Summary
The M-protein is the major reference measure for response in multiple myeloma (MM) and its correct interpretation is key to clinical management. The emergence of oligoclonal banding is recognized as a benign finding in the post-autologous stem cell transplantation setting (ASCT) for MM but its significance during non-myeloablative therapy is unknown. In a study of the immunomodulatory combination BiRD, (lenalidomide and dexamethasone with clarithromycin), we frequently detected the emergence of mono- and oligo-clonal immunoglobulins unrelated to the baseline diagnostic M-protein. The new M-proteins seen on serum immunofixation electrophoresis were clearly different in either heavy or light chain component(s) from the original M-spike protein and were called atypical serum immunofixation patterns (ASIPs). Overall, 24/72 (33%) patients treated with BiRD developed ASIPs. Patients who developed ASIPs compared with patients treated with BiRD without ASIPs, had a significantly greater overall response (100% vs. 85%) and complete response rates (71% vs. 23%). ASIPs were not associated with new clonal plasma cells or other lymphoproliferative processes, and molecular remissions were documented. This is the first time this phenomenon has been seen with regularity in non-myeloablative therapy for MM. Analogous to the ASCT experience, ASIPs do not signal incipient disease progression, but rather herald robust response.
Keywords: lenalidomide, multiple myeloma, atypical serum immunofixation patterns, M-protein
Introduction
Multiple myeloma (MM) is a neoplasm characterized by malignant plasma cells that produce excessive quantities of a single monoclonal immunoglobulin (Ig), M-protein, also called paraprotein (Munshi et al., 2001). M-protein (either an intact antibody with both heavy- and light-chain components, or light chains alone) is a highly specific tumor marker used in the diagnosis, prognosis, and treatment of MM and other plasma cell dyscrasias (Rajkumar et al., 2006; Mead et al., 2004). Relative reduction or increase in monoclonal Ig levels, as detected on serum protein electrophoresis and immunofixation, are the backbone of the International Uniform Response Criteria (IURC) designed to guide the management of patients with MM (Durie et al., 2006). An important issue complicating the management of MM, however, is interpretation of unrelated Ig bands which appear during MM therapy.
The phenomena of transient abnormal protein banding and oligoclonal M-spike appearance on serum immunofixation studies has been anecdotally noted previously, and was formally reported following bone marrow recovery after high-dose therapy for MM and for solid organ transplantation (Mitus et al., 1989; Hovenga et al., 2000; Zent et al., 1998; Myara et al., 1991; Touchard et al., 1997; Deteix et al., 1985). The abnormal or oligoclonal banding was originally attributed to either immune reconstitution (in the case of stem cell rescue after high-dose therapy) or immune system dysregulation during highly immunosuppressive therapy following solid organ transplantation (Gerritsen et al., 1994; Fumoux et al., 1993). In MM, those patients with abnormal protein banding following autologous transplantation were found to have a greater tumor reduction, higher rate of complete response (CR), and longer overall survival (Hovenga et al., 2000; Zent et al., 1998). Acknowledging this finding, the MM response criteria outlined by Bladé et al., (1998) allows for the presence of oligoclonal banding consistent with oligoclonal immune reconstitution in the definition of CR (Bladé et al., 1998). Likewise, the recently updated criteria for disease response outlined by the myeloma working group in 2006 strictly defines a threshold of a rise in at least 0·5 g/dl of M-protein as progressive disease (Durie et al., 2006). However, the same committee defines relapse from CR as any reappearance of immunofixation positivity on two or more consecutive measurements, which would apply to many of the post-transplantation patients with abnormal protein banding (Durie et al., 2006).
Here we report the appearance of emergent M-spikes, which we call atypical serum immunofixation patterns (ASIPs), occurring outside the setting of myeloablative therapy or organ transplantation in patients with MM treated with the lenalidomide and dexamethasone with clarithromycin combination (BiRD) (Niesvizky et al., 2007). ASIPs were defined as having one or more Igs present on serum immunofixation with either a clearly different heavy- or light-chain component from the original M-protein. We characterize the baseline diagnostic monoclonal Ig, the new mono- and oligo-clonal Ig patterns, the clinical context of their appearance, and the associated response to induction therapy as compared with those patients who did not develop ASIPs undergoing the same treatment. We also performed serial bone marrow histological and molecular analysis to evaluate new clonal plasma or other B-cell populations, in order to explain the phenomena and reconcile our findings with the autologous stem cell transplantation experience, and the IURC for MM.
