Abstract
Purpose
Radiation pneumonitis is a major toxicity following thoracic radiotherapy with no method available to accurately predict the individual risk. This is a prospective study to evaluate exhaled nitric oxide as a predictive biomarker for radiation pneumonitis in esophagus cancer patients.
Patients and Methods
34 patients prescribed neoadjuvant chemoradiotherapy for esophagus cancer were enrolled in this trial. Each received respiratory surveys and exhaled nitric oxide (NO) measurements before, at the end of, and 1 to 2 months after completing radiotherapy. Pneumonitis toxicity was scored using the Common Terminology Criteria for Adverse Events version 4.0. The demographics, dosimetric factors, and exhaled NO were evaluated for correlation with symptomatic patients (scores ≥2).
Results
28 patients were evaluable, all received 50.4 Gy with concurrent chemotherapy. Pneumonitis toxcity scores were: 1 grade 3, 3 grade 2, 7 grade 1, and 17 grade 0. Dosimetric factors were not predictive of symptoms. Exhaled NO measured before, at completion, and at restaging were 17.3(8.5; 5.5 - 36.7), 16.0(14.2; 5.8 - 67.7), 14.7(6.2; 5.5 - 28.0) parts per billion respectively. The ratio of exhaled NO at the end of radiotherapy versus pre-treatment was 3.4(1.7 - 6.7) for the symptomatic and 0.8(0.3 - 1.3) for the asymptomatic (P = 0.0017). Elevation in exhaled NO preceded peak symptoms by 33(21 - 50) days. The time to peak symptoms was found to be inversely related to the exhaled NO elevation.
Conclusions
Elevation in the exhaled NO at the end of radiotherapy was found to predict for radiation pneumonitis symptoms.
Keywords: Radiation pneumonitis, nitric oxide, esophagus cancer
1. Introduction
Radiation pneumonitis is the dose limiting toxicity in thoracic radiotherapy for lung cancer 1, 2 and a major cause of mortality in the treatment of mesothelioma 3-5. It occurs after radiotherapy for esophagus cancer 6, 7 and for breast cancer8. Radiation pneumonitis is an inflammatory reaction within irradiated normal lung tissue in response to radiation injury9, 10. Bronchoalveolar lavage11 and lung biopsies12 have shown that acute radiation pneumonitis is characterized by leukocyte infiltration. Leukocyte migration into the radiation injured site begins and is propagated by pro-inflammatory cytokines produced by macrophages, epithelial cells, pneumocytes, and fibroblasts13. Radiation pneumonitis occurs beginning after the initiation of radiotherapy to six months after completion of thoracic radiotherapy, with cough, shortness of breath, fever, and/or changes in pulmonary function14, 15. Severe radiation pneumonitis is often fatal, with a mortality rate among non-small cell lung cancer patients who experienced severe symptoms reported to approach 50%16.
The differential diagnosis of radiation pneumonitis includes lymphangitic malignancy spread, respiratory infection, exacerbation of underlying pulmonary disease, pulmonary emboli, cardiac disease, and drug toxicity17. The difficulty in the diagnosis of radiation pneumonitis stems from an overlap of symptoms (cough, shortness of breath, low grade fever, etc.) with those from the differential diagnosis also commonly present in patients with thoracic malignancies. Studies using dosimetric parameters, such as the percentage of lung volume irradiated ≥ 20 Gy (V20) or mean lung dose (MLD), to assess the risk of radiation pneumonitis complications have had poor predictive power for individual risk 2, 18, 19. The range of these dosimetric parameters for asymptomatic and symptomatic patients often completely overlap19, 20 [8F]-2-fluoro-2- deoxyglucose positron emission tomography (18F-FDG PET) imaging provides an assessment of pneumonitis, pulmonary inflammation appears as enhanced 18F-FDG uptake in response to inflammatory stimuli21-23. A metabolic response to radiation has been reported24, beginning early in the radiotherapy course25 and reaching its peak response up to three months following completion26. There is a significant correlation between clinical symptoms and the metabolic response measured by 18F-FDG PET imaging6. However, this metabolic response occurs concurrently with clinical symptoms. An inexpensive biomarker that predicts for radiation pneumonitis before clinical symptoms is needed.
Exhaled nitric oxide (NO) is an inexpensive non-invasive marker of pulmonary inflammation that has been studied in many acute and chronic lung diseases27, 28. Exhaled NO is useful in assessing inflammatory status and response to therapy in moderate to severe asthma and is becoming routine clinical practice in the pediatric asthmatic population29. NO has been studied in the irradiated lung in animal models and humans. In an irradiated lung mouse model, alveolar macrophages produced NO after irradiation, and the expression of inducible NO synthase (iNOS) in both alveolar macrophages and alveolar epithelial cells was increased30, 31. In a prospective patient study of 29 patients undergoing thoracic radiotherapy for lung cancer, Koizami et al.32 found the exhaled NO level rose > 3 times pretreatment levels in five patients. Three of these five patients developed symptomatic radiation pneumonitis, and no other patient showed signs of radiation pneumonitis. However, the 2-dimensional treatment planning used and the lack of clinically available NO breath analyzers limited the studies wider acceptance. These studies suggest exhaled NO is a potential biomarker of radiation pneumonitis.
