Abstract
Purpose.
To determine whether previously shown photodynamic (PD)-induced inhibition of specific photoreceptor outer segment (POS) phagocytosis by ARPE-19 cells is associated with reductions in receptor proteins mediating POS phagocytosis, and if PD treatment with merocyanine-540 (MC-540) produces additional effects leading to its inhibition of nonspecific phagocytosis.
Methods.
ARPE-19 cells preloaded with MC-540 or rose bengal (RB) were sublethally irradiated with green light. Phagocytosis of POS was measured by flow cytometry and POS receptor proteins (Mer tyrosine kinase receptor [MerTK] and integrin subunits αv and β5) and β-actin were quantified by Western blotting at 0.5 and 24 hours after irradiation, with comparison to samples from nonsensitized control cultures. The intact integrin heterodimer αvβ5 was quantified by immunoprecipitation followed by blotting. The distribution of N-cadherin, ZO-1, and F-actin was visualized by fluorescence microscopy.
Results.
Mild PD stress mediated by both photosensitizers that elicits no significant morphologic changes produces transient and recoverable reductions in MerTK. The individual αv and β5 integrin subunits are also reduced but only partially recover. However, there is sufficient recovery to support full recovery of the functional heterodimer. Light stress mediated by MC-540 also reduced levels of actin, which is known to participate in the internalization of particles regardless of type.
Conclusions.
After PD treatment POS receptor protein abundance and phagocytosis show a coincident in time reduction then recovery suggesting that diminution in receptor proteins contributes to the phagocytic defect. The additional inhibition of nonspecific phagocytosis by MC-540–mediated stress may result from more widespread effects on cytosolic proteins. The data imply that phagocytosis receptors in RPE cells are sensitive to oxidative modification, raising the possibility that chronic oxidative stress in situ may reduce the efficiency of the RPE's role in photoreceptor turnover, thereby contributing to retinal degenerations.
Using sublethal photodynamic stress mediated by merocyanine-540 or rose bengal in ARPE-19 cells, we ask whether the impairments in phagocytosis could be explained by photic stress-induced changes in the abundance of receptor proteins, Mer tyrosine kinase receptor and the αvβ5 integrin, that mediate photoreceptor outer segment uptake.
Introduction
An important function of the retinal pigment epithelium (RPE) is the efficient phagocytosis of photoreceptor outer segments (POS), which are periodically shed in a circadian rhythm.1,2 Interaction between RPE cells and POS of the retina in the process of photoreceptor renewal is essential for photoreceptor function and survival such that impairments in the process at the level of the RPE can result in retinal degenerations.3
RPE cells reside in a location and perform functions that make them susceptible to oxidative stress, including stress from irradiation with visible light.4,5 One mechanism whereby light damages the RPE is by the formation of reactive species generated by the endogenous photosensitizer lipofuscin.6–8 Lipofuscin is a photoreactive pigment that increases in the RPE with age, and is believed to increase cell susceptibility to photic stress. Light stress mediated by lipofuscin accumulation has been implicated in several hereditary retinal degenerations9,10 and in the pathogenesis of AMD.7,8,11,12
We previously modeled light stress and its effect on RPE cell functions using the exogenous photosensitizing dyes merocyanine-540 (MC-540) and rose bengal (RB),13 which were selected for their cellular localization and absorption characteristics.14–18 We found that photodynamic (PD) treatment of ARPE-19 cells mediated by these dyes inhibits the specific phagocytosis of POS. Subthreshold PD stress induced by MC-540 also inhibits the nonspecific phagocytosis of polystyrene beads. In both cases inhibition of phagocytosis was transient and recoverable by 24 hours.13
Here, we used a similar model to ask whether impairments in phagocytosis could be explained by photic stress-induced changes in the abundance of receptor proteins that mediate POS uptake. Among the RPE plasma membrane proteins known to mediate POS binding and internalization are the Mer tyrosine kinase receptor (MerTK)19 and the αvβ5 integrin.20–22 αvβ5 is the only integrin receptor that displays an apical localization21,23,24 and is the primary RPE receptor involved in recognition and binding during POS phagocytosis21,25,26 while MerTK mediates engulfment.27 POS binding via the αvβ5 integrin receptor initiates a signaling cascade that leads to the tyrosine phosphorylation of MerTK, whose deficiency almost completely abolishes engulfment of bound POS.27,28 Using sublethal PD stress mediated by MC-540 or RB in ARPE-19 cells, we report here that MerTK receptor protein is reduced, then recovers 24 hours after irradiation in the presence of either photosensitizer, coincident in time with inhibition, and recovery of POS phagocytosis. Individual integrin receptor subunits αv and β5 are also reduced immediately after PD treatment, but recover variably at 24 hours. Despite the variability, however, subunit recovery appears sufficient to support full recovery of the functional heterodimer αvβ5. Light stress mediated by MC-540 has more widespread effects than by RB, including diminution of the cytoskeletal protein actin, which participates in internalization of particles regardless of type. Together these results suggest that phagocytosis receptors in RPE cells are sensitive to photic stress. The outcomes also support the hypothesis that chronic oxidative stress in situ, over time may reduce the efficiency of the RPE's role in photoreceptor turnover, thereby predisposing to retinal degenerations associated with aging.
Methods
Cell Cultures and Induction of Oxidative Stress
Cultures of the human RPE cell line ARPE-19 (American Type Culture Collection, Rockville, MD) were maintained using biweekly feedings with Minimal Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich Co., St. Louis, MO). Control experiments were also performed using RPE cultures established from human donor eyes that were also propagated in MEM-FBS, as described previously.29
For oxidative stress experiments, ARPE-19 cells were seeded in 24-well plates at confluent density, using two to four replicate wells per treatment group, and sublethal stress was induced 21 days later by previously described protocols.13 Briefly, cells were exposed to 8 μM MC-540 or 600 nM RB in serum free medium for 0.5 hours at 37°C, then washed three times in Hank's Balanced Salt Solution containing calcium and magnesium ions (HBSS; Invitrogen, Carlsbad, CA). Cultures were then irradiated for 30 minutes (for MC-540) or 15 minutes (for RB) with green light derived from four 40 W cool-white fluorescent tubes at a fluence rate of 1.1 mW/cm2 and covered with a green filter (520–570 nm) (Lee Filters, Central Way Walworth Industrial Estate Andover, Hampshire, England). At 0.5 and 24 hours after PD treatment, oxidative stress was confirmed as sublethal by analysis of propidium iodide fluorescence, and phagocytic activity was analyzed by flow cytometry, as described below. At the same time intervals, cell extracts were prepared for Western blotting or immunoprecipitation to quantify POS receptor proteins, and the distribution of N-cadherin, ZO-1, and F-actin was visualized by fluorescence microscopy.
Cell Viability and Morphologic Analyses
To confirm that PD stress was sublethal, an imaging assay was used to identify dead cells by nuclear staining with the membrane impermeant fluorescent dye propidium iodide (PI; Sigma-Aldrich Co.). For this protocol, cells were refed after PD treatment with fresh MEM-FBS and five microscope fields per culture well were preselected by capturing digital bright field images to confirm consistent culture morphology. PI (final concentration 100 μM) was added to the medium and fluorescence images (555-nm excitation/617-nm emission) were captured of the selected fields at 0.5 and 24 hours to detect PI-positive nuclei. Digitonin (final concentration 1 mM; Sigma-Aldrich Co.) was then added to permeabilize membranes and fluorescence images were captured again after 30 minutes to quantify total cell number. The number of viable cells (PI negative) was determined per field and expressed as a percent of the total cell number per field. Data are expressed as the mean percent (±SD) viable cells in replicate culture wells within an experiment.