Materials and methods
A total of 72 adult patients with symptomatic Durie-Salmon stage II or III MM participated in a prospective clinical trial of lenalidomide in combination with dexamethasone and clarithromycin, as reported previously (Niesvizky et al., 2007). In this protocol, patients were treated with: lenalidomide 25 mg daily for 21 days out of a 28-day cycle; dexamethasone 40 mg once weekly; and clarithromycin 500 mg twice daily on every day of the cycle. The serum protein electrophoretic pattern, serum immunofixation, and serum free light chain analysis after each 28-day cycle of BiRD were serially reviewed for each patient. Bone marrow examination, karyotype analysis, and fluorescence in situ hybridization (FISH) testing were performed centrally at New York Presbyterian Hospital laboratories before study enrollment and to confirm CR, when appropriate, as described previously (Niesvizky et al., 2007).
ASIP definitions
The serum immunofixation gels were compared in order to establish qualitative and electrophoretic mobility distinctions in a blinded fashion. We defined an ASIP as the appearance of new mono- or oligo-clonal Ig protein bands, which had either light chain or heavy chain component(s) different from the baseline diagnostic M-protein. Oligoclonal banding was defined as the development of two or more concurrent monoclonal-type bands on the serum electrophoretic pattern, with either a different heavy or light chain component from the original M-protein band.
Molecular clonal analysis by polymerase chain reaction
Methanol-fixed MM cells were lysed and DNA was extracted using Qiamp® DNA mini kit (Qiagen, Valencia, CA, USA). For IgH clonality analysis, a semi-nested polymerase chain reaction (PCR) method was used to amplify the sequences between the framework 3 of the V region and J segments. (See Supplementary methods section for full description of IgH nested PCR primers and conditions.) Multiplex IgK clonality analyses were conducted with the BIOMED-2 assay (InVivoScribe Technologies, San Diego, CA, USA) using the enclosed protocol, standard reagents, and conditions. All specimens were run in duplicate.
Study end points
The time to appearance of a unique ASIP was measured as the time elapsed from Day 1 of BiRD therapy until the time of that first ASIP appearance on serum immunofixation. The duration of a unqiue ASIP was measured as the elapsed time in months from that particular ASIP appearance to that the same ASIP had disappeared The appearance of different ASIPs were recorded and treated as separate events and therefore the same patient could have multiple ASIPs with different time to first appearance and/or different durations. ASIP time to appearance and duration werecorrelated with the quality of response, the time to first and maximum response, the time to progression, and overall survival. MM disease response criteria were adopted from the new IURC defined by Durie et al., (2006).
Statistical analysis
The primary efficacy analyses were performed according to the intent-to-treat principle, which included all enrolled subjects. Fisher’s exact test and Student’s t-test (or Wilcoxon rank-sum test) were used for associating categories of response with potential risk factors such as: development of ASIPs; no ASIP development; chromosomal abnormalities; International Staging System stage; the presence of lactate dehydrogenase; and prior monoclonal gammopathy of undetermined significance. Spearman’s rank correlation coefficient was determined to relate the duration of BiRD therapy at the time of the first ASIP appearance with treatment response in patients. All P-values are 2-sided with statistical significance evaluated at the 0·05 alpha level. All analyses were performed in MedCalc version 9·3·6·0 (MedCalc Software, Mariakerke, Belgium).
Results
Ig patterns in patients with MM treated with BiRD
The clinical characteristics of the 72 BiRD study patients as well as the subgroup of patients that developed ASIPs are shown in Table I. Overall, 24 patients (33%) developed ASIPs during the course of therapy. An example ASIP for a patient in the study is shown in Fig 1A, which illustrates the change in the immunoelectrophoretic pattern over time.
Table I.
