoxifen | 10540-29-1 | P | P | self | SERM | |
|
| ||||||
| toremifene | 89778-27-8 | P | P | Tamoxifen | SERM | |
|
| ||||||
| afimoxifene | 68392-35-8 | P | P | Tamoxifen | SERM | |
|
| ||||||
| PPT | 263717-53-9 | neither | Selective ER alpha agonist | |||
|
| ||||||
| DPN | 1428-67-7 | neither | Selective ER beta agonist | |||
|
| ||||||
| R,R THC | 138090-06-9 | neither | ER alpha agonist/beta antagonist | |||
|
| ||||||
| 17 β estradiol | 50-28-2 | neither | ER agonist | |||
|
| ||||||
| fulvestrant | 129453-61-8 | neither | General ER antagonist | |||
CAS # is the Chemical Abstract Service Registry number.
Shaded box with the letter P indicates that a protective effect was observed with that protective compound-toxin combination. Neo=neomycin 1 hr exposure, Gent 1=gentamicin 1 hr exposure, Gent 6=gentamicin 6 hr
Indicates whether chemical structure is similar to either raloxifene or tamoxifen, the two compounds originally found in the drug screen.
Known receptor binding partners and the modulatory results of binding: SERM = selective estrogen receptor modulator with both agonist and antagonist effects with multiple estrogen receptors, ER = estrogen
Figure 5.
Five selective estrogen receptor modulators (SERMs) protect hair cells from neomycin, while only raloxifene and MPP protect hair cells from long-term gentamicin exposure. Dose response curves show mean % hair cell survival ± 1 SEM following exposure to 200 μM neomycin or 50 μM gentamicin and tamoxifen (A), toremifene (B), afimoxifene (C), raloxifene (D), and MPP (E; 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride). Larvae were pretreated with protective compound for 1 hr before co-exposure of protective compound and neomycin for 1 hr or co-exposure with gentamicin for 6 hr, and then stained with DASPEI to count hair cells. Each group is n >9 fish. Treating with protective compound had a significant effect (1-way ANOVA, p < 0.05) on neomycin toxicity for all groups and a significant effect (1-way ANOVA, p < 0.05) on gentamicin toxicity for raloxifene and MPP but no significant effect for tamoxifen, toremifene and afimoxifene.
4. Discussion
4.1 Multiple mechanisms of cell death with aminoglycosides and cisplatin
There are several possible mechanisms by which protective compounds could prevent toxin-induced cell death. The protective compound may interact directly with the target of the toxin and thereby interfere with the initiation of events that lead to cell death, or interact with molecular components downstream of the initiating event but prior to a commitment to cell death. Alternatively protective compounds may affect the ability of toxins to reach their cellular targets by blocking entry of the toxin into the cell or transit within the cell, or altering efflux of the toxin. An additional possibility is that protective compounds may directly interact with a toxin. The pretreatment requirement of methiothepin and phenoxybenzamine suggests that these compounds need time to reach an intracellular target rather than interacting directly with the toxins. This required time may reflect entry route of the protective compound or transit within the cell. While we cannot rule out that some protective drugs may interact directly with toxins, the observation that many protect against multiple aminoglycosides on different time scales and two protect additionally against cisplatin leads us to favor the hypothesis that at least some of these protective drugs act intracellularly.
The ability of the compounds to protect against different subsets of toxins may reflect differences in the mechanisms by which toxins kill hair cells. Differences between the aminoglycosides and their mechanisms of action are poorly understood. Selimoglu et al. (2003) suggested that kanamycin is less ototoxic than gentamicin; Smith et al. (1977) found no significant difference between amikacin and gentamicin ototoxicity; Dulon et al. (1986) found that clearance of amikacin, gentamicin and netilmicin from the cochlea did not correlate with reported differences in toxicity. Previously, we showed that at least two distinct series of cellular events contribute to gentamicin-induced hair cell death (Owens, et al., 2009). (Note that some initial events may not be intrinsic to the cell death process per se but rather be perturbations in the cell that trigger the cell death process). One pathway is rapid (~1hr) while the second pathway is slower (6–24 hr) and may disrupt slower processes in hair cells, such as transcription or translation. Neomycin induces a rapid series of events solely or predominantly, while gentamicin induces both pathways fairly equally and kanamycin induces predominantly the slower pathway(s). The sentinel mutation in the cc2d2a gene protects zebrafish lateral line hair cells from neomycin and short-term gentamicin exposure (~1hr) but not long-term (6hr) gentamicin exposure indicating that the rapid, short-term and slower, long-term events are molecularly distinct (Owens, et al., 2009). In addition, changes in extracellular calcium levels were found to alter sensitivity to neomycin and short-term gentamicin toxicity but not long-term gentamicin toxicity (Coffin, et al., 2009).