To evaluate the morphologic effects of PD treatment, cells were processed for fluorescence microscopy and stained for junctional proteins N-cadherin and ZO-1, and for the cytoskeletal protein F-actin by our previously described methods.30 For immunostaining of N-cadherin, a rabbit polyclonal primary antibody was used (1:200 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); for ZO-1 the primary antibody was a mouse monoclonal (1:200 dilution; Invitrogen, Camarillo, CA). Fluor-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). F-actin was visualized by staining with TRITC-conjugated phalloidin (1:500 dilution; Sigma-Aldrich Co.).
Phagocytosis Assay
As explained with the Results, ARPE-19 cultures used here were in later confluency (21 days post plating) than in our previous study.13 To confirm that the previously observed phagocytosis inhibition resulting from PD treatment also occurs in these post confluent cultures, phagocytosis was re-analyzed at the later culture interval using the previously described protocol.13 Briefly, control and photodynamically-treated cells were challenged 0.5 hours after light irradiation with FITC-labeled POS (3.2 × 108 POS/mL), incubated for 5.5 hours at 37°C, then washed three times in PBS. The cells were detached by trypsinization and suspended in PBS containing 10% FBS. The percentage of fluorescence-positive events (λexcitation = 488 nm, λemission = 525 nm) from 10,000 unfixed cells per sample was analyzed on a FACSCalibur instrument (BD Biosciences, San Jose, CA) using CellQuest (BD Biosciences) software. Data are expressed as normalized phagocytosis (±SD) for paired control (untreated) and photosensitized cultures. The rate of phagocytosis by untreated cells was taken as 100%.
Western Blot Analysis of POS Receptor Proteins
For quantitative Western blotting, cell extracts were prepared from control and PD-treated ARPE-19 cultures by direct lysis in SDS-containing electrophoresis buffer,31 supplemented with a 1% protease inhibitor cocktail (Sigma-Aldrich, Co.), 1% β-mercaptoethanol, and 1.5% bromophenol blue dye. Lysates from replicate wells were loaded volume equivalent on 10% polyacrylamide gels, separated by electrophoresis, and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA) by standard protocols. Immunoblots were probed with primary antibodies to the MerTK receptor (1:1000 dilution; Abcam, Cambridge, MA), the αv integrin subunit (1:1000 dilution; BD Bioscience), the β5 integrin subunit (1:1000 dilution; Santa Cruz Biotechnology), β-actin (1:20,000 dilution; Sigma-Aldrich, Co.), catalase (1:20,000 dilution; Abcam), or glyceraldehye-3-phosphate dehydrogenase (GAPDH; 1:20,000 dilution; Advanced Immunochemical Inc., Long Beach, CA). Appropriate secondary antibodies were horseradish peroxidase-conjugated (1:5000 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or from LI-COR (1:20,000 dilution; LI-COR Biosciences, Lincoln, NE). Detection was by enhanced chemiluminescence (ECL; GE Healthcare, Buckinghamshire, UK) or the LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences) according to the manufacturers' protocols. Quantitative densitometry of the immunoblots was performed and band density is given in arbitrary units and expressed as the mean density (±SD) from replicate culture wells within an experiment. All experiments were performed a minimum of three times. Consistent outcomes were obtained and representative experiments are shown.
Immunoprecipitation Analysis of the Integrin Heterodimer
The intact functional integrin heterodimer αvβ5 in control and PD-treated cells was quantified by immunoprecipitation followed by immunoblotting. To generate protein extracts for immunoprecipitation, samples were lysed for 30 minutes at 4°C on a rotating shaker in 50 mM Tris-HCl pH7.8, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1% Triton X-100, freshly supplemented with 1 mM phenylmethylsufonyl fluoride (PMSF), and a 1% protease inhibitor cocktail. Immunoprecipitates were prepared by incubation of precleared lysates (100 μg of protein per sample) for 2 hours at 4°C with 1.5 μg αvβ5 heterodimer-specific antibody (P1F6 antibody; Abcam) or with control nonimmune mouse Immunoglobulin G (Ig G; Jackson ImmunoResearch Laboratories Inc.), followed by agitation overnight at 4°C in the presence of protein G-agarose beads (Upstate-Millipore, Temecula, CA). After four washes with lysis buffer, samples were eluted by boiling under reducing conditions in SDS-containing electrophoresis buffer followed by Western blot analysis using primary antibodies to the αv or β5 integrin subunits as described above.
Results
Post Confluent ARPE-19 Cultures Express POS Receptor Proteins MerTK and αv and β5 Integrins
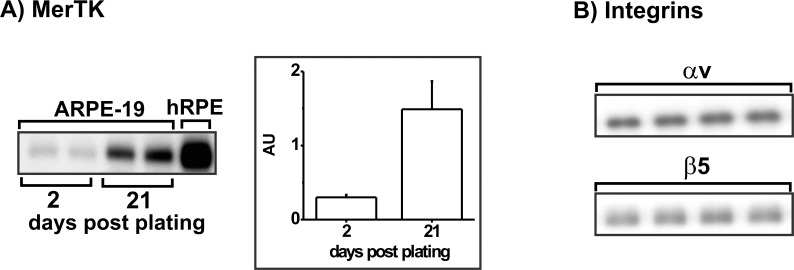
The goal in this investigation was to extend our previous observation that PD treatment reversibly impairs phagocytosis in the cell line ARPE-1913 by testing the hypothesis that phagocytosis receptor proteins are stress susceptible in this model. Since ARPE-19 cells have been reported to lack the receptor MerTK,32 we conducted preliminary experiments to determine expression of our proteins of interest in untreated ARPE-19 cultures, planned for use as baseline controls. Consistent with the earlier report,32 MerTK protein levels were low, but not absent, in confluent ARPE-19 cultures when analyzed by Western blot quantification of extracts prepared from cells at 2 days post plating (Fig. 1A). However, MerTK protein showed a time-in-culture–dependent increase, becoming 5-fold higher in post confluent cultures at day 21 post plating (Fig. 1A). The protein detected in day 21 ARPE-19 cell extracts was confirmed as MerTK by demonstrating co-electrophoresis with MerTK in similarly prepared extracts from cultures of human donor RPE (Fig. 1A). Day 21 ARPE-19 culture extracts prepared for detection of MerTK also contain easily detected levels of αv and β5 integrin subunits (Fig. 1B).
Figure 1.
Western blot analysis of MerTK and integrin subunits αv and β5 in ARPE-19 cultures at two intervals post plating. (A) Left: Western blot of MerTK in extracts of duplicate cultures of ARPE-19 cells at 2 days (lanes 1, 2) and 21 days (lanes 3, 4) post plating. Also shown is MerTK in day 21 cultures of human donor RPE cells (lane 5). Right: quantitative densitometry of the MerTK blotting signals for ARPE-19 blots shown at the left. Data are shown graphically with band density given in arbitrary units (AU); error bars indicate SD. Outcomes differ significantly (paired t-test, P < 0.001). (B) Western blots of αv and β5 integrin subunits in extracts of quadruplicate ARPE-19 cultures on day 21 post plating.