Patient characteristics at initiation of lenalidomide therapy (N = 72)
| Characteristic | BiRD Patients (n=72) | Patients with ASIPs (n=24) |
|---|---|---|
| Median age, years (range) | 63 (36–83) | 69 (45–83) P=0.07 |
| Sex, female, n (%) | 31 (43·1) | 7 (29.1) |
| Median beta-2 microglobulin, mg/dl (range) | 3·0 (1·1–17·6) | 3·3 (1·1–12·8) P=0.66 |
| Median albumin, g/dl (range) | 3·6 (2·3–4·9) | 3·1 (2·3–3·8) P=0.88 |
| Median hemoglobin, g/dl (range) | 10·9 (7·5–15·0) | 10·9 (7·8–15·0) P=0.69 |
| Durie-Salmon stage, n (%) | ||
| IIA | 32 (44·4) | 9 (37·5) |
| IIB | 1 (1·4) | 0 |
| IIIA | 36 (50·0) | 14 (58·3) |
| IIIB | 3 (4·2) | 1 (4·2) |
| International Staging System stage, n (%) | ||
| I | 33 (45·8) | 10 (41·2) |
| II | 26 (36·1) | 9 (37·5) |
| III | 13 (18·1) | 5 (20·8) |
| Monoclonal protein, n (%) | ||
| IgG-κ | 29 (40·3) | 8 (33·3) |
| IgG-λ | 15 (20·8) | 6 (25·0) |
| IgA-κ | 11 (15·3) | 4 (16·7) |
| IgA-λ | 5 (6·9) | 1 (4·2) |
| Free κ | 9 (12·5) | 5 (20.8) |
| Free λ | 3 (4·2) | 0 |
Fig 1.
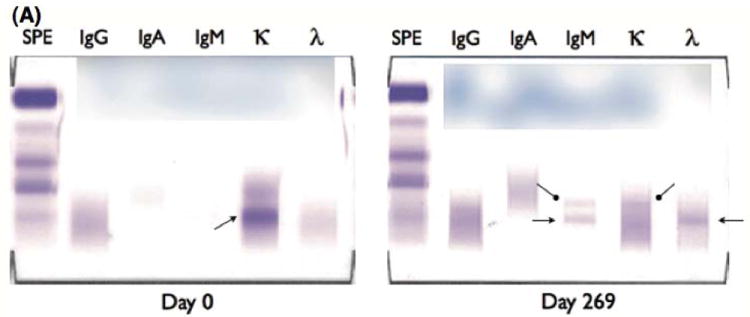
(A) Atypical serum immunofixation patterns (ASIPs). Depicted are two serum immunofixation electrophoresis (S-IFE) gels done at different time points for a patient. The patient was diagnosed with a free light chain κ monoclonal protein at the initiation of therapy as shown on the Day 0 S-IFE gel (left panel) above. During clarithromycin, lenalidomide, and dexamethasone (BiRD) therapy, the free light chain κ level as well as the initial hypogammaglobulinemia normalized. The patient developed an ASIP by Day 269 (right panel) of BiRD therapy, showing new monoclonal IgM-κ (rounded arrows), and monoclonal IgM-λ (pointed arrows) protein. A repeated serum immunofixation study performed at Day 271 confirmed the continued presence of the ASIP witnessed above (data not shown).
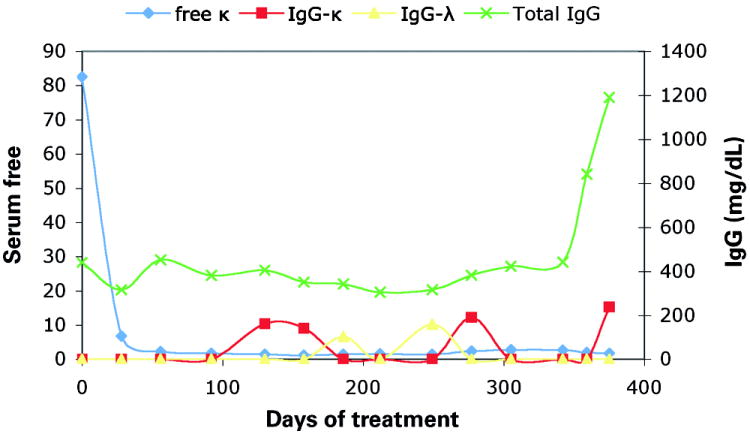
(B) ASIP time course. This figure is an illustrative example of the changes seen in serial immunofixation studies over time witnessed for a patient with free light chain κ multiple myeloma, treated with BiRD therapy. There is a dramatic initial response with a drop in the level of serum free κ light chain to normal level within 50 days. Over time, relatively small IgG-κ and IgG-λ monoclonal bands are observed to appear and then disappear. Throughout the patient’s course of treatment, until nearly Day 350, there is a state of hypogammaglobulinemia, as manifested by a low total IgG level.