In the present study, we identified seven drugs that protect hair cells against neomycin and gentamicin, but only three that also protect hair cells from kanamycin suggesting that kanamycin toxicity may employ some different cell death pathways from neomycin and gentamicin. On the other hand, all of the compounds that provided protection from kanamycin also protected hair cells from gentamicin and neomycin, suggesting that at least some components of the cell death mechanisms are shared. This could reflect a differential affinity of the protective drugs or aminoglycosides for a common target. Alternatively we cannot rule out that protective drugs interact differentially with toxins, or that multiple shared pathways are triggered differentially by the toxins.
All compounds that protect against neomycin also protect against short-term gentamicin, further supporting the hypothesis that neomycin and gentamicin can trigger a common, rapidly-acting cell death pathway. We also noted that several protective compounds with similarities in their known pharmaceutical functions differ in their responses to short-term and long-term exposure to gentamicin. For instance, fluoxetine and paroxetine are both selective serotonin reuptake inhibitors (SSRIs), but only paroxetine protects from long-term gentamicin exposure. These findings support the idea that gentamicin kills hair cells by at least two distinct mechanisms.
Most of the aminoglycoside-protective compounds identified in this screen were not protective against cisplatin toxicity. Previously, we have found that the zebrafish mutant sentinel and the chemical compound PROTO-1 protect hair cells from neomycin but not cisplatin (Owens, et al., 2008). Studies in several animal systems have suggested that cisplatin acts in a cumulative manner and via a different mechanism than aminoglycosides (Bokemeyer, et al., 1998; Helson, et al., 1978; Ou, et al., 2007). The paucity of compounds that protect hair cells from both aminoglycosides and cisplatin is consistent with previous genetic and pharmacological data suggesting that these toxins kill hair cells by the different mechanisms (e.g., Owens, et al., 2008). Two compounds protect against cisplatin-induced hair cell death: paroxetine is also protective with neomycin and gentamicin, and benzamil is protective with all tested toxins. Benzamil, along with amiloride, is a member of the pyrazine carboxamide class of diuretics. Amiloride and its derivative, hexamethylene amiloride, protect zebrafish lateral line hair cells from aminoglycosides (Coffin, et al., 2009; Ou, et al., 2009) However, amiloride did not protect hair cells from cisplatin toxicity when tested across a wide range of amiloride concentrations. This suggests that benzamil, but not amiloride, may inhibit a target common to aminoglycoside- and cisplatin-induced pathways or may inhibit two distinct cell death-related events. Oxidative stress is proposed to be involved in both cisplatin and aminoglycoside-induced hair cell death (Rybak, et al., 2007; Wu, et al., 2000). Whether paroxetine and benzamil alter the response of hair cells to oxidative stress is unknown.
4.2 Hair cell protection: an off-target effect of pharmaceuticals
We hypothesized that screening a library of FDA-approved drugs would identify compounds that protect hair cells by some known mechanisms and therefore reveal new insights about how hair cell death occurs. Compounds in the same drug class protected hair cells from different combinations of toxins, suggesting that their known mechanisms of action do not explain the compounds’ protection of hair cells.
Raloxifene and tamoxifen, are both selective estrogen receptor modulators (SERMs) that each protect zebrafish lateral line hair cells from neomycin. Previous studies suggest that estrogen receptors might affect hair cell function. Estrogen receptors are expressed in hair cells of the inner ear of mice and rats (Stenberg, et al., 1999), birds (Noirot, et al., 2009), and in zebrafish lateral line hair cells (Tingaud-Sequeira, et al., 2004). Froehlicher et al. (2009) showed that reduction of estrogen receptor function interferes with hair cell development in zebrafish. In chick, Hawkins et al. (2007) observed up-regulation of estrogen receptor signaling in the inner ear following neomycin exposure. Nakamagoe et al. (2010) demonstrated that estradiol protects hair cells from gentamicin toxicity in rat cochlear explants. Meltser et al. (2008) found that DPN, an ER-β agonist, protects murine hair cells from acoustic trauma perhaps via BDNF. Thus, SERMs may protect hair cells from neomycin damage via their known mechanism of action.