In this study, we evaluate exhaled NO as a predictive biomarker for radiation pneumonitis in patient receiving neoadjuvant chemoradiotherapy for esophagus cancer in a prospective study. Patient symptoms are surveyed using a standard respiratory questionnaire33 and they receive exhaled NO measurements prior to the start of, at the completion of, and 6 weeks after thoracic radiotherapy.
2. Materials and Methods
2.1. Study Overview
Thirty-four patients scheduled to begin esophagus cancer treatment in the Department of Radiation Oncology at the University of Texas M. D. Anderson Cancer Center (MDACC) between March 23, 2009 and December 21, 2009 were enrolled in this MDACC Institutional Review Board approved prospective study (2008-0632). Each patient signed an informed consent and was entered into the clinical research database prior to study entry. Esophagus cancer patients at our institution undergo induction and/or neoadjuvant chemoradiotherapy, interval restaging, and surgery34. Patients on this study received measurement of their concentration of exhaled NO prior to beginning radiotherapy, on the last day of radiotherapy, and at their restaging follow-up visit. Since thoracic surgical intervention would interfere with the interpretation of clinical symptoms, patients completed this study at their restaging follow-up visit prior to surgery. At each of the three study encounters, they were asked to complete a respiratory questionnaire and confirm their current medications list. A measurement of their exhaled nitric oxide concentration was also made.
2.2. Radiation Treatment Plans
Each patient received a treatment planning session in which CT images of the entire thorax and upper-abdomen. Gross target delineation and margin generation was performed in a consistent manner as our group has reported35. The radiation dose was calculated using an average CT calculated from a 4-dimensional CT (4D CT) image set 36, 37. The radiation dose distributions were calculated with lung heterogeneity corrections for all cases38, 39. Mean lung dose (MLD), the percentage volume of lung irradiated to 5, 10, 20, and 30 Gy (V5, V10, V20, and V30), were obtained from the radiation treatment plans as dosimetric parameters to estimate the volume of lung irradiated.
2.3. Measurement of exhaled Nitric Oxide Concentration
The patients received exhaled NO testing using the Food and Drug Administration approved NIOX Minno (Aerocrine, New Providence, NJ) within 2 weeks prior to the start of radiotherapy, on the day of its completion, and on their restaging follow-up visit after the completion after radiotherapy. The patients received exhaled breath testing following the American Thoracic Society (ATS) guidelines40 standard techniques. The subjects were seated comfortably, a sterile single-use mouthpieces and filter was used for each subject at each session. Each patient inserted the mouthpiece and inhaled over 2 to 3 seconds through the mouth to total lung capacity (TLC), or near TLC if TLC was difficult, and then exhaled immediately. Since the NO concentration has a marked dependence on the flow rate, the flow rate monitored is by the NIOX Minno to maintain a 3 L/min flow rate on exhalation. The NIOX Minno creates a mild back pressure (8-10 mmHg) to close the posterior vellum and prevent nasal air contamination. The measurements were made in triplicate, with data acquired during three breaths at each time point and recorded. Exhaled NO values were then calculated as the mean of the three values. There was at least 2 minutes of rest prior to each measurement. Measurements were taken at the start of radiation therapy, during the last week of radiation treatment, and at the time of the follow-up PET imaging session.
2.4. Respiratory Questionnaire & Assessment
At each of the three assessment time points, patients enrolled in this study were asked to complete the Americanized St. George's Respiratory Questionnaire33. Their current medications list was confirmed with the patients and the documents entered into the patients' electronic file for this study. The clinical and research records were combined to score the pneumonitis toxicity using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (CTCAEv4.0)41. Clinically symptomatic pneumonitis was defined as grade 2 or higher.
2.5. Statistical analysis
Continuous variables (mean lung dose, V5, V10, V20, V30, radiation dose, time between radiotherapy and restaging, and age) were summarized in the form of mean (SD; range). Categorical variables (toxicity, tumor location, and tumor histology) were summarized in the form of frequency tables. A Wilcoxon rank sum test was used to compare the dosimetry parameters (MLD, mean lung dose; CTV, clinical target volume; V5 – V30, percentage of lung volume that received > 5 - 30 Gy) between the asymptomatic and symptomatic groups. P-values of 0.05 or less were considered statistically significant. The Wilcoxon rank test was also used to compare exhaled NO ratios of end versus before RT. The exhaled NO ratio of end versus before treatment was tested as classifier to predict symptomatic patients using a k-nearest neighbor classifier with k=1-5 and an 80/20 cross-validation scheme. Simulations were run 1000 times to predict the average error, where 80/20 is the percentage of data used to train/test the classifier.