The baseline monoclonal Ig, the altered protein banding pattern witnessed, the duration of treatment until the time of the first appearance of an ASIP, and the treatment response for each patient with one or more ASIPs was recorded. (See Supplementary table for a case-by-case compilation of all observed ASIPs.) The ASIPs that were seen were diverse in their type, number, and duration of monoclonal Ig(s) detected. The median duration of ASIP appearance was relatively prolonged at 236 days, yet varied widely per individual patient, with a range from as short as 27 days to as long as 758 days. A total of 7 patients (29%) exhibited ASIPs with monoclonal Ig bands that frequently disappeared and reappeared periodically throughout the course of treatment, indicating that the protein level fluctuated near the lower limits of detection by immunofixation. An example ASIP time course is shown in Fig 1B which shows alternating M-proteins appearing and disappearing over time in a reciprocal fashion; similar patterns was also seen in other patients with an ASIP during the course of treatment. The appearance of oligoclonal banding over time was also a common phenomenon, occurring in 10 patients (42%). ASIPs tended to appear after a prolonged course of therapy, with the median first appearance after 6 months of treatment. At the last follow-up, 10 patients (42%) still had evidence of an ASIP. The isotype distribution of original and newly detected monoclonal Ig for patients who went on to develop ASIPs is depicted in Fig 2A,B. The new Ig distribution was roughly equivalent to the original study population with a preponderance of IgG-κ. However, the development of a new IgM band as part of the ASIPs was noted, with 12 patients (50%) developing either IgM-κ, IgM-λ, or both, on repeat serum electrophoretic testing. In summary, ASIPs were commonly seen after prolonged courses of BiRD therapy, they persisted and fluctuated in level for years, and were protean in their Ig makeup.
Fig 2.
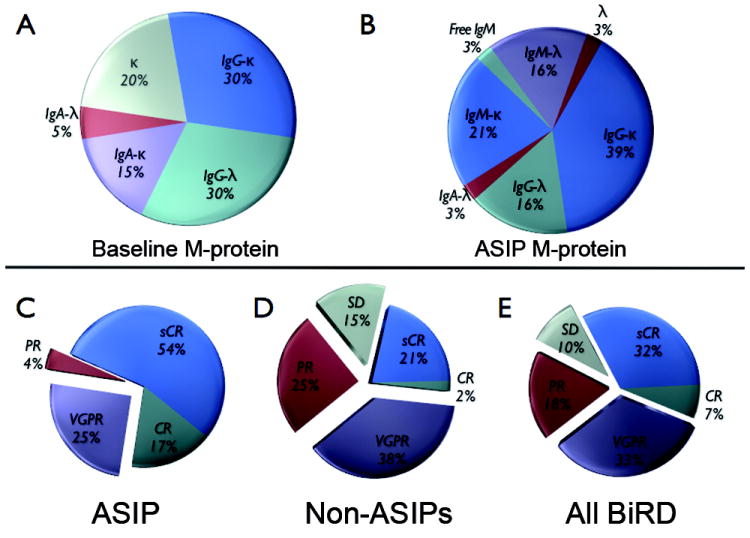
Analysis of monoclonal protein production and response for patients with atypical serum immunofixation patterns (ASIPs). The distribution of M-protein for patients at the initiation of lenalidomide and dexamethasone with clarithromycin (BiRD) therapy (A), and the relative distribution of M-proteins as witnessed on ASIPs (B) are shown. The maximum response rates achieved with BiRD therapy are compared in patients who developed ASIPs (C), those who did not develop ASIPs (D), and to the entire cohort of BiRD-treated patients on study (E). CR, complete response; PR, partial response; sCR, stringent CR; SD, stable disease; and VGPR, very good PR.
