Abstract
Purpose.
To investigate whether high glucose (HG) alters expression of connexin 30.2 (Cx30.2) and influences gap junction intercellular communication (GJIC) in retinal endothelial cells and promotes vascular lesions characteristic of diabetic retinopathy (DR).
Methods.
Western blot analysis and immunostaining were performed to determine Cx30.2 protein expression and localization in rat retinal endothelial cells (RRECs) grown in normal (N; 5 mM) or HG (30 mM) medium for 7 days. Concurrently, GJIC was assessed in cells grown in N or HG medium and in cells transfected with Cx30.2 siRNA. Similarly, retinal Cx30.2 expression was assessed in nondiabetic and diabetic rats. Additionally, the effect of reduced Cx30.2 on development of acellular capillaries (ACs) and pericyte loss (PL) was studied in retinas of Cx30.2 knockout mice.
Results.
Cx30.2 was identified in RRECs in vitro and in vascular cells of retinal capillaries. RRECs grown in HG exhibited significantly reduced Cx30.2 protein levels consistent with decreased Cx30.2 immunostaining compared with those grown in N medium. Cells grown in HG and cells transfected with Cx30.2 siRNA exhibited significantly diminished dye transfer compared with N or nontransfected cells. Importantly, Cx30.2 protein level and immunostaining were decreased in diabetic retinas compared with nondiabetic retinas. Retinal capillaries of Cx30.2 knockout mice exhibited increased numbers of ACs and PL compared with those of wild-type mice.
Conclusions.
These results indicate that HG- or diabetes-induced downregulation of Cx30.2 expression and decrease in GJIC activity play a critical role in the development of retinal vascular lesions in early DR.
The study reports a novel finding that diabetes-induced downregulation of a gap junction protein, connexin 30.2, and subsequent reduction in cell–cell communication may play an important role in triggering retinal vascular cell loss, a hallmark of early diabetic retinopathy.
Introduction
Diabetic retinopathy (DR) is a leading cause of vision impairment and blindness in adults.1–3 A prominent characteristic lesion of early DR is apoptotic cell loss in the retinal microvasculature.4 Studies have shown that cell–cell communication is necessary for maintenance of retinal vascular homeostasis, and that impaired intercellular communication may contribute to apoptosis in retinal vascular cells.5 However, it is unknown whether changes in Cx30.2 expression and gap junction intercellular communication (GJIC) activity participate in retinal pathology associated with the development and progression of DR.
Connexins form gap junction channels that connect the cytoplasms of adjacent cells and enable direct passage of molecules such as ions, metabolites, and second messengers less than 1 kD.6 The connexin protein family consists of 20 distinct members in the mouse genome and 21 members in the human genome,7 some of which exhibit tissue-specific localization.8 The presence of gap junctions in the retinal microvasculature has been previously documented.9 Although four different connexins, Cx37, Cx40, Cx43, and Cx45, have been documented in different layers of the retinal vasculature, their presence has been shown to vary between vascular beds, between species, and during development.10,11 In the rodent retina, it has been demonstrated that Cx43 and Cx37 form extensive gap junction channels that facilitate cell-to-cell coupling of the microvasculature and promote maintenance of retinal vascular homeostasis.12–15
Recently, it has been shown that Cx30.2, also known as gap junction protein delta 3 (GJD3), is expressed in the mouse retina.16 The murine Cx30.2 consists of 278 amino acids and has four transmembrane regions similar to other connexins.17 It shares 79% identical residues and 84% similar residues with the human Cx31.9,17 and is expressed in vascular smooth muscle, heart, testes, brain,17 and retina.16 Additionally, a study has shown that Cx30.2 may facilitate coupling of retinal ganglion and amacrine cells.16
Chronic hyperglycemia in diabetes is associated with the development and progression of pathologic changes in the retinal capillaries involving Cx43 downregulation. Our previous studies indicate that the high-glucose (HG) condition reduces Cx43 expression and impairs cell–cell communication in retinal pericytes and endothelial cells,18,19 and that HG-induced downregulation of Cx43 expression can trigger apoptosis in retinal endothelial cells.5 Other tissues such as corpus cavernosum exhibit reduced Cx43 in diabetes20 and decreased expression of two connexins, Cx30 and Cx43, has been reported in the inferior colliculus of diabetic rats.21
Most cells in general express more than one connexin. Since Cx30.2 has been reported to be expressed in the vasculature of different tissues, in this study, we determined whether Cx30.2 is expressed in the retinal vasculature and whether HG alters its expression and GJIC activity. Currently, it is unknown whether high glucose or diabetes alters Cx30.2 expression, and whether subsequent changes in GJIC activity contributes to retinal pathology in diabetic retinas. Additionally, we investigated whether Cx30.2 expression is altered in an animal model of diabetes, and studied the effects of Cx30.2 downregulation in the retinal vasculature of Cx30.2 knockout mice.