3. Results
3.1. Patient characteristics
Of the 34 consented patients 4 patients withdrew from the study prior to completing radiotherapy and their scheduled tests. Three patients missed one of the evaluation sessions, either due to instrument failure or schedule. One patient did not return for follow-up or restaging visits. The characteristics of the evaluable patients who completed the study are summarized in Table 1. All patients received 50.4 Gy (or Co-60 GE), 10 received proton therapy and the remainder x-ray based intensity modulated radiotherapy (IMRT). The primary esophagus tumors from all cases were mid-thoracic or lower esophagus cancer primaries. The median time between the completion of radiotherapy and the follow-up visit was 36 days (range: 23 to 56 days).
Table 1. Evaluable Patients Characteristics.
| Characteristics | n |
|---|---|
| Age (y) | |
| Median | 62 |
| Range | 41 - 75 |
| Gender | |
| Male/Female | 25/3 |
| Stage | |
| I | 1 (3.6) |
| IIA | 10 (35.7) |
| III | 12 (42.9) |
| IVA | 4 (14.3) |
| IV B | 1 (3.6) |
| Location | |
| Middle | 5 (17.9) |
| Middle to GE junction | 3 (10.7) |
| Lower | 7 (25.0) |
| GE junction | 13 (46.4) |
| CTV (mL) | |
| Median | 379 mL |
| Range | 157 – 1095 mL |
| Prescription dose (Gy) | |
| Median | 50.4 Gy (or Co-60 GE) |
| Range | 50.4 Gy |
| Radiation type | |
| IMRT/Protons | 18/10 |
| Time between completion of radiotherapy to PET imaging session (days) | |
| Median | 36 days |
| Range | 23 to 53 days |
| Chemotherapy | |
| Induction prior to radiotherapy | 17 (60.7) |
| Concurrent with radiotherapy | 28 (100) |
Abbreviations: GE = Gastro-esophageal, CTV = clinical target volume, Co-60 GE = cobalt-60 Gray Equivalents.
Percentages are presented in parenthesis.
3.2. Exhaled Nitric Oxide
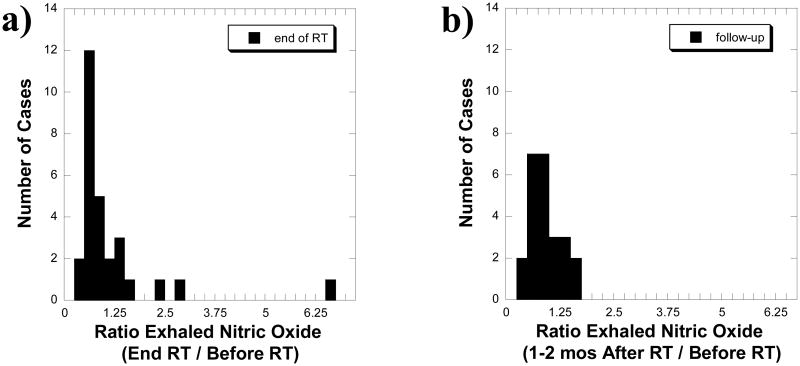
The concentration of exhaled NO measured before radiotherapy, at completion of radiotherapy, and at restaging were 17.3(8.5; 5.5 - 36.7), 16.0(14.2; 5.8 - 67.7), and 14.7(6.2; 5.5 - 28.0) parts per billion (ppb) respectively. Each measurement was made in triplicate with an average standard error of 0.8 ppb. Ratios for each case were formed with respect to the pre- radiotherapy baseline and are given in histogram format in Figure 1 for the radiotherapy completion and restaging time points. For a small subset, there is a transient rise in the exhaled NO over baseline at the end of radiotherapy that nearly completely resolves by the restaging visit.
Figure 1. Ratio of exhaled nitric oxide versus timepoint.
a) A histogram is shown of the ratio of exhaled nitric oxide concentrations at the completion of versus that obtained before radiotherapy (RT) from 28 cases. b) A histogram of the ratio of exhaled nitric oxide concentration obtained at the first follow-up visit (23 to 53 days after RT) versus that obtained before RT from 24 cases. All symptomatic cases are represented at both time points.
3.3. Respiratory Symptoms
Pneumonitis toxicity was assessed from medical records, including a review of the radiographic studies, and the respiratory surveys. The toxicity scores derived using the CTCAEv4.041 were: grade 0 - 17 patients, grade 1 - 7 patients, grade 2 - 3 patients, grade 3 - 1 patient, grade 4 or 5 - none. One patient who was hospitalized for dyspnea received a grade 3 toxicity score, his restaging 18F-FDG PET imaging study is shown overlain the treatment planning isodose distribution in Figure 2. Using the scoring system of Hicks et al.42 this corresponds with a grade 3 pneumonitis metabolic response. There is enhanced FDG uptake within his lungs in the radiation treatment field consistent with radiation pneumonitis. Three patients had both radiographic and clinical respiratory symptoms affecting their instrumental activities of daily living were given a score of 2. Seven patients, who had only radiographic findings within the radiation treatment field or minor clinical symptoms, were scored as grade 1 toxicity. Patients were stratified based on clinical score as symptomatic (≥ 2, 4 patients) or asymptomatic (0 or 1, 24 patients). For the four symptomatic patients, toxicity grade ≥ 2, the times to peak respiratory symptoms were recorded.