Stress Induced by Sublethal PD Treatment Mediated by MC-540 or RB Transiently Reduces POS Receptor Proteins
Given that our intent was to determine whether PD treatment diminishes POS receptor proteins, we sought to maximize protein abundance under control conditions to increase the likelihood of detecting reductions. Following from the observation above that the proteins slated for analysis are well expressed in day 21 post confluent ARPE-19 cultures, all assays were performed at this time post plating. Several preliminary experiments were performed to confirm that post confluent cultures produced outcomes consistent with those previously obtained in earlier stage cultures.13
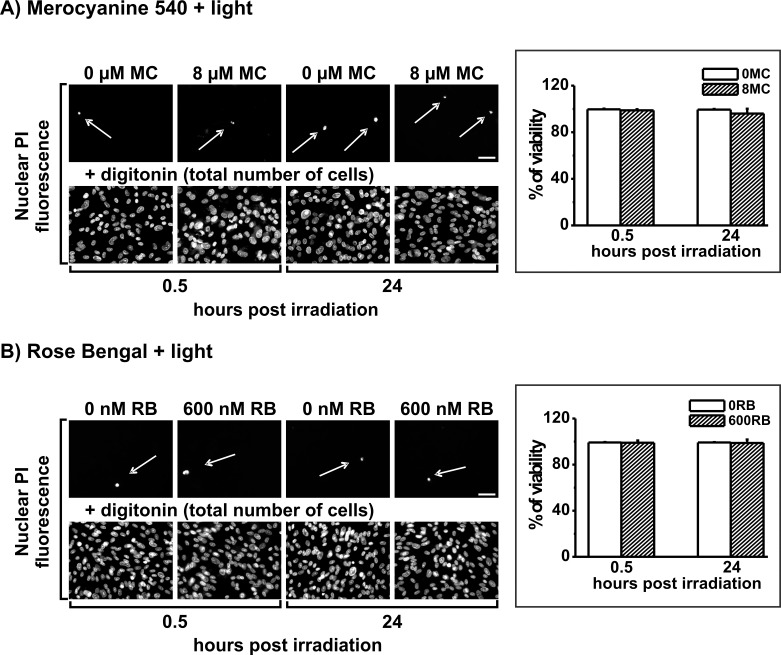
Initial experiments showed that the protocol used previously for subthreshold PD treatment was also sublethal in day 21 cultures: no cytotoxicity was associated with photosensitizer delivery alone or with light irradiation alone (not shown). Combining photosensitizer delivery with light irradiation for PD treatment also elicited no significant cytotoxicity for either photosensitizer (Fig. 2). For PD treatment mediated by MC-540, pretreatment with 8 μM of the agent, followed by 30 minute irradiation with green light, produced no significant lethality when analyzed shortly after irradiation (0.5 hours) or longer term (24 hours) (Fig. 2A). Similar outcomes were observed for PD treatment mediated by RB; pretreatment with 600 nM RB followed by 15 minute irradiation had no significant effect on cell survival at 0.5 hours or 24 hours (Fig. 2B).
Figure 2.
ARPE-19 cell survival after PD treatment mediated by (A) MC-540 or (B) RB determined by image analysis of nuclear PI fluorescence at 0.5 and 24 hours after light irradiation. PI positive nuclei show dead cells without and with each sensitizer at the concentrations indicated (upper rows), and total cells in the field after digitonin permeabilization (lower rows). Shown at right is a graphical display of cell survival quantified from five microscope fields per treatment group for nonsensitized (white bars) or sensitized (hatched bars) cells; data are the mean percent surviving cells (error bars indicate SD). Outcomes without and with PD treatment do not differ significantly for either photosensitizer at either time point (t-test analyses). Scale bar: 20 μm.
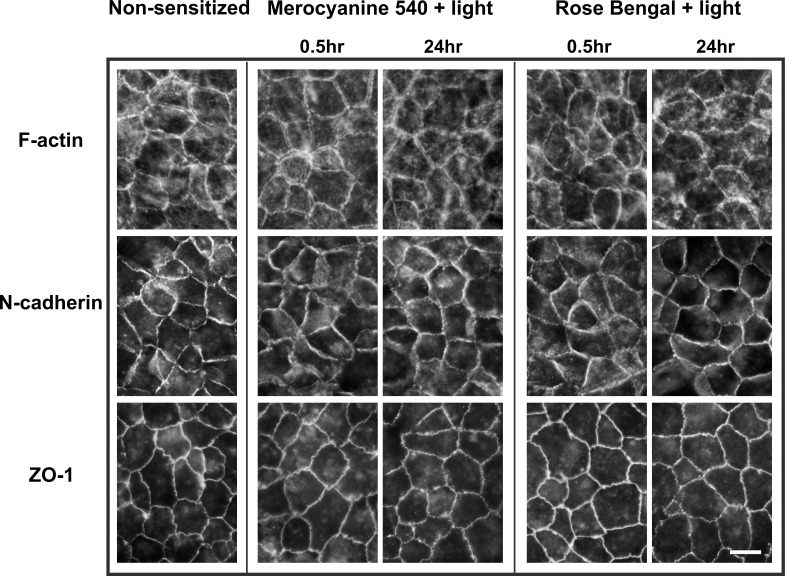
In addition to confirming sublethality, morphologic analyses were performed to determine whether PD treatment produced significant alterations in the distribution of proteins known to be sensitive to redistribution by oxidative stress. Known stress sensitive proteins in the RPE include cell–cell adhesion proteins N-cadherin and ZO-1, and cytoskeletal actin.33,34 None of these proteins showed prominent redistributions in ARPE-19 cells on PD treatment with either photosensitizer (Fig. 3). N-cadherin and ZO-1 demonstrated a junctional distribution in the post confluent cultures prior to and after PD treatment at both 0.5 and 24 hours post irradiation. Similarly, the major F-actin distribution pattern was circumferential both before and after PD treatment. These outcomes indicate that the PD treatment is not only sublethal, but also too mild to elicit prominent morphologic effects.
Figure 3.
Fluorescence microscopy of control (nonsensitized) ARPE-19 cultures, and cultures PD treated using MC-540 or RB and stained for F-actin, N-cadherin, or ZO-1 at 0.5 or 24 hours after irradiation. None of the proteins was prominently redistributed from cell–cell borders by PD treatment with either photosensitizer. Scale bar: 10 μm.
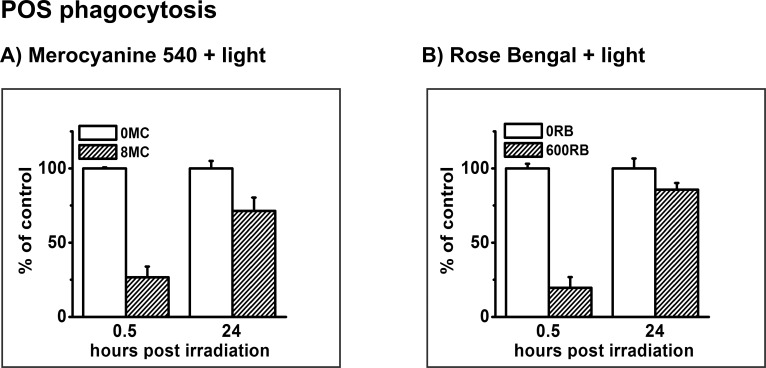
The key observation leading to the study here was that PD treatment produces a transient inhibition of specific POS phagocytosis in ARPE-19 cultures in early confluency13 so phagocytosis experiments were repeated here to confirm similar outcomes with post confluent cultures. Consistent with the results of the previous study, delivery of POS to post confluent cultures at 0.5 hours after irradiation in the presence of either photosensitizer inhibited POS uptake over the subsequent 5.5 hours, and the uptake function was largely recovered when POS delivery occurred at 24 hours (Fig. 4). Specific POS phagocytosis was reduced to 27% of uptake by untreated controls when phagocytosis was initiated 0.5 hours after PD treatment mediated by MC-540, and recovered to 72% when phagocytosis was initiated at 24 hours (Fig. 4A). For RB-mediated PD treatment, POS uptake was reduced to 20% of controls and recovered to 86% of controls when phagocytosis was initiated at 0.5 or 24 hours after irradiation, respectively (Fig. 4B). Further, as previously observed in early stage cultures,13 recoverable inhibition of the phagocytosis of nonspecific particles (polystyrene beads) was also observed in post confluent cultures following PD treatment mediated only by MC-540 (not shown).
Figure 4.