Materials and Methods
Cell Culture
Rat retinal endothelial cells (RRECs) confirmed positive for von Willebrand factor (vWF), an endothelial cell marker,22 were used in this study, and were isolated as described.23 Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco/Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis, MO), antibiotics, and antimycotics. Experiments were performed on cells from passages three to six. To examine the localization of Cx30.2 and the effect of HG on its expression in RRECs, cells were grown on coverslips and 35-mm dishes in normal (N) or HG medium for 7 days until confluency. Cells were then subjected to immunostaining and total protein analyzed using Western blot (WB) analysis. To assess GJIC activity, RRECs were grown on coverslips in N or HG medium, and a subset of cells was transfected with small interfering RNA (siRNA) after 5 days in culture. Scrape load dye transfer (SLDT) was performed on day 6 or day 7, when cells reached confluency. Each experiment was repeated at least three times.
Immunofluorescence Microscopy
Cells grown on coverslips in either N or HG medium were fixed with ice-cold methanol for 30 seconds and blocked with 2% bovine serum albumin (BSA; Invitrogen, Carlsbad, CA) in 1xPBS for 20 to 30 minutes to prevent nonspecific antibody binding. Next, cells were incubated overnight in a moist chamber at 4°C with 2% BSA–PBS primary antibody solution containing rabbit anti-Cx30.2T17 at 1:250 dilution. After incubation, cells were washed with 1xPBS and placed in a dark chamber at 37°C for 2 hours in 2% BSA–PBS secondary antibody solution containing rhodamine-conjugated antirabbit IgG (Jackson ImmunoResearch, West Grove, PA) at 1:600 dilution. Cells were then washed with 1xPBS and mounted in a commercial antifade reagent (SlowFade; Molecular Probes/Invitrogen, Carlsbad, CA). To stain the nuclei, 0.9% 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Indianapolis, IN) was applied during mounting. Negative control samples were processed in the same manner without addition of the primary antibody. Digital images of the cells were captured with a digital camera (Nikon F1; Nikon, Tokyo, Japan) attached to a fluorescence microscope (Nikon Diaphot; Nikon). Cx43 signals appeared as fluorescent punctate dots and were present between adjacent retinal microvascular endothelial cells. At least 10 random fields were scored for the punctate dots from each sample, and fluorescence intensity was assessed using NIH ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available in the public domain at http://rsbweb.nih.gov/ij/index.html).
Cell Protein Isolation and Western Blot Analysis
Total protein was isolated from RRECs grown in either N or HG medium as described.24 Briefly, samples were washed with PBS and lysed with buffer containing 10 mM Tris (pH 7.4) (Sigma), 1 mM EDTA, and 0.1% Triton X-100 (Sigma). Samples were homogenized and protein content was determined using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Proteins were separated by electrophoresis in 10% SDS-polyacrylamide gel (American Bioanalytical, Natick, MA). Samples were then transferred onto PVDF membranes, blocked with 5% nonfat dry milk in Tris-buffered saline (TBS; American Bioanalytical) for 2 hours, and incubated overnight at 4°C with rabbit anti-Cx30.2T17 at 1:300 dilution. Membranes were washed with TBS containing 0.1% Tween-20 (TTBS) and incubated with secondary anti-rabbit IgG conjugated with alkaline phosphatase (Cell Signaling, Danvers, MA). After a second set of washings with TTBS, protein levels of Cx30.2 were detected with a detection system for protein analysis (ImmunoStar Chemiluminescent Protein Detection System; BioRad, Hercules, CA). Densitometric analysis of WB signals was performed at nonsaturating exposures using NIH ImageJ software (National Institutes of Health).