Figure 2. [18F]-Fluorodeoxyglucose (FDG) PET Response to Radiotherapy (RT).
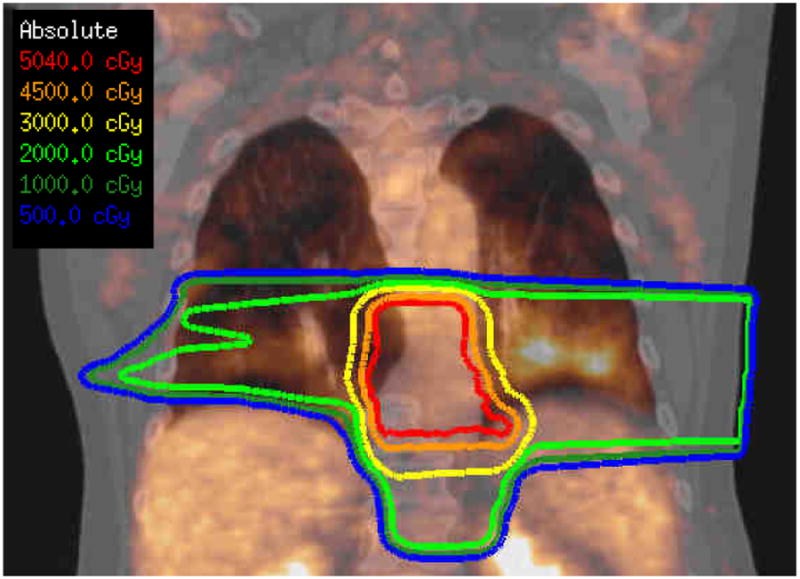
The radiation isodose distribution is shown in coronal section for the case with the most severe symptoms and the largest exhaled nitric oxide (NO) concentration ratio. The post-treatment FDG PET imaging study obtained 33 days after completion of radiotherapy is overlain. Note the FDG-avid lung regions within the irradiated lung. This patient experienced CTCAEv4.0 grade 3 pneumonitis. This patient's exhaled NO concentration was elevated (ratio equals; 6.7) one month prior to the above FDG PET imaging study and 3 weeks before his hospitalization for radiation pneumonitis related symptoms.
3.4. Prediction of Clinical Symptoms
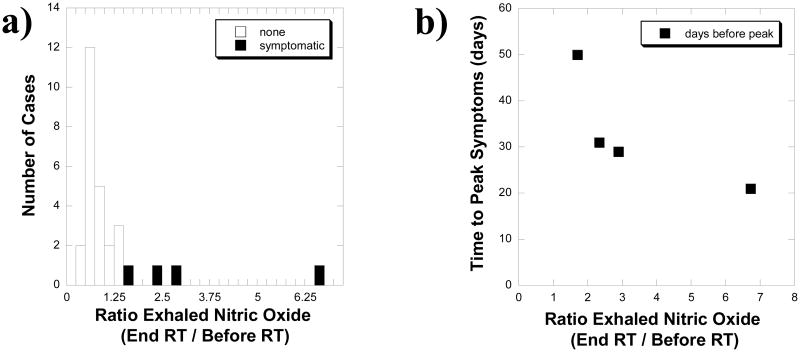
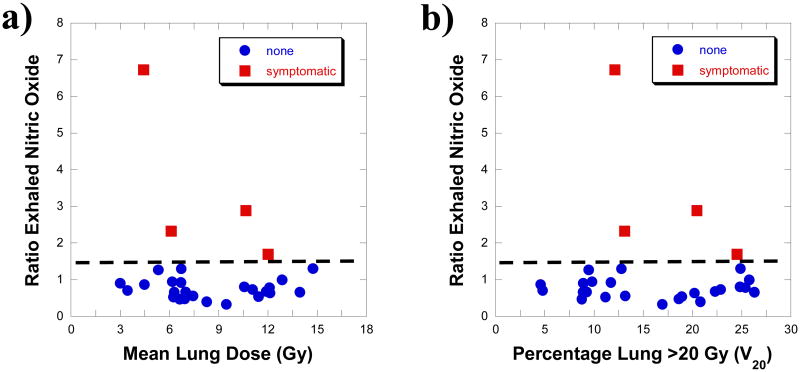
The dosimetric parameters, the MLD and V5 though V30, for asymptomatic and symptomatic cases are summarized in Table 2. The range of values for the asymptomatic cases included and exceeded the range for the symptomatic cases. There was no statistical difference in the dosimetric parameters detected between the two groups. The exhaled NO ratios, start versus end of radiotherapy, are separated into symptomatic and asymptomatic cases in Figure 3a. Every symptomatic case was higher than all of the asymptomatic cases, there were no symptomatic cases with a ratio of ≤ 1.5 and vice versa. A scatter plot of the time from the end of radiotherapy to peak symptoms versus exhaled NO ratio is given in Figure 3b. For each of these symptomatic cases peak symptoms were defined during a clinical encounter such as hospitalization for dyspnea. For every symptomatic case the greater the exhaled NO ratio at the end of radiation treatment, the shorter the time to peak symptoms. The exhaled NO ratio was plotted against the MLD and V20 in Figure 4 for the symptomatic (red) and asymptomatic (blue) cases. There was no dosimetric parameter that could meaningfully separate the two groups, however an exhaled NO ratio of 1.5 completely separated the symptomatic from asymptomatic groups.