Flow cytometric analysis of specific phagocytic activity of FITC-labeled POS in control (nonsensitized, white bars) or PD-treated cultures (hatched bars), measured 0.5 hours + 5.5 hours and 24 hours + 5.5 hours after light irradiation in the presence of (A) MC-540 or (B) RB. Data are normalized to control cells. Values are means; error bars indicate SD. POS-FITC uptake differed significantly between untreated and PD-treated cultures using both photosensitizers at both time points (t-test analyses, P < 0.05).
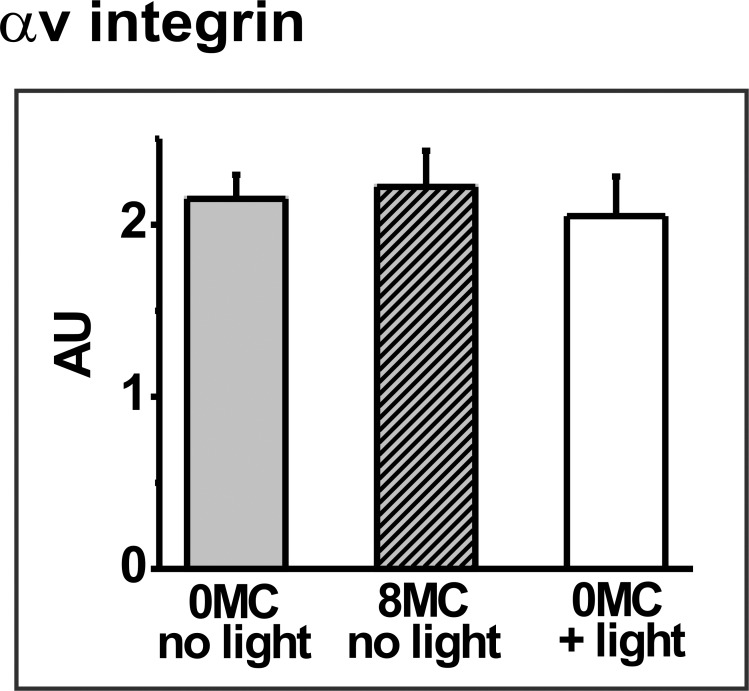
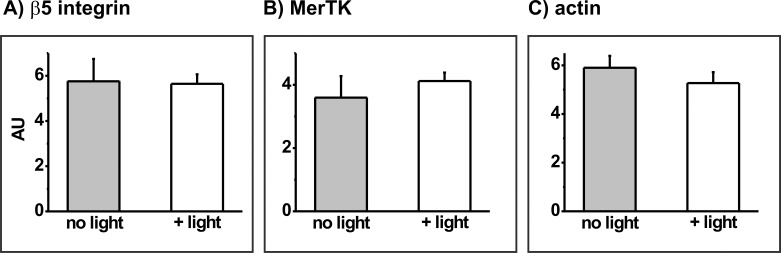
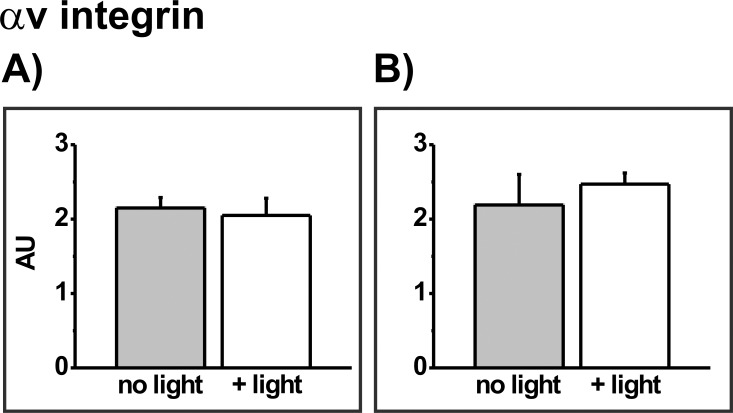
To determine whether mild PD treatment of ARPE-19 cells sufficient to transiently inhibit phagocytosis affects levels of POS receptor proteins, experiments were initially performed to confirm that neither photosensitizer alone, nor light alone, affected either the receptor proteins or the cytoskeletal protein actin. Incubation of cells with 8 μM MC-540 or 600 nM RB in the dark did not affect the abundance of any protein of interest; results for the αv integrin subunit at 0.5 hours after photosensitizer treatment are used for illustration (Fig. 5). Light irradiation alone in the absence of photosensitizer also had no effect on protein abundance; results for the αv integrin subunit are included in Figure 5 and outcomes for the other proteins are shown in Figure 6. Further, no delayed effects of photosensitizer alone or light alone on protein abundance were observed; to illustrate, results for light irradiation alone on the αv integrin subunit at 0.5 and 24 hours after light treatment are compared (Fig. 7).
Figure 5.
Densitometric analysis of the blotting signal for the αv integrin subunit in control (untreated) ARPE-19 cultures (grey bar), in nonirradiated cultures preloaded with 8 μM MC-540 (hatched grey bar), or in cultures light irradiated for 30 minutes without photosensitizer (white bar). Data are the mean band densities, given in AU, from extracts prepared 0.5 hours after light treatment for triplicate culture wells from each group in a representative experiment; error bars indicate SD. Outcomes do not differ significantly (t-test analyses).
Figure 6.
Densitometric analysis of the blotting signal for (A) the β5 integrin subunit, (B) MerTK, or (C) actin in sensitizer-free ARPE-19 cultures without (grey bars) or with light irradiation for 30 minutes (white bars). Data are the mean band densities, given in AU, from extracts prepared 0.5 hours after light treatment of triplicate culture wells from each group in a representative experiment; error bars indicate SD. Outcomes do not differ significantly without or with light irradiation for any protein (t-test analyses).
Figure 7.
Densitometric analysis of the blotting signal for the αv integrin subunit in sensitizer-free ARPE-19 cultures without (grey bars) or with (white bars) light irradiation for 30 minutes to compare extracts taken at (A) 0.5 and (B) 24 hours after light treatment. Data are the mean band densities, given in AU, from extracts of triplicate culture wells from each group in a representative experiment; error bars indicate SD. Outcomes do not differ significantly without or with light irradiation at either time point (t-test analyses).
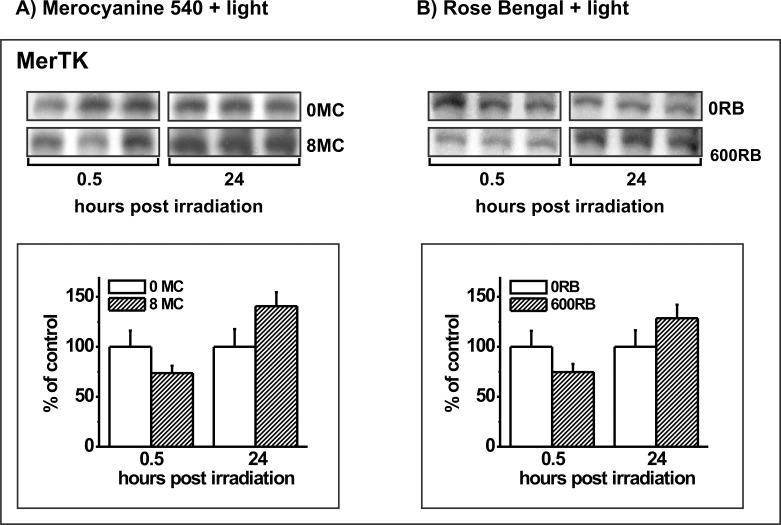
To determine whether photodynamic stress affects POS receptor proteins, ARPE-19 cells lacking photosensitizers and cells preloaded with photosensitizers MC-540 or RB were light irradiated followed by Western blot analysis of extracts after 0.5 or 24 hours. In our previous investigation,13 and confirmed here (Fig. 4), delivery of POS to cells at 0.5 hours post irradiation is followed by impaired phagocytic function, while delivery at 24 hours is followed by recovered function. In order for receptor protein abundance to be a significant contributor to the observed impairment and recovery of phagocytic function, the protein amount has to be affected at or near the time of POS challenge. PD treatment mediated by MC-540 was found to produce an immediate (at 0.5 hours) 27% decrease in the abundance of MerTK relative to nonirradiated cultures (Fig. 8A). An immediate 25% reduction in MerTK protein expression was also seen in cells sensitized with RB (Fig. 8B). MerTK protein levels fully recovered after 24 hours in cells PD treated with either MC-540 or RB (Figs. 8A, 8B).