Correlation of ASIPs with clinical response rate
Patients who developed ASIPs had a significantly better response to BiRD versus non-ASIP patients (P = 0·0001), (Figure 2). Every patient with an ASIP achieved at least a partial response (PR) compared with an 85% response rate in the non-ASIP group. Further analysis showed that the very good PR or better rate (96% vs. 60%; P = 0·0017) and CR rate (71% vs. 23%; P = 0·0002) were also greater for the ASIP versus non-ASIP patients, respectively. Many patients with an ASIP continued to have tumor-burden reduction with further cycles of BiRD treatment even after the ASIP first appeared. The extent of BiRD therapy prior to development of first ASIP did not correlate with response rate (P = 0·50). To confirm the response to treatment, bone marrow aspirates were repeatedly tested for residual disease by karyotype and FISH analysis when possible. Overall, 18 pre- and post-treatment samples were evaluable out of the 24 patients with an ASIP (Table III – should be in supplement). In all cases, there was resolution of prior karyotypic abnormalities, with the exception of a persistent pericentric inversion of chromosome 9 in a patient. All FISH detected abnormalities at the beginning of treatment had resolved in patients with an ASIP, except for a patient who had continued hyperdiploidy of chromosome 11. Deletion of chromosome 13 [del(13q)] and t(4;14) were present in 39% and 5%, respectively, of the ASIP patients. These FISH detected abnormalities did not significantly impact the clinical response that was achieved (P = 0·5612), with 6 out of 8 of the tested patients with either del(13q) or t(4;14) achieving a CR or better. Event-free survival (EFS) and progression-free survival (PFS) differences between the ASIP and non-ASIP groups did not achieve significance due to the low number of events overall (data not shown). Furthermore, univariate statistical analysis failed to show a relationship between the development of ASIPs and other known MM prognostic factors, including β-2 microglobulin, ISS, Durie Salmon stage, albumin, LDH, CRP, and age (Table 1, additional data not shown). Thus, ASIPs are associated with marked disease response rates, seemingly independent of initial chromosomal abnormalities as well as other known MM prognostic factors, and patients who develop ASIPs often continue to have tumor reduction with ongoing BiRD therapy.
Table III.
Cytogenetic analyses of patients with atypical serum immunofixation pattern development pre- and post-treatment
| Patient | Pre-treatment karyotype | Post-treatment karyotype | Pre-treatment FISH | Post-treatment FISH | Maximum response |
|---|---|---|---|---|---|
| 1 | N/A | N | N | N | VGPR |
| 3 | N | N | 13q14del; trisomy and tetrasomy 11 | N | CR |
| 4 | N | N | Tetrasomy 11; 13q14del | N | sCR |
| 5 | N | N | N | N | sCR |
| 6 | N | N | N | N/A | CR |
| 9 | N | N | 13q14del | N | CR |
| 10 | N | N | VGPR | ||
| 11 | N | N | 13q14del; trisomy 11 | N | sCR |
| 12 | inv(9)* | inv(9)* | N | N | CR |
| 13 | N | N | t(4;14) | N | sCR |
| 14 | N | N | 13q14del | N | sCR |
| 15 | N | N | N | N | sCR |
| 16 | N | N | 13q14del; trisomy 4; trisomy 11 | N | PR |
| 17 | Loss of Y | N | Trisomy and tetrasomy 11 | Tetrasomy 11 | sCR |
| 18 | add(1)(p13); del(3) t(1;3)(p32;p21), +9,+11, −13, +15 and +21; trisomy 7, 9, 11, 15 | N | Trisomy 11 | N | sCR |
| 20 | N | N | Trisomy 11 | N | sCR |
| 21 | N | N/A | 13q14del, t(11;14) | N | VGPR |
| 22 | N | N | Trisomy 11 | N | sCR |
The inv(9) chromosomal rearrangement is commonly found in the general population and is not known to have any phenotypic association.