Based on our findings, raloxifene and tamoxifen appear to diminish cell death by distinct, off-target mechanisms of action. Of the SERMs tested here, only those that are structurally similar to either raloxifene or tamoxifen showed any protective capabilities. In addition, their ability to protect hair cells from short-term and long-term gentamicin exposure correlated with structure (Table 2, Fig. 6). Tamoxifen, toremifene and afimoxifene share a common chemical core (Fig. 6A) and protect hair cells from short-term gentamicin exposure. Raloxifene and MPP are structurally similar and distinct from tamoxifen (Fig. 6B) and protect against both short-term and long-term gentamicin exposure. However the other SERMs tested had dissimilar structures from either tamoxifen or raloxifene (Fig. 6C) and showed no protection. These observations suggest that although several compounds in the same drug class protect hair cells, they are doing so through distinct and unknown structure-related mechanisms.
Figure 6.
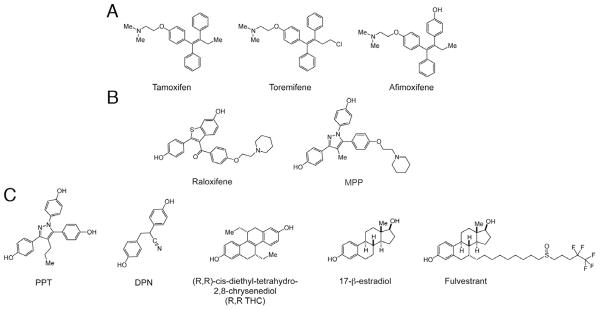
Chemical structures of estrogen receptor ligands tested for protective effects in this study. Those that protected against aminoglycosides had similar structural elements to either tamoxifen (toremifene, afimoxifene; A) or raloxifene (MPP; B) while the other compounds (PPT, DPN, R,R THC, 17-beta estradiol and fulvestrant; C) had noticeably different structures.
In addition to the SERMs, we found divergence in protective profile of two SSRIs, fluoxetine and paroxetine, and between two sodium-calcium exchangers, benzamil and amiloride (discussed in 4.1 and 4.3). Based on these differences it is unlikely that these compounds protect hair cells via effects on their defined pharmaceutical targets. Clinically, SSRIs are used to treat psychological disorders by blocking serotonin transport channels and maintaining serotonin in the synapse (Iversen, et al., 2006). Serotonin receptors and transporters are expressed in the zebrafish brain (Norton, et al., 2008; Wang, et al., 2006) but lateral line expression has not been examined. Airhart et al. (2007) showed that zebrafish exposed to fluoxetine exhibit movement disorders, but whether this effect is related to hair cell function in lateral line, vestibular or hearing organs or to other systemic effects is unknown. Fluoxetine can inhibit potassium currents in guinea pig hair cells, an effect not mediated by the serotonin reuptake channel, but rather by blocking the SK-type potassium channel (Bian, et al., 2002; Terstappen, et al., 2003). This suggests that an off-target effect, blockage of potassium channels, may afford hair cells protection from toxins. Paroxetine also has targets other than the serotonin transport channel; paroxetine was shown to interact with yeast proteins that metabolize RNA and with a P4-type ATPase, NEO-1, which confers neomycin resistance in yeast (Ericson, et al., 2008).