Figure 8.
Western blot analysis of MerTK protein in ARPE-19 cells lacking photosensitizers (controls) or in cells preloaded with (A) 8 μM MC-540 or (B) 600 nM RB and analyzed 0.5 and 24 hours after light irradiation. Blots from triplicate cultures for each group are shown. Graphs show densitometric analysis of the bands for control (white bars) or PD-treated cultures (hatched bars) with the band densities in the treated groups expressed as a percent of their respective controls. Error bars indicate SD. MerTK signals differ significantly without and with both photosensitizers at the 0.5 hour time point, and without and with MC-540 at the 24 hour time point (t-test analysis, P < 0.05).
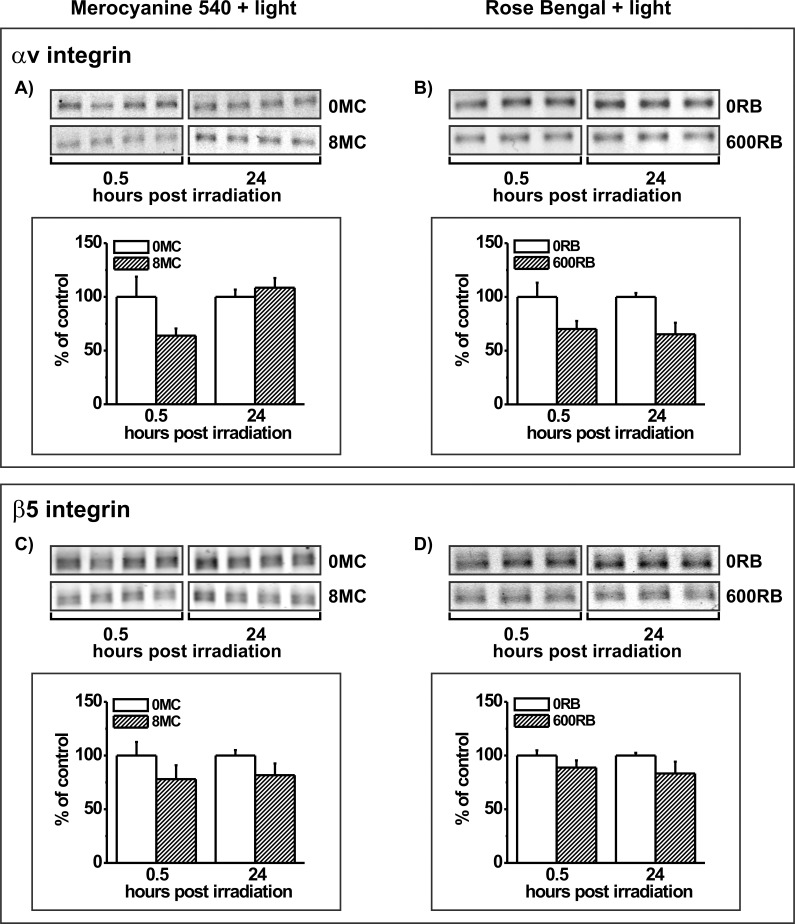
The effects of PD treatment of ARPE-19 cells on integrin receptor proteins differed for the two subunits and from the effects on MerTK. Both integrin subunit proteins were diminished in cells at 0.5 hours after PD treatment with both photosensitizers, but the reduction was greater for the αv subunit (reduced 36% and 30% for MC-540 and RB, respectively) than for the β5 subunit (reduced 20% and 13% for MC-540 and RB, respectively) (Fig. 9). The most interesting difference, however, was in recovery. Full recovery at 24 hours after treatment was seen only for the αv subunit in cells following PD treatment with MC-540 (Fig. 9A). Levels of αv subunit did not recover after PD treatment with RB (Fig. 9B), nor did the small reductions in β5 subunit abundance recover after PD treatment with either MC-540 (Fig. 9C) or RB (Fig. 9D).
Figure 9.
Western blot analysis of (A, B) αv integrin and (C, D) β5 integrin subunits in ARPE-19 cells lacking photosensitizers (controls) or in cells preloaded with (A, C) 8 μM MC-540 or (B, D) 600 nM RB, and analyzed at 0.5 and 24 hours after light irradiation. Blots from triplicate cultures for each group are shown. Graphs show densitometric analysis of the bands for control (white bars) or PD-treated cultures (hatched bars) with the band densities in the treated groups expressed as a percent of their respective controls. Error bars indicate SD. αv integrin signals differ significantly without and with both photosensitizers at the 0.5 hour time point, and without and with RB at 24 hours (t-test analyses, P < 0.05). β5 integrin signals differ significantly without and with both photosensitizers at both time points (t-test analyses, P < 0.05).
Stress Induced by Sublethal Photodynamic Treatment Induces a Transient but Recoverable Reduction in the Functional Integrin Heterodimer
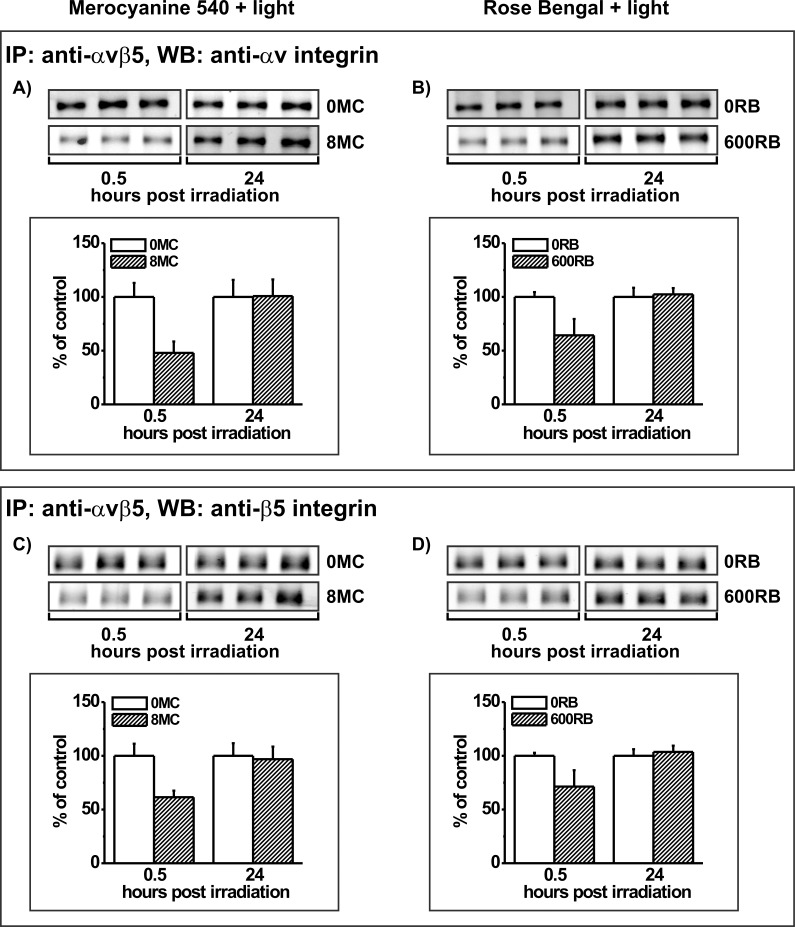
With the exception of the αv integrin subunit after PD treatment mediated by MC-540, integrin subunits failed to recover at 24 hours after being subjected to photic stress (Fig. 9). This observation appears to conflict with our observation that POS phagocytic function, to which the αvβ5 integrin contributes, shows recovery. However, since the functional protein mediating phagocytosis is the heterodimeric form,21,35 we tested the effects of photic stress on the abundance of the functional integrin heterodimer. Immunoprecipitation of the αvβ5 integrin using a heterodimer-specific antibody was performed using extracts from control and PD-treated ARPE-19 cells followed by Western blotting for the integrin subunits (Fig. 10). Predictably from the analyses of individual integrin subunits (Fig. 9), αv and β5 subunits in heterodimers showed reductions at 0.5 hours after PD treatment mediated by both photosensitizers (Fig. 10). The αv integrin in heterodimer immunoprecipitates at 0.5 hours was reduced 53% relative to control levels in cells PD treated with MC-540 (Fig. 10A), and 36% in cells PD treated with RB (Fig. 10B). Respective outcomes for the β5 integrin were 39% reduction using MC-540 (Fig. 10C) and 29% reduction using RB (Fig. 10D). The reductions observed at 0.5 hours were reversible such that by 24 hours, recovery of integrin receptor subunits αv and β5 in functional heterodimers was observed for both photosensitizing protocols (Fig. 10).