CR, complete response; FISH, fluorescence in situ hybridization; N, normal; N/A, sample insufficient to run test or sample unavailable; PR, partial response; sCR, stringent CR; VGPR, very good PR.
Bone marrow and molecular analysis
All bone marrow samples that we analyzed to confirm CR and stringent CR had, by definition, resolution of the original plasmacytosis, and no repeat bone marrow biopsy at the time of ASIP had histologic or flow cytometry evidence for lymphocytosis or clonal lymphocyte expansion. All prior cytogenetic abnormalities (Table III – move to supplement please) were not detected on repeat karyotype and FISH analysis after ASIP detection.
To further assess for the presence of new plasma cell clones and the presence of minimal residual disease, which could produce the witnessed M-protein bands, Ig gene rearrangement analysis of 7 available patient samples pre- and post-development of ASIPs was performed by both Ig heavy chain and light chain multiplex PCR. None of the tested sample pairs showed the emergence of a new clonal B-cell gene rearrangement to account for the presence of the emergent Ig proteins. (See Supplementary figure for PCR analysis results.) Interestingly, in 4 out of 7 patients (57%) we witnessed the loss of the original plasma cell clone signal, showing that the original tumor burden had been reduced beyond the lower limits of molecular detection using the above method. Thus, we failed to find evidence for either a new clonal plasma cell or B-cell population in the bone marrow to account for ASIP production.
Discussion
Transient low-level abnormal or oligoclonal Ig banding is frequently seen after high-dose myeloablative therapy for MM, and has been associated with good prognostic outcomes (Myara et al., 1991). By convention, these bands are often ignored and do not signify disease relapse by the European Group for Blood and Marrow Transplantation criteria (Bladé et al., 1998). We now extend this observation to the non-myeloablative setting and report on the frequent development of ASIPs in a cohort of patients with MM receiving the novel, highly effective combination therapy BiRD. Overall, 33% of patients treated with BiRD developed ASIPs on serial serum immunofixation testing. The protein banding patterns seen in ASIPs varied widely in Ig isotype and duration of appearance. We also found that ASIPs had a good prognostic significance, with 76% of patients going on to achieve a CR after the emergence of an ASIP. FISH and karyotypic analyses confirmed these CRs with elimination of chromosomal abnormalities, including those associated with poor disease outcome, such as del(13) and t(4;14). Furthermore, PCR studies revealed molecular CR in 4 out of 7 tested patients. The discovery of what appears to be loss of the original plasma cell clone on PCR study has been shown in prior studies to correlate with increased survival (Corradini et al., 1999; Corradini et al., 2003; Martinelli et al., 2000). Although we have not achieved a statistically significant difference in median EFS and PFS at this time due to lack of overall events, the depth of remission patients with ASIPs achieved, is encouraging for durable response.
Abnormal protein banding after high-dose therapy in MM is posited to result from immune reconstitution after high-dose chemotherapy. However, as we report this observation following a non-myeloblative regimen, we searched for other potential sources for the ASIPs. These protein bands could potentially arise as a result of de-differentiation from the original MM cell clone, or may identify the emergence of new unrelated neoplastic clones. We, however, found no evidence of emergent neoplastic clonal populations of B-cells or plasma cells in the bone marrow or the peripheral blood of patients with ASIPs through karyotype, FISH, and selected PCR analysis. Prior studies of transient oligoclonal banding in the MM post-transplantation setting have shown that the protein bands occurred more frequently in patients who achieved a CR after high-dose chemotherapy (Hovenga et al., 2000; Zent et al., 1998). The fact that ASIPs were witnessed in patients who had a robust response to therapy suggests that major malignant plasma cell cytoreduction is a contributory aspect to ASIP development. In a prior phase 2 trial conducted at our institution using the combination of clarithromycin, thalidomide, and dexamethasone, there were no such instances of ASIPs as reported here, and the response rates were not as profound (Coleman et al., 2002). This illustrates that prior to the advent of new highly effective myeloma regimens, CRs were achieved in only a small percentage of patients and perhaps there was no opportunity for ASIP development.