Table 2.
Dosimetric parameters for asymptomatic and symptomatic cases.
| Parameter | Asymptomatic (4 cases) | Symptomatic (26 cases) | P-value |
|---|---|---|---|
|
| |||
| MLD | 7.0 (3.0 - 14.7) | 8.4 (4.4 - 12.0) | 0.69 |
| V5 | 37.9 (11.9 - 63.8) | 39.2 (18.5 - 56.1) | 0.93 |
| V10 | 24.2 (8.5 - 43.8) | 28.8 (15.9 - 39.9) | 0.93 |
| V20 | 13.2 (4.6 - 26.3) | 16.8 (11.8 - 24.5) | 0.56 |
| V30 | 7.3 (1.3 - 18.8) | 6.2 (2.3 - 12.2) | 0.78 |
Median value with range given in parentheses for each entry. P-values calculated using the Wilcoxon rank sum test. P-values of 0.05 or less were considered statistically significant.
Abbreviations: MLD = mean lung dose; V5 = percentage of lung receiving ≥5 Gy; V10 = percentage of lung receiving ≥10 Gy; V20 = percentage of lung receiving ≥20 Gy; V30 = percentage of lung receiving ≥30 Gy.
Figure 3. Exhaled nitric oxide versus symptoms and radiotherapy (RT) type.
a) A histogram is shown of the ratio of exhaled nitric oxide concentrations at the completion of versus that obtained before radiotherapy from 28 cases. The symptomatic cases are shown in solid black and the asymptomatic in solid white. Symptomatic cases were those with CTCAEv4.0 grade 2 or higher pneumonitis. b) Scatter plot of time from the end of radiotherapy to peak symptoms versus exhaled NO ratio. For every symptomatic case the greater the exhaled NO ratio at the end of radiation treatment, the shorter the time to peak symptoms. For each of these symptomatic cases peak symptoms were defined during a clinical encounter such as hospitalization for dyspnea.
Figure 4. Toxicity analysis using exhaled nitric oxide (NO).
a) Plot of toxicity outcome by exhaled NO ratio and MLD. The dashed line (ratio exhaled NO = 1.5) separates those with grade 2 or higher toxicity from the asymptomatic cases. b). Plot of toxicity outcome by exhaled NO ratio and V20. The same line delineates a high risk region for those with grade 2 or higher radiation pneumonitis toxicity. The two volumetric parameters (MLD & V20) alone or combined had poor predictive power.
A comparison of the exhaled NO ratios (end versus before) between the asymptomatic versus the symptomatic group found that symptomatic patients had a higher ratio than asymptomatic patients (P-value < 0.0017). Additionally, the exhaled NO ratio (end versus before) was tested as a classifier to predict symptomatic patients. A k-nearest neighbor classifier with k=1-5 and an 80/20 cross-validation scheme run 1000 times to predict the average error, where 80/20 is the percentage of data used to train/test the classifier, resulted in a very small average error rate (8%).
4. Discussion
In this report, we report the findings of our prospective study designed to test the ability of exhaled NO concentration measurements, using a point-of-service NO breath analyzer, to predict symptomatic radiation pneumonitis. We found a transiently elevated exhaled NO ratio at the end of radiotherapy in each patient who subsequently develop symptomatic radiation pneumonitis. We found no dosimetric parameter predictive of radiation pneumonitis symptom status, similar to the recently reported RTOG-0117 findings of Bradley et al.43. This elevation in exhaled NO was predictive and occurred weeks to months before peak symptoms developed in each case. We demonstrated that the exhaled NO ratio can be used as a classifier to predict symptomatic patients with a small average error rate (8%). These findings in esophagus cancer patients undergoing thoracic radiotherapy are similar to those of Koizumi et al.32, who studied lung cancer patients, and may be generalized broadly to all thoracic radiotherapy patients.