Figure 10.
Immunoprecipitates (IP) of the integrin heterodimer αvβ5 followed by Western blotting (WB) for the (A, B) αv integrin or (C, D) β5 integrin subunits. IPs were prepared from ARPE-19 cells lacking photosensitizers (controls) or cells preloaded with (A, C) 8 μM MC-540 or (B, D) 600 nM RB and analyzed 0.5 and 24 hours after light irradiation. Blots from triplicate cultures for each group are shown. Graphs show densitometric analysis of the bands for control (white bars) or PD-treated cultures (hatched bars) with the band densities in the treated groups expressed as a percent of their respective controls. Error bars indicate SD. αv and β5 integrin signals differ significantly without and with photosensitizers at the 0.5 hour time point (t-test analyses, P < 0.05).
Photodynamic Treatment with MC-540 But Not RB Induces Transient Reductions in Actin
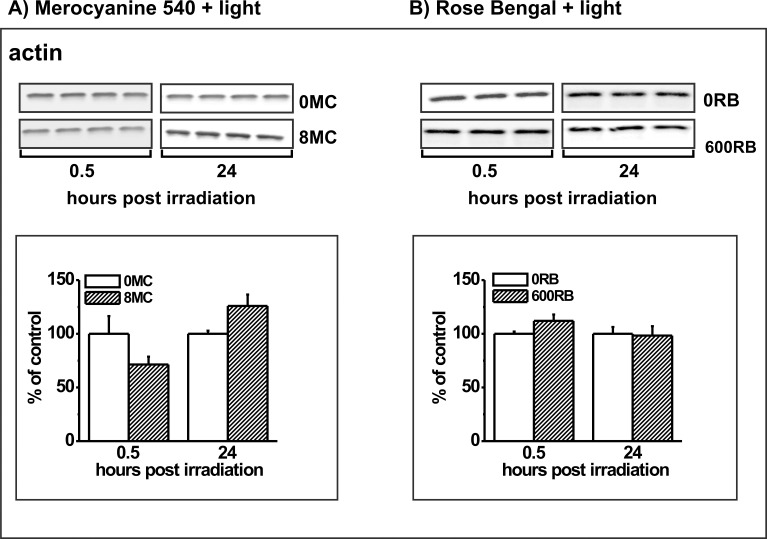
Photodynamic stress mediated by MC-540, but not by RB, reduces the nonspecific phagocytosis of polystyrene beads in addition to the specific phagocytosis of POS in ARPE-19 cells.13 We speculated that the more indiscriminate phagocytosis inhibition by MC-540 might be explained by more widespread photodynamic damage to ARPE-19 cells when using this photosensitizer. We, therefore, tested the effects of PD treatment on widely expressed cytosolic proteins: the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, the antioxidant enzyme catalase, and the cytoskeletal protein β-actin. For all three proteins, PD treatment with MC-540, but not RB, produced transient reductions (at 0.5 hours) that were restored at 24 hours; data for actin are shown (Fig. 11). In this experiment, PD treatment with MC-540 reduced actin levels by 29% (Fig. 11A). Recovery of actin levels at 24 hours often showed increases relative to the 24 hour control; here actin was 25% higher in the PD-treated cultures after recovery (Fig. 11).
Figure 11.
Western blot analysis of β-actin in ARPE-19 cells lacking photosensitizers (controls) or in cells preloaded with (A) 8 μM MC-540 or (B) 600 nM RB and analyzed at 0.5 and 24 hours after light irradiation. Blots from replicate cultures for each group are shown. Graphs show densitometric analysis of the bands for control (white bars) or PD-treated cultures (hatched bars) with the band densities in the treated groups expressed as a percent of their respective controls. Error bars indicate SD. Actin signals differ significantly without and with MC-540 at both time points (t-test analyses, P < 0.05).
Discussion
We previously reported that the specific phagocytosis of POS by ARPE-19 cells is significantly inhibited by sublethal photic stress induced by PD treatment using MC-540 or RB.13 PD treatment with MC-540 also inhibited the nonspecific phagocytosis of polystyrene beads. The inhibitory effects were reversible after 24 hours, suggesting transient stress-induced effects on phagocytic machinery. The investigation here was aimed at determining whether PD stress diminished phagocytosis receptor proteins MerTK and αvβ5 integrin, key molecules involved in POS phagocytosis by RPE cells that functionally interact to coordinate particle uptake.19–22,35,36
The underlying rationale for this study lies in the importance of efficient RPE phagocytosis for photoreceptor survival, and the importance of oxidative stress to the RPE, including photic stress, as a risk factor for AMD. Several studies have shown that deficiencies in the MerTK or αvβ5 integrin receptors abolish efficient POS phagocytosis by RPE cells, and cause retinal degenerations in animal models such as the RCS rat or the β5 null mouse.3,35,37 It is conceivable that sublethal photic stress to the RPE, which has been implicated in the etiology of AMD,4,11,12,38 could promote retinal degeneration in part by rendering phagocytosis less efficient. RPE cells contain multiple endogenous photosensitizers that could mediate light stress; lipofuscin is the most widely recognized,6–8,39 but there are others as well such as retinoids,4 porphyrins or flavins,11,40,41 mitochondrial cytochrome c oxidase,42,43 and melanin.44–46 Since the abundance and subcellular localization of endogenous photosensitizers is variable or unknown, to investigate photic stress and phagocytic function in RPE cells we employed a well-controlled model using ARPE-19 cells and selected photosensitizing dyes,13 taking advantage of their previously described properties.14–18,47,48 Photodynamic damage is usually thought to be mediated by singlet oxygen, but both dyes used here can generate strongly oxidizing radicals that can also play a significant role in oxidation of target molecules.49,50 Other properties of photosensitizing dyes that are particularly useful for the study conducted here are the efficient excitation with green light that is not itself phototoxic, the ability to limit lethality by selection of dye concentration and irradiation time and, importantly, the ability to localize oxidative stress largely to cell membranes where the dyes localize and where phagocytosis occurs.