Recent reports demonstrate that in normal bone marrow, there is a plasma cell compartment that has a capped, or finite, population of cells. In order for new, normal, plasma cells to occupy this bone marrow niche after antigenic stimulation, older plasma cells must be replaced (Odendahl et al., 2005; Radbruch et al., 2006). It may be possible that BiRD therapy (or high-dose chemotherapy) clears the bone marrow of malignant plasma cells in such a rapid and complete manner that an environment is created that allows benign plasma cells to expand within the predefined niche without competition. Certainly, the wide spectrum and variability of the ASIP protein isotypes, the recent confirmation that normal plasma cells express the inhibitory FcγRIIb receptor which suppresses normal polyclonal Ig expression, and the finding that administration of tetanus toxoid vaccine after stem cell transplantation induces oligoclonal banding patterns, all support the theory of rapid oligoclonal plasma cell expansion after the clearance MM cells as a potential cause for ASIP generation (Gerritsen et al., 1994; Xiang et al., 2007).
We report that ASIPs do not necessarily solely appear after high-dose therapy for MM, but may appear in highly effective non-myeloablative therapy as well. To the best of our knowledge, this is also the first report of the achievement of molecular remission during the course of IMiD therapy. We propose that the abnormal protein banding seen may stem from the massive plasma cytoreduction that can be achieved either by the high-dose therapy or highly effective non-myeloablative novel combination therapy. Regardless of the means used to rid the marrow of malignant plasma cells, ASIPs may develop as benign plasma cell reconstitution takes place. Similar to the high-dose therapy experience, ASIP emergence during immunomodulatory-based therapy for MM has a good prognostic significance. Alternatively, ASIPs may represent an unexplained effect of the immunomodulatory therapy itself that may somehow mimic the immune reconstitution state seen after high-dose therapy for MM. If either theory is correct, we should expect the issue of ASIP interpretation to become more frequent with the increasing popularity of more effective induction regimens for MM using immunomodulatory therapy.
Supplementary Material
Table II.
Summary of novel protein band appearance characteristics (N = 24)
| Novel protein band isotype | n (%frequency) | Median duration, days (range) |
|---|---|---|
| IgM-κ | 9 (20·0) | 46·5 (26–315) |
| IgM-λ | 7 (15·6) | 29·7 (22–315) |
| IgG-κ | 18 (40·0) | 76 (27–581) |
| IgG-λ | 8 (17·8) | 54 (23–581) |
| IgA-κ | 0 | |
| IgA-λ | 1 (2·2) | 28 |
| Free κ | 0 | |
| Free λ | 1 (2·2) | 26 |
| Free heavy chain (IgM) | 1 (2·2) | 315 |
|
| ||
|
Duration of treatment at time of first ASIP appearance, days
| ||
| Median (range) | 178 (27–724) | |
|
| ||
|
Duration of ASIP appearance, days
| ||
| Median (range) | 236 (27–758) | |
ASIP, atypical serum immunofixation pattern.
Acknowledgments
Conflicts of interest
This work was supported in part by The Leukemia & Lymphoma Society SCOR grant and an NCI K23 Award: CA109260-01. The authors would like to thank The Hermione Foundation. Funding support for this study was also provided by the Celgene Corporation, Summit, NJ, USA.