4.3 Mechanotransduction and toxin entry
Several studies have suggested that aminoglycosides enter hair cells via a mechanotransduction-dependent process (Gale, et al., 2001; Steyger, et al., 2003; Marcotti, et al., 2005; Santos, et al., 2006; Alharazneh, et al., 2011). Amiloride, hexamethylene amiloride, and benzamil (see 4.1) have been shown to block mechanotransduction in hair cells (Jorgensen and Ohmori, 1988; Tang, et al., 1988; Rusch, et al., 1994). Inhibiting mechanotransduction by breaking tip links protects hair cells from subsequent aminoglycoside toxicity (Assad, et al., 1991; Zhao, et al., 1996; Gale, et al., 2001). Mice and zebrafish with mutations that inactivate mechanotransduction are resistant to aminoglycosides (Richardson et al. 1997, 1999; Seiler and Nicolson 1999, Kros, et al., 2001). Amiloride and hexamethylene amiloride were shown to reduce uptake of gentamicin (Ou, et al., 2009; Coffin, et al., 2009). Although benzamil has been described as a mechanotransduction channel blocker, we observe that in the presence of benzamil, some gentamicin enters lateral line hair cells within three minutes of exposure. The route of gentamicin entry may alter access to molecular targets. Perhaps mechanotransduction-dependent entry of gentamicin results in hair cell death, while entry via other routes, such as apical endocytosis, does not.
It is important to note that the relationship that we observed between the ability of a compound to protect against long-term gentamicin and reduction of labeled gentamicin uptake is complex. Four of the compounds (benzamil, phenoxybenzamine, raloxifene and ractopamine) that protect hair cells against long-term gentamicin exposure significantly decreased GTTR uptake. Paroxetine and methiothepin that are protective against gentamicin (and other aminoglycosides) appeared to reduce GTTR uptake, but this uptake reduction was not statistically significant. Other compounds protective against gentamicin had similar levels of GTTR uptake as compared to GTTR only controls or showed an apparent (but not significant) increase in GTTR uptake. On the other hand, we only evaluated GTTR uptake with compounds that were first identified as inhibiting aminoglycoside toxicity of hair cells, and therefore we would not identify compounds that significantly block GTTR uptake but do not protect hair cells from aminoglycoside toxicity. Additionally our uptake assay is relatively insensitive. It is not clear whether a modest reduction in aminoglycoside uptake would explain a robust protection of hair cells over long time periods. Further studies that use a more discriminative assay and comparisons with labeled neomycin and kanamycin in addition to GTTR might shed further light on these issues.
Cisplatin has been shown to enter cells via the copper transporter CTR1 (Ishida, et al., 2002; Pabla & Dong, 2008) and via the organic cation transporter OCT2 (Ciarimboli, et al., 2010). It is not known how cisplatin entry relates to mechanotransduction or whether cisplatin might also enter hair cells in a mechanotransduction-dependent manner. The ability of benzamil to protect against cisplatin toxicity in lateral line hair cells may be due to benzamil’s ability to block mechanotransduction with a somewhat higher affinity than amiloride (Rusch, et al., 1994). Interestingly, neither of the two compounds that blocked GTTR uptake most effectively, ractopamine and raloxifene, were protective against cisplatin toxicity. As these protective compounds may block mechanotransduction-dependent uptake of GTTR, this might suggest that cisplatin does not rely on the same route to enter hair cells. It will be interesting to determine whether protective compounds reduce cisplatin toxicity by blocking its entry into cells and whether cisplatin uptake is a mechanotransduction-dependent process.
5. Conclusions
We believe that comprehensive screening of compounds that protect lateral line hair cells in zebrafish in vivo is a first step toward finding therapies to prevent hearing loss in humans. The protective compounds presented here are FDA-approved for use in humans and strongly protect zebrafish hair cells with multiple ototoxic drugs; to our knowledge they are the first compounds shown to have these properties. We hope that testing in mammals will reveal protective effects in the inner ear as well.
Supplementary Material
Highlights.
The zebrafish lateral line system is used as an in vivo drug-screening platform.
10 medicinal drugs protect hair cells from aminoglycoside or cisplatin damage.
Some protective compounds block gentamicin uptake into mechanosensory hair cells.
Protective compounds from the same drug class protect against different toxins.
Drugs may not act via their canonical targets in protecting hair cells.
Acknowledgments
We would like to thank David White and staff for maintenance of the zebrafish facilities, Eli Ocheltree for assistance with tissue culture experiments and Glen MacDonald for advice on microscopy. Funding for this work was provided by NIH/NIDCD grants DC05897 and DC04661.
Abbreviations
- dpf
days post-fertilization
- EM
embryo medium
- GTTR
gentamicin-Texas Red
- MIC
minimum inhibitory concentration
- MBC
minimum bactericidal concentration
- SERM
selective estrogen receptor modulator
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna L. Vlasits, Email: avlasits@berkeley.edu.