Prior to initiating this investigation, we considered the question of whether ARPE-19 cells express the MerTK receptor, which appears to vary in expression depending upon culture conditions.32,51 The expression of cell type-specific proteins is often heterogeneous in cultured RPE cells and in many cases dependent upon time in culture, which determines the extent of culture differentiation.52 We, therefore, asked whether MerTK protein in ARPE-19 cells is sensitive to culture time and found that the levels of MerTK protein expression increase in post confluency. ARPE-19 cells, therefore, have value for studying receptor-mediated POS phagocytosis, especially if post confluent cultures are used as was done here.
Aside from its importance in mediating specific POS phagocytosis by RPE, a rationale for considering that photodynamic stress may affect MerTK is a recent report indicating that this receptor protein is sensitive to oxidative stress induced by another treatment, subthreshold exposure to H2O2, which also inhibited the phagocytic function of ARPE-19 cells.53 The mechanism of inhibition in this model was attributed to blockade of focal adhesion kinase (FAK) and MerTK signaling.53 MerTK abundance was apparently not affected53 and it was not clear whether loss of MerTK function was due to stress-induced changes in the receptor protein itself or to stress-induced mitochondrial dysfunction,54,55 leading to local reductions in energy-dependent activities such as receptor phosphorylation. Here, we, therefore, focused on the question of whether photodynamic stress affects the abundance of receptor proteins MerTK, the individual αv and β5 integrin subunits, and the intact integrin heterodimer αvβ5 in a time frame that could explain our observed photic, stress-induced phagocytosis inhibition. Receptor proteins were found to be reduced by 0.5 hours after irradiation, which coincides with the time of onset of phagocytosis in the previous and current investigation that showed inhibition of phagocytic activity.13 Receptor proteins were further shown to recover by 24 hours after irradiation, the time at which phagocytic function was restored. MerTK protein showed full recovery and both integrin subunits showed sufficient recovery to support full recovery of the functional integrin heterodimer. It, therefore, appears that RPE phagocytosis receptors are sensitive to oxidative stress induced by PD treatment, and that the transient depletion of receptor proteins MerTK and αvβ5 integrin contributes to stress-induced impairments in POS phagocytosis.
It remains to be determined how PD stress produces diminution of the receptor proteins, but it is unlikely that the outcome results from diminished synthesis since the proteins were rapidly depleted, showing significant reductions within 30 minutes. Perhaps a more likely explanation is that oxidative stress induced oxidative modifications in the receptor proteins affecting their ability to mediate POS phagocytosis and targeting them for degradation. At this time, there is no evidence to indicate that POS receptors are specifically susceptible to oxidative modification. Protein oxidation increases with RPE aging and in the β5−/− mouse several proteins involved in POS phagocytosis (αv, β5, CD36, MerTK) were analyzed for 4-hydroxynonenal (HNE) adduct formation, but none was detected.56 Other types of oxidative modifications in phagocytosis receptor proteins have not yet been explored. It should also be noted that transient diminution of receptor protein abundance may not be the only mechanism whereby PD stress produces transient inhibition of phagocytic function. Photic stress may also affect MerTK phosphorylation as reported for H2O2-induced stress,53 but diminished phosphorylation is difficult to show conclusively on a background of diminished total receptor protein, and if found could be indirect, resulting from stress effects on energy production rather direct effects on the receptor protein itself. PD stress could also reduce phagocytosis efficiency by affecting receptor distribution. However, the PD stress protocol used here is too mild to produce morphologically detectable changes in the distribution of membrane-associated (N-cadherin, ZO-1) or cytosolic proteins (F-actin) that are known to be sensitive to stress-induced reorganization.33,34
In our earlier study of phagocytosis inhibition by PD treatment, the photosensitizer MC-540 had greater effects than RB, producing inhibition of the nonspecific phagocytosis of beads in addition to the specific phagocytosis of POS.13 The difference in outcome could be due to the cellular localization of the photosensitizers.14–18 Both MC-540 and RB localize to membranes, notably surface membranes, which presumably accounts for the ability of PD treatment using both dyes to affect membrane receptors mediating phagocytosis. Additionally, MC-540 is more competent to penetrate to intracellular membranes, which would increase the likelihood of affecting cytosolic proteins as well. Using PD treatment with MC-540, we observed reductions in cytosolic proteins including β-actin. Actin is particularly relevant here because this cytoskeletal protein is known to participate in RPE phagocytosis,57,58 and disruption of actin microfilaments, as has been shown to occur during rubella virus infection of cultured RPE cells, for example, renders cells defective in the phagocytosis of latex beads.59 Reductions in actin due to treatment using MC-540 could, therefore, contribute to our observation of inhibited nonspecific phagocytosis using this dye, but given its widespread distribution, MC-540 could also mediate the loss of other stress-sensitive proteins that participate in particle uptake.
Overall we conclude that POS receptor proteins MerTK and αvβ5 integrin in ARPE-19 cells are susceptible to sublethal photodynamic oxidative stress. The proteins show a transient reduction and recovery after PD treatment that coincides with a transient reduction and recovery in phagocytic function. The observations support the hypothesis that chronic photic stress to the RPE in situ could reduce phagocytic efficiency, which over time would have consequences for the health and survival of retinal photoreceptors that depend upon efficient RPE phagocytosis during the essential process of photoreceptor renewal.
Footnotes
Supported by Grants R01EY019664 and P30EY01931 from the National Eye Institute (JMB), Grants 2661/B/P01/2010/39 from the Poland National Science Centre, and Grant 3P04A00825 from the Poland State Committee for Scientific Research (TS), and an unrestricted grant from Research to Prevent Blindness, Inc. This investigation was conducted in a facility constructed with support from National Center for Research Resources Grant C06 RR-RR016511 from the National Institutes of Health. The Faculty of Biochemistry, Biophysics, and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from European Union Grant POIG.02.01.00-12-064/08 – ‘Molecular biotechnology for health.'