References
- Bladé J, Samson D, Reece D, Apperley J, Björkstrand D, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. British Journal of Haematology. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Coleman M, Leonard J, Lyons L, Pekle K, Nahum K, Pearse R, Niesvizky R, Michaeli J. BLT-D (clarithromycin [Biaxin], low-dose thalidomide, and dexamethasone) for the treatment of myeloma and Waldenstrom’s macroglobulinemia. Leukemia & Lymphoma. 2002;43:1777–1782. doi: 10.1080/1042819021000006303. [DOI] [PubMed] [Google Scholar]
- Corradini P, Cavo M, Lokhorst H, Martinelli G, Terragna C, Majolino I, Valagussa P, Boccadoro M, Samson D, Bacigalupo A, Russell N, Montefusco V, Voena C, Gahrton G Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- Corradini P, Voena C, Tarella C, Astolfi M, Ladetto M, Palumbo A, Van Lint MT, Bacigalupo A, Santoro A, Musso M, Majolino I, Boccadoro M, Pileri A. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. Journal of Clinical Oncology. 1999;17:208–215. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- Deteix P, Chapuis-Cellier C, Ghais Z. Systemic survey of immunoglobulin abnormalities: frequency and evolution in organ transplant recipients. Transplantation Proceedings. 1985;17:2651–2654. [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Fumoux F, Guigou V, Blaise D, Maraninchi D, Fougereau M, Schiff C. Reconstitution of human immunoglobulin VH repertoire after bone marrow transplantation mimics B-cell ontogeny. Blood. 1993;81:3153–3157. [PubMed] [Google Scholar]
- Gerritsen EJ, Van Tol MJ, Van’t Veer MB, Wels JM, Khouw IM, Touw CR, Jol-Van Der Zijde CM, Hermans J, Rumke HC, Radl J, Vossen JM. Clonal dysregulation of the antibody response to tetanus-toxoid after bone marrow transplantation. Blood. 1994;84:4374–4382. [PubMed] [Google Scholar]
- Hovenga S, de Wolf JT, Guikema JE, Klip H, Smit JW, Smit Sibinga CT, Bos NA, Vellenga E. Autologous stem cell transplantation in multiple myeloma after VAD and EDAP courses: a high incidence of oligoclonal serum Igs post transplantation. Bone Marrow Transplantation. 2000;25:723–728. doi: 10.1038/sj.bmt.1702194. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Dispenzieri A, Kyle RA. Monoclonal gammopathy of undetermined significance, Waldenstrom macroglobulinemia, AL amyloidosis, and related plasma cell disorders: diagnosis and treatment. Mayo Clinic Proceedings. 2006;81:693–703. doi: 10.4065/81.5.693. [DOI] [PubMed] [Google Scholar]
- Martinelli G, Terragna C, Zamagni E, Ronconi S, Tosi P, Lemoli RM, Bandini G, Motta MR, Testoni N, Amabile M, Ottaviani E, Vianelli N, de Vivo A, Gozzetti A, Tura S, Cavo M. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. Journal of Clinical Oncology. 2000;18:2273–2281. doi: 10.1200/JCO.2000.18.11.2273. [DOI] [PubMed] [Google Scholar]
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. British Journal of Haematology. 2004;126:348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- Mitus AJ, Stein R, Rappeport JM, Antin JH, Weinstein HJ, Alper CA, Smith BR. Monoclonal and oligoclonal gammopathy after bone marrow transplantation. Blood. 1989;74:2764–2768. [PubMed] [Google Scholar]
- Munshi N, Tricot G, Barlogie B. Plasma cell neoplasms. In: DeVita V Jr, Hellman S, Rosenberg S, editors. Principles and Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2465–2499. [Google Scholar]
- Myara I, Quenum G, Storogenko M, Tenenhaus D, Guillemain R, Moatti N. Monoclonal and oligoclonal gammopathies in heart-transplant recipients. Clinical Chemistry. 1991;37:1334–1337. [PubMed] [Google Scholar]
- Niesvizky R, Jayabalan DS, Christos PJ, Furst JR, Naib T, Ely S, Jalbrzikowski J, Pearse RN, Zafar F, Pekle K, Larow A, Lent R, Mark T, Cho HJ, Shore T, Tepler J, Harpel J, Schuster MW, Mathew S, Leonard JP, Mazumdar M, Chen-Kiang S, Coleman M. BiRD (Biaxin® [clarithromycin]/Revlimid® [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dörner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature Reviews Immunology. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Touchard G, Pasdeloup T, Parpeix J, Hauet T, Bauwens M, Dumont G, Aucouturier P, Preud’homme JL. High prevalence and usual persistence of serum monoclonal immunoglobulins evidenced by sensitive methods in renal transplant recipients. Nephrology, Dialysis, Transplantation. 1997;12:1199–1203. doi: 10.1093/ndt/12.6.1199. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nature Immunology. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- Zent CS, Wilson CS, Tricot G, Jagannath S, Siegel D, Desikan KR, Munshi N, Bracy D, Barlogie B, Butch AW. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood. 1998;91:3518–3523. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