Julian A. Simon, Email: jsimon@fhcrc.edu.
David W. Raible, Email: draible@uw.edu.
Edwin W Rubel, Email: rubel@uw.edu.
Kelly N. Owens, Email: kowens@u.washington.edu.
References
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–664. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GMG, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Bian JT, Yeh JZ, Aistrup GL, Narahashi T, Moore EJ. Inhibition of K+ currents of outer hair cells in guinea pig cochlea by fluoxetine. Eur J Pharmacol. 2002;453:159–166. doi: 10.1016/s0014-2999(02)02421-4. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Berger C, Hartmann J, Kollmannsberger C, Schmoll H, Kuczyk M, Kanz L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–62. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett RE. Animal models of aminoglycoside antibiotic ototoxicity. Clin Infect Dis. 1983;5:S294–S303. [Google Scholar]
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnoff-Dinnesen A, Schinkel AH, Koepsel H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto-and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–80. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, Raible DW. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish. 2010;7:3–11. doi: 10.1089/zeb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Gorner P, Munz H. The mechanosensory lateral line: neurobiology and evolution. New York: Springer-Verlag; 1989. [Google Scholar]
- Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Aran JM, Zajic G, Schacht J. Comparative uptake of gentamicin, netilmicin, and amikacin in the guinea pig cochlea and vestibule. Antimicrob Agents Chemother. 1986;30:96–100. doi: 10.1128/aac.30.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson E, Gebbia M, Heisler LE, Wildenhain J, Tyers M, Giaever G, Nislow C. Off-target effects of psychoactive drugs revealed by genome-wide assays in yeast. PLoS Genet. 2008;4:e1000151. doi: 10.1371/journal.pgen.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Pettit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Fermin C, Park J, Cohen G. Pre-and post-natal ototoxicities of kanamycin and streptomycin in the chick. Paper presented at the Third Midwinter Meeting of ARO Abstracts 59.1980. [Google Scholar]
- Fleischman RW, Stadnicki SW, Ethier MF, Schaeppi U. Ototoxicity of cis-dichlorodiammine platinum (II) in the guinea pig. Toxicol Appl Pharmacol. 1975;33:320–332. doi: 10.1016/0041-008x(75)90098-8. [DOI] [PubMed] [Google Scholar]
- Froehlicher M, Liedtke A, Groh K, López-Schier H, Neuhauss SCF, Segner H, Eggen RI. Estrogen receptor subtype [beta] 2 is involved in neuromast development in zebrafish (danio rerio) larvae. Dev Biol. 2009;330:32–43. doi: 10.1016/j.ydbio.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Gale J, Marcotti W, Kennedy H, Kros C, Richardson G. FM1–43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. The J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Giari L, Dezfuli BS, Astolfi L, Martini A. Ultrastructural effects of cisplatin on the inner ear and lateral line system of zebrafish (danio rerio) larvae. J Appl Toxicol. 2011;32:293–299. doi: 10.1002/jat.1691. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One. 2007:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helson L, Okonkwo E, Anton L, Cvitkovic E. Cis-platinum ototoxicity. Clin Toxicol. 1978;13:469–478. doi: 10.3109/15563657808988252. [DOI] [PubMed] [Google Scholar]
- Hinshaw HC, Feldman WH, Pfuetze KH. Streptomycin in treatment of clinical tuberculosis. Am Rev Tuberc. 1946;54:191–203. doi: 10.1164/art.1946.54.3.191. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Simon JA, Ou HC. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J Assoc Res Otolaryngol. 2011;12:719–728. doi: 10.1007/s10162-011-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147:S82–S88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen F, Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol. 1988;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaus S. The influence of calcium on the ototoxicity of aminoglycosides. Acta Otolaryngol. 1992;112:83–87. doi: 10.3109/00016489209100787. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Kros C, Marcotti W, Van Netten S, Self T, Libby R, Brown S, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2001;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64:166–174. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–70. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- Nakamagoe M, Tabuchi K, Uemaetomari I, Nishimura B, Hara A. Estradiol protects the cochlea against gentamicin ototoxicity through inhibition of the JNK pathway. Hear Res. 2010;261:67–74. doi: 10.1016/j.heares.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252:49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Nicolson T, Rusch A, Friedrich RW, Granato M, Ruppersberg JP, Nusslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanotransduction: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- Norton WHJ, Folchert A, Bally-Cuif L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Comp Neurol. 2008;511:521–542. doi: 10.1002/cne.21831. [DOI] [PubMed] [Google Scholar]