The rate of symptomatic RP was lower in this study than in our prior retrospective study of esophagus cancer patients reported by McCurdy et al. 44, where the overall incidence of symptomatic RP was 58%. In that retrospective study which included 139 cases and one case of fatal RP, the MLD was 12.3 Gy versus 7.2 Gy in the present series. The lower lung doses in this prospective study are due to the progressive use of technology, IMRT and proton therapy versus 3D-RT planning, as well as reduced planning margins due to the use of image guidance. Subgroup analysis would not yield additional findings given the small sample size in this study. The incidence of symptomatic RP in the current study falls within the range of 13 to 37% reported in the literature review by Rodrigues et al. 45. In a recent retrospective study of RP after stereotactic body radiotherapy (SBRT) in the setting of prior thoracic radiotherapy, a crude symptomatic RP incidence of 50% was found 46. In that study the biologically equivalent dose received was 112.5 Gy to the target, however, the lung dosimetry parameters were not reported. Hua et al. 20 reported a 1-year cumulative incidence of symptomatic RP of 8.2% in a retrospective study of 122 pediatric patients who received thoracic radiotherapy. The prescription radiation dose for this cohort ranged from 15 Gy (Hodgkin lymphoma) to 70 Gy (sarcoma). Symptomatic cases were found even in the Hodgkin lymphoma patients who received treatment at the lower dose range. We hypothesize exhaled NO will increase in all symptomatic thoracic radiotherapy patients and are planning a phase II study to test our hypothesis.
Previous studies have evaluated inflammatory biomarkers from blood samples to predict radiation pneumonitis symptoms. Plasma transforming growth factor β1 (TGF- β1) was found to be a predictive marker of symptomatic radiation pneumonitis in a study by Anscher et al.47. In that study of 73 patients, those who had no elevation above pre-radiotherapy and an absolute plasma concentration ≤ 7.5 ng/mL were unlikely to develop symptoms with a sensitivity and positive predictive value of 90%. However, subsequent studies attempting to reproduce these results were mixed; a table illustrating the mixed results of TGF- β1 studies can be found in Fleckenstein et al.48. Soluble intercellular adhesion molecule 1 (sICAM-1) was evaluated prospectively by Ishii et al.49 in 30 lung cancer patients. 12 patients developed symptomatic pneumonitis, serum levels of sICAM-1 at the end of radiotherapy were elevated in the 7 out of 12 patients from the symptomatic group. In 10 out of 12 patients serum levels of sICAM-1 was elevated at the onset of symptoms. Arpin et al.50 found, in a study of 96 patients, a significant rise in interleukin-6 (IL-6) among the radiation pneumonitis group at 2 weeks into treatment, however there was a large variation in IL-6 values in the radiation pneumonitis group (113.7(SD 474.7) versus 6.7(SD 27.3), P = 0.025) making individual risk assessment difficult. Each of these test have the drawback of requiring blood samples and consist of tests that are not broadly available.
Exhaled NO is useful in assessing inflammatory status and response to therapy in moderate to severe asthma and is becoming routine clinical practice in that population29. The instrument used in this study was designed for point of service use for both adult and pediatric populations; it was simple to use, no technical training required, and gave reproducible readings. The sensitivity and specificity of this test could not be fully assessed due to the limited number of symptomatic cases. This study was also limited by the number exhaled NO measurements made. To address these issues, this study is currently being expanded to recruit a larger cohort of esophagus and lung cancer patients, with weekly assessments to allow earlier detection of the rise in exhaled NO.
We found in this study, patients who developed an elevation in their exhaled NO, are at high risk to develop clinical radiation pneumonitis symptoms. Previously investigators have shown amifostine51 or inhaled beclamethasone52 can reduce the incidence of radiation pneumonitis in locally advanced lung cancer patient receiving thoracic radiotherapy. However, the perceived risk of reduced cancer control and the medication side-effects resulted in a reluctance to treat prophylactically all thoracic radiotherapy with these medications. Now that a high risk group can be identified prior to peak symptoms, studies to mitigate the subsequent development of radiation pneumonitis symptoms in this group are needed. Genome-wide studies should also be applied to this high risk group to identify genetic determinant that contribute to the enhanced pulmonary inflammatory response to radiation expressed by this group.
5. Conclusions
In this prospective study, elevation in the exhaled NO concentration at the end of radiotherapy was found to predict for subsequent symptoms. Every symptomatic patient was found to have a higher ratio of exhaled NO over baseline than any of the asymptomatic cases, there were no symptomatic cases with a ratio of ≤ 1.5 and vice versa. The time to peak symptoms was found to be inversely related to the rise in exhaled NO. Serial exhaled NO may be utilized to identify a group at high risk to the subsequent development of symptomatic radiation pneumonitis.