Disclosure: M.M. Olchawa, None; A.M. Herrnreiter, None; C.M.B. Skumatz, None; M. Zareba, None; T.J. Sarna, None; J.M. Burke, None
References
- 1. Bok D, Young RW. Phagocytic properties of the retinal pigment epithelium. In: Zinn KM, Marmor MF. eds The Retinal Pigment Epithelium. Cambridge, MA: Harvard University Press; 1979: 148–174 [Google Scholar]
- 2. LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976; 194: 1071–1074 [DOI] [PubMed] [Google Scholar]
- 3. Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976; 192: 799–801 [DOI] [PubMed] [Google Scholar]
- 4. Hunter JJ, Morgan JI, Merigan WH, Sliney DH, Sparrow JR, Williams DR. The susceptibility of the retina to photochemical damage from visible light. Prog Retin Eye Res. 2012; 31: 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Organisciak DT, Sarna T. Genetic age and light induced degenerations of the retina and retinal pigment epithelium. In: Coohill TP, Valenzeno DP. eds Photobiology for the 21st Century. Overland Park, Kansas: Valdenmar Publishing Company; 2001: 81–100 [Google Scholar]
- 6. Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993; 19: 201–204 [DOI] [PubMed] [Google Scholar]
- 7. Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995; 270: 18825–18830 [DOI] [PubMed] [Google Scholar]
- 8. Wassell J, Davies S, Bardsley W, Boulton M. The photoreactivity of the retinal age pigment lipofuscin. J Biol Chem. 1999; 274: 23828–23832 [DOI] [PubMed] [Google Scholar]
- 9. Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000; 97: 7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998; 19: 241–247 [DOI] [PubMed] [Google Scholar]
- 11. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115–134 [DOI] [PubMed] [Google Scholar]
- 12. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999; 5: 32 [PMC free article] [PubMed] [Google Scholar]
- 13. Olchawa M, Szewczyk G, Zareba M, et al. Sub-lethal photodynamic damage to ARPE-19 cells transiently inhibits their phagocytic activity. Photochem Photobiol. 2010; 86: 772–780 [DOI] [PubMed] [Google Scholar]
- 14. Croce AC, Wyroba E, Bottiroli G. Distribution and retention of rose bengal and disulphonated aluminium phthalocyanine: a comparative study in unicellular eukaryote. J Photochem Photobiol. 1992; 16: 319–330 [DOI] [PubMed] [Google Scholar]
- 15. Easton TG, Valinsky JE, Reich E. Merocyanine 540 as a fluorescent probe of membranes: staining of electrically excitable cells. Cell. 1978; 13: 475–486 [DOI] [PubMed] [Google Scholar]
- 16. Kochevar IE, Bouvier J, Lynch M, Lin CW. Influence of dye and protein location on photosensitization of the plasma membrane. Biochim Biophys Acta. 1994; 1196: 172–180 [DOI] [PubMed] [Google Scholar]
- 17. Sieber F. Merocyanine 540. Photochem Photobiol. 1987; 46: 1035–1042 [DOI] [PubMed] [Google Scholar]
- 18. Sieber F. Elimination of residual tumor cells from autologous bone marrow grafts by dye-mediated photolysis: preclinical data. Photochem Photobiol. 1987; 46: 71–76 [DOI] [PubMed] [Google Scholar]
- 19. D'Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000; 9: 645–651 [DOI] [PubMed] [Google Scholar]
- 20. Lin H, Clegg DO. Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998; 39: 1703–1712 [PubMed] [Google Scholar]
- 21. Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997; 94: 12932–12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miceli MV, Newsome DA, Tate DJ Jr. Vitronectin is responsible for serum-stimulated uptake of rod outer segments by cultured retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997; 38: 1588–1597 [PubMed] [Google Scholar]
- 23. Nandrot EF, Chang Y, Finnemann SC. Alphavbeta5 integrin receptors at the apical surface of the RPE: one receptor, two functions. Adv Exp Med Biol. 2008; 613: 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson DH, Johnson LV, Hageman GS. Vitronectin receptor expression and distribution at the photoreceptor-retinal pigment epithelial interface. J Comp Neurol. 1995; 360: 1–16 [DOI] [PubMed] [Google Scholar]
- 25. Finnemann SC, Silverstein RL. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001; 194: 1289–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007; 104: 12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002; 277: 17016–17022 [DOI] [PubMed] [Google Scholar]
- 28. Chaitin MH, Hall MO. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci. 1983; 24: 812–820 [PubMed] [Google Scholar]
- 29. Burke JM, Skumatz CM. Autofluorescent inclusions in long-term postconfluent cultures of retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998; 39: 1478–1486 [PubMed] [Google Scholar]
- 30. Youn YH, Hong J, Burke JM. Endogenous N-cadherin in a subpopulation of MDCK cells: distribution and catenin complex composition. Exp Cell Res. 2005; 303: 275–286 [DOI] [PubMed] [Google Scholar]
- 31. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680–685 [DOI] [PubMed] [Google Scholar]
- 32. Carr AJ, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009; 15: 283–295 [PMC free article] [PubMed] [Google Scholar]
- 33. Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45: 675–684 [DOI] [PubMed] [Google Scholar]
- 34. Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun. 2006; 342: 372–378 [DOI] [PubMed] [Google Scholar]
- 35. Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004; 200: 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003; 22: 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962; 14: 73–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cruickshanks KJ, Klein R, Klein BE. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch Ophthalmol. 1993; 111: 514–518 [DOI] [PubMed] [Google Scholar]
- 39. Rozanowska M, Wessels J, Boulton M, et al. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998; 24: 1107–1112 [DOI] [PubMed] [Google Scholar]
- 40. Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006; 84: 4–15 [DOI] [PubMed] [Google Scholar]
- 41. Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006; 51: 461–481 [DOI] [PubMed] [Google Scholar]
- 42. Putting BJ, van Best JA, Zweypfenning RC, Vrensen GF, Oosterhuis JA. Spectral sensitivity of the blood-retinal barrier at the pigment epithelium for blue light in the 400-500 nm range. Graefes Arch Clin Exp Ophthalmol. 1993; 231: 600–606 [DOI] [PubMed] [Google Scholar]
- 43. Putting BJ, van Best JA, Vrensen GF, Oosterhuis JA. Blue-light-induced dysfunction of the blood-retinal barrier at the pigment epithelium in albino versus pigmented rabbits. Exp Eye Res. 1994; 58: 31–40 [DOI] [PubMed] [Google Scholar]
- 44. Sarna T. Properties and function of the ocular melanin–a photobiophysical view. J Photochem Photobiol B. 1992; 12: 215–258 [DOI] [PubMed] [Google Scholar]
- 45. Sarna T, Burke JM, Korytowski W, et al. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res. 2003; 76: 89–98 [DOI] [PubMed] [Google Scholar]
- 46. Zadlo A, Burke JM, Sarna T. Effect of untreated and photobleached bovine RPE melanosomes on the photoinduced peroxidation of lipids. Photochem Photobiol Sci. 2009; 8: 830–837 [DOI] [PubMed] [Google Scholar]
- 47. Kalyanaraman B, Feix JB, Sieber F, Thomas JP, Girotti AW. Photodynamic action of merocyanine 540 on artificial and natural cell membranes: involvement of singlet molecular oxygen. Proc Natl Acad Sci U S A. 1987; 84: 2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valenzeno DP, Trudgen J, Hutzenbuhler A, Milne M. Singlet oxygen involvement in photohemolysis sensitized by merocyanine-540 and rose bengal. Photochem Photobiol. 1987; 46: 985–990 [DOI] [PubMed] [Google Scholar]
- 49. Lambert C, Sarna T, Truscott TG. Rose bengal radicals and their activity. J Chem Soc Faraday Trans. 1990; 86: 3879–3882 [Google Scholar]
- 50. Sarna T, Pilas B, Lambert C, Land EJ, Truscott TG. The role of free-radicals in photosensitized oxidation reactions induced by merocyanine 540. J Photochem Photobiol A: Chem. 1991; 58: 339–347 [Google Scholar]
- 51. Hall MO, Abrams TA, Burgess BL. Integrin alphavbeta5 is not required for the phagocytosis of photoreceptor outer segments by cultured retinal pigment epithelial cells. Exp Eye Res. 2003; 77: 281–286 [DOI] [PubMed] [Google Scholar]
- 52. Burke JM. Epithelial phenotype and the RPE: is the answer blowing in the Wnt? Prog Retin Eye Res. 2008; 27: 579–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qin S, Rodrigues GA. Roles of alphavbeta5, FAK and MerTK in oxidative stress inhibition of RPE cell phagocytosis. Exp Eye Res. 2012; 94: 63–70 [DOI] [PubMed] [Google Scholar]
- 54. Ballinger SW, Van HB, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999; 68: 765–772 [DOI] [PubMed] [Google Scholar]
- 55. Godley BF, Jin GF, Guo YS, Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to H(2)O(2). Exp Eye Res. 2002; 74: 663–669 [DOI] [PubMed] [Google Scholar]
- 56. Yu CC, Nandrot EF, Dun Y, Finnemann SC. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking alphavbeta5 integrin. Free Radic Biol Med. 2012; 52: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burnside B, Laties AM. Actin filaments in apical projections of the primate pigmented epithelial cell. Invest Ophthalmol. 1976; 15: 570–575 [PubMed] [Google Scholar]
- 58. Burnside MB. Possible roles of microtubules and actin filaments in retinal pigmented epithelium. Exp Eye Res. 1976; 23: 257–275 [DOI] [PubMed] [Google Scholar]
- 59. Williams LL, Lew HM, Shannon BT, et al. Phagocytosis of latex beads is defective in cultured human retinal pigment epithelial cells with persistent rubella virus infection. Am J Pathol. 1993; 142: 451–461 [PMC free article] [PubMed] [Google Scholar]