- Ou HC, Cunningham LL, Francis SP, Brandon CS, Simon JA, Raible DW, Rubel EW. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (danio rerio) lateral line and mouse (mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10:191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (danio rerio) lateral line. Hear Res. 2007;233:46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Santos F, Raible DW, Simon JA, Rubel EW. Drug screening for hearing loss: Using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today. 2010;15:265–271. doi: 10.1016/j.drudis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KOC, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253:32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, Macdonald G, Rubel EW, Raible DW, Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502:522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH. Ototoxicity in patients receiving cisplatin: Importance of dose and method of drug administration. Cancer Treat Rep. 1982;66:19–23. [PubMed] [Google Scholar]
- Richardson G, Forge A, Kros C, Fleming J, Brown S, Steel K. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J Neurosci. 1997;17:9506–19. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Marcotti W, Becker D, Williams DS, Thorpe J, Fleming J, Brown SD, Steel KP. A missense mutation in myosin VIIA prevents aminoglycoside accumulation in early postnatal cochlear hair cells. Ann N Y Acad Sci. 1999;884:110–124. [PubMed] [Google Scholar]
- Rosenberg B. Charles F. Kettring prize. Fundamental studies with cisplatin. Cancer. 1985;55:2303–2316. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Robbins C, Owens K, Raible D, Simon J. Assoc Res Otolaryngol. Vol. 266. Baltimore, Maryland: 2011. PROTO1 provides robust protection against kanamycin-induced hearing loss in rats; p. Abstracts 34. [Google Scholar]
- Rusch A, Kros C, Richardson G. Block by amiloride and its derivatives of mechano-electrical transduction in outer hair cells of mouse cochlear cultures. J Physiol. 1994;474:75–86. doi: 10.1113/jphysiol.1994.sp020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- Selimoglu E, Kalkandelen S, Erdogan F. Comparative vestibulotoxicity of different aminoglycosides in the guinea pigs. Yonsei Med J. 2003;44:517–522. doi: 10.3349/ymj.2003.44.3.517. [DOI] [PubMed] [Google Scholar]
- Smith CR, Baughman KL, Edwards CQ, Rogers JF, Lietman PS. Controlled comparison of amikacin and gentamicin. N Engl J Med. 1977;296:349–353. doi: 10.1056/NEJM197702172960701. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors [alpha] and [beta] in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Steyger P, Peters S, Rehling J, Hordichok A, Dai C. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol. 2003;4:565–578. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Terstappen GC, Pellacani A, Aldegheri L, Graziani F, Carignani C, Pula G, Virginio C. The antidepressant fluoxetine blocks the human small conductance calcium-activated potassium channels SK1, SK2 and SK3. Neurosci Lett. 2003;346:85–88. doi: 10.1016/s0304-3940(03)00574-3. [DOI] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Andre M, Forgue J, Barthe C, Babin PJ. Expression patterns of three estrogen receptor genes during zebrafish (Danio rerio) development: Evidence for high expression in neuromasts. Gene Expr Patterns. 2004;4:561–568. doi: 10.1016/j.modgep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wanamaker HH, Slepecky NB, Cefaratti LK, Ogata Y. Comparison of vestibular and cochlear ototoxicity from transtympanic streptomycin administration. Am J Otol. 1999;20:457–464. [PubMed] [Google Scholar]
- Wang Y, Takai R, Yoshioka H, Shirabe K. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J Exp Med. 2006;208:267–274. doi: 10.1620/tjem.208.267. [DOI] [PubMed] [Google Scholar]
- Wayne P. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute; 2008. p. 100. [Google Scholar]
- Wikler MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard Clinical and Laboratory Standards Institute. 2006. [Google Scholar]
- Williams J, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurotol. 2000;7:171–174. doi: 10.1159/000058305. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA. 1996;93:15469–74. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.