Acknowledgments
We extend our gratitude to the thoracic radiation oncology faculty, thoracic surgeons, and gastrointestinal medical oncologists at M. D. Anderson whose patients were the focus of this study. The authors thank Dr. Mersiha Hadziahmetovic who performed the initial calibration and clinical acceptance testing of the NO breath analyzer. The authors also thank Mr. Jan Pagilagan who served as the data coordinator for this study and Ms. Toni Williams who provided administrative support. We most sincerely thank the National Institutes of Health (NIH) and the National Cancer Institute (NCI) who provided support for this project through NIH/NCI Grant R21CA141833. Dr. Martinez was supported by a post-doctoral training grant, NCI grant R25T-CA90301. Additional funding for this study was provided from The University of Texas M. D. Anderson Cancer Center's Physician Scientist Program.
Footnotes
Conflict of Interest Notification: The authors have no commercial or financial interests related to this study to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradley J. A review of radiation dose escalation trials for non-small cell lung cancer within the Radiation Therapy Oncology Group. Semin Oncol. 2005;32:S111–S113. doi: 10.1053/j.seminoncol.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Bradley JD, Hope A, Naqa IE, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:984–995. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640–645. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen CA, Nøttrup TJ, Berthelsen AK, et al. Pulmonary toxicity following IMRT after extrapleural pneumonectomy for malignant pleural mesothelioma. Radiat Oncol. 2009;92:96–99. doi: 10.1016/j.radonc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Rice DC, Smythe R, Liao Z, et al. Dose-Dependent Pulmonary Toxicity After Postoperative Intensity-Modulated Radiotherapy for Malignant Pleural Mesothelioma. Int J Radiat Oncol Biol Phys. 2007;69:350–357. doi: 10.1016/j.ijrobp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Hart JP, McCurdy MR, Ezhil M, et al. Radiation pneumonitis: correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys. 2008;71:967–971. doi: 10.1016/j.ijrobp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCurdy M, McAleer MF, Wei W, et al. Induction and Concurrent Taxanes Enhance Both the Pulmonary Metabolic Radiation Response and the Radiation Pneumonitis Response in Patients With Esophagus Cancer. Int J Radiat Oncol Biol Phys. 2010;76:816–823. doi: 10.1016/j.ijrobp.2009.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taghian AG, Assaad SI, Niemierko A, et al. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93:1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 9.Fajardo LF, Berthrong M, Anderson RE. Radiation Pathology. New York: Oxford Univ. Press; 2001. [Google Scholar]
- 10.Roberts CM, Foulcher E, Zaunders JJ, et al. Radiation pneumonitis: A possible lymphocyte- mediated hypersensitivity reaction. Ann Intern Med. 1993;118:696–700. doi: 10.7326/0003-4819-118-9-199305010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Cordier JF, Mornex JF, Lasne Y, et al. Bronchoalveolar lavage in radiation pneumonitis. Bull Eur Physiopathol Respir. 1984;20:369–374. [PubMed] [Google Scholar]
- 12.Mark E. Lung biopsy interpretation. Baltimore, MD: Williams & Wilkins; 1984. [Google Scholar]
- 13.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 14.Gross NJ. Pulmonary effects of radiation therapy. Ann Intern Med. 1977;86:81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- 15.McDonald S, Rubin P, Phillips TL, et al. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys. 1995;31:1187–1203. doi: 10.1016/0360-3016(94)00429-O. [DOI] [PubMed] [Google Scholar]
- 16.Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54:735–741. doi: 10.1016/s0360-3016(02)02994-2. [DOI] [PubMed] [Google Scholar]
- 17.Movsas BMD, Raffin TAMD, Epstein AHMD, et al. Pulmonary radiation injury. Chest. 1997;111:1061–1076. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 18.Kocak Z, Borst GR, Zeng J, et al. Prospective assessment of dosimetric/physiologic-based models for predicting radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2007;67:178–186. doi: 10.1016/j.ijrobp.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 20.Hua C, Hoth KA, Wu S, et al. Incidence and correlates of radiation pneumonitis in pediatric patients with partial lung irradiation. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.07.1709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones HA, Clark RJ, Rhodes CG, et al. In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med. 1994;149:1635–1639. doi: 10.1164/ajrccm.149.6.7516252. [DOI] [PubMed] [Google Scholar]
- 22.Chen DL, Rosenbluth DB, Mintun MA, et al. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol. 2006;100:1602–1609. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 23.Chen DL, Ferkol TW, Mintun MA, et al. Quantifying pulmonary inflammation in cystic fibrosis with positron emission tomography. Am J Respir Crit Care Med. 2006;173:1363–1369. doi: 10.1164/rccm.200506-934OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerrero T, Johnson V, Hart J, et al. Radiation pneumonitis: Local dose versus [18F]- fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys. 2007;68:1030–1035. doi: 10.1016/j.ijrobp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 25.De Ruysscher D, Houben A, Aerts HJWL, et al. Increased 18F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): A prospective pilot study. Radiat Oncol. 2009;91:415–420. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Teo BK, Abelson J, Teo A, et al. Time Interval to FDG PET/CT after Mediastinal Radiation Impacts the Dose Response of Pneumonitis Related Metabolic Activity. Int J Radiat Oncol Biol Phys. 2008;72:S67–S68. [Google Scholar]
- 27.Gustafsson LE, Leone AM, Persson MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 28.Kharitonov SA, Barnes PJ. Exhaled Biomarkers. Chest. 2006;130:1541–1546. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]
- 29.Smith AD, Cowan JO, Brassett KP, et al. Use of Exhaled Nitric Oxide Measurements to Guide Treatment in Chronic Asthma. N Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 30.Nozaki Y, Hasegawa Y, Takeuchi A, et al. Nitric oxide as an inflammatory mediator of radiation pneumonitis in rats. Am J Physiol Lung Cell Mol Physiol. 1997;272:L651–658. doi: 10.1152/ajplung.1997.272.4.L651. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji C, Shioya S, Hirota Y, et al. Increased production of nitrotyrosine in lung tissue of rats with radiation-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;278:L719–725. doi: 10.1152/ajplung.2000.278.4.L719. [DOI] [PubMed] [Google Scholar]
- 32.Koizumi M, Yamazaki H, Toyokawa K, et al. Influence of thoracic radiotherapy on exhaled nitric oxide levels in patients with lung cancer. Jpn J Clin Oncol. 2001;31:142–146. doi: 10.1093/jjco/hye028. [DOI] [PubMed] [Google Scholar]
- 33.Barr JT, Schumacher GE, Freeman S, et al. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22:1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 34.Ajani JA, Komaki R, Putnam JB, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer. 2001;92:279–286. doi: 10.1002/1097-0142(20010715)92:2<279::aid-cncr1320>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Liao Z, Liu H, Komaki R. Target delineation for esophageal cancer. Journal of Women's Imaging. 2003;5:177–186. [Google Scholar]
- 36.Vedam SS, Keall PJ, Kini VR, et al. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol. 2003;48:45–62. doi: 10.1088/0031-9155/48/1/304. [DOI] [PubMed] [Google Scholar]
- 37.Pan T, Lee TY, Rietzel E, et al. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med Phys. 2004;31:333–340. doi: 10.1118/1.1639993. [DOI] [PubMed] [Google Scholar]
- 38.Ahnesjo A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989;16:577–592. doi: 10.1118/1.596360. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Zhao KL, Guerrero TM, et al. 4D CT-based Treatment Planning for Intensity- Modulated Radiation Therapy and Proton Therapy for Distal Esophagus Cancer. Int J Radiat Oncol Biol Phys. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ATS/ERS. ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 41.CTEP. Common Terminology Criteria for Adverse Events Version 4.0. 2009 doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Hicks RJ, Mac Manus MP, Matthews JP, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: Inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60:412–418. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Bradley JD, Bae K, Graham MV, et al. Primary Analysis of the Phase II Component of a Phase I/II Dose Intensification Study Using Three-Dimensional Conformal Radiation Therapy and Concurrent Chemotherapy for Patients With Inoperable Non-Small-Cell Lung Cancer: RTOG 0117. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.27.1205. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCurdy M, McAleer MF, Wei W, et al. Induction and Concurrent Taxanes Enhance both the Pulmonary Metabolic Radiation Response and the Radiation Pneumonitis Response in Patients with Esophagus Cancer. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.02.059. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues G, Lock M, D'Souza D, et al. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer--a systematic review. Radiotherapy and Oncology. 2004;71:127–138. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Kelly P, Balter PA, Rebueno N, et al. Stereotactic Body Radiation Therapy for Patients with Lung Cancer Previously Treated with Thoracic Radiation. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.09.070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anscher MS, Kong FM, Andrews K, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41:1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 48.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using Biological Markers to Predict Risk of Radiation Injury. Semin Radiat Oncol. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Ishii Y, Kitamura S. Soluble intercellular adhesion molecule-1 as an early detection marker for radiation pneumonitis. Eur Respir J. 1999;13:733–738. doi: 10.1034/j.1399-3003.1999.13d06.x. [DOI] [PubMed] [Google Scholar]
- 50.Arpin D, Perol D, Blay J-Y, et al. Early Variations of Circulating Interleukin-6 and Interleukin-10 Levels During Thoracic Radiotherapy Are Predictive for Radiation Pneumonitis. J Clin Oncol. 2005;23:8748–8756. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- 51.Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369–1377. doi: 10.1016/j.ijrobp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Pagel J, Mohorn M, Kloetzer KH, et al. The inhalation versus systemic prevention of pneumonitis during thoracic irradiation. Strahlenther Onkol. 1998;174:25–29. doi: 10.1007/BF03038224. [DOI] [PubMed] [Google Scholar]