Real-Time PCR Analysis
Cx30.2 mRNA expression was determined in retinal endothelial cells grown in either normal or HG medium using RT-PCR. Briefly, total RNA was extracted using a commercial kit for fast purification of high-quality total RNA (RNeasy Mini Kit; Qiagen, Valencia, CA). For reverse transcription, 1 μg of the total RNA was converted to first-strand complementary DNA in 20 μL reactions using a commercial kit (AffinityScript Q-PCR cDNA Synthesis Kit; Agilent Technologies, La Jolla, CA), which was subsequently diluted five times. For PCR sample preparation, 2 μL of diluted cDNA was mixed in 20 μL reaction volume with 0.5 μM primer and SYBR master enzyme mix (SABiosciences, Frederick, MD). The reaction was initiated at 95°C for 10 minutes, followed by 40 cycles through 95°C for 15 seconds and 60°C for 1 minute. All reactions were performed in duplicate and normalized to the expression of an endogenous housekeeping gene hypoxanthine phosphoribosyl transferase (HPRT). All CT values were in the range of 25 to 30 cycles. Amplification curves were analyzed using commercial sequence detection system software (SDS version 1.9.1 software; Applied Biosystems, Foster City, CA). The forward and reverse primers used for PCR amplification of Cx30.2 target cDNA were 5′-TCA TGC TGA TCT TCC GCA TCC-3′ and 5′-GAA GCG GTA GTG GGA CACC-3′, respectively, with a product size of 148 bp.
Cell Transfection
RRECs grown on coverslips in N medium were transfected with Cx30.2 siRNA (product: Rn_RGD:1,308,942_3, gene accession: XM_001,081,399; Qiagen, Valencia, CA) or scrambled siRNA (AllStars Negative Control siRNA; Qiagen) after 5 days. Cells were transfected at 70% to 80% confluency in reduced serum medium (Opti-MEM; Invitrogen) in the presence of 8 μM of a transfection reagent (Lipofectin; Gibco, Grand, Island, NY) and either 0.04 μM Cx30.2 siRNA or 0.04 μM scrambled siRNA for 4 hours. Cells were then returned to N medium and analyzed 2 days after transfection. The target sequences for Cx30.2 siRNA and scrambled siRNA were 5′-CTG GCT GGT GAT CAT GCT GAT-3′ and 5′-CAG GGT ATC GAC GAT TAC AAA-3′, respectively.
SLDT Assay
To assess the effects of high glucose on GJIC activity, SLDT assay was used following the procedure described by el-Fouly et al.25 Four groups of cells were analyzed: cells grown in N medium, those grown in HG medium, and those grown in N medium and transfected with either Cx30.2 siRNA or scrambled siRNA. Briefly, confluent RRECs on coverslips were washed with 1xPBS containing 0.01% Ca2+ and Mg2+. Next, several random cuts were made on cell monolayers using a sharp razor blade. Cells were exposed to a solution containing 0.2% DAPI and washed with 1xPBS containing 0.01% Ca2+ and Mg2+. Cells were then fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), mounted with a commercial antifade reagent (SlowFade; Molecular Probes/Invitrogen), and photographed with a digital camera (Nikon F1; Nikon) attached to a fluorescence microscope (Nikon Diaphot; Nikon). The number of dye-coupled cell layers on either side of the scrape was counted in at least five random areas from each SLDT preparation to evaluate GJIC activity.
Diabetic Rat Model and Cx30.2 Knockout Mice
All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. In general, eyes destined for retinal trypsin digests (RTDs) were stored in 4% paraformaldehyde, whereas those destined for WB were immediately subjected to retinal isolation and protein extraction. Retinas from six 7-month-old Sprague-Dawley (SD; Charles River Laboratories, Wilmington, MA) rats and six diabetic rats of the same age with 3 months of diabetes were used in this study for assessment of Cx30.2 immunostaining and Cx30.2 protein expression by WB. Diabetes was induced in six SD rats, by injecting streptozotocin intraperitoneally at a dose of 55 mg/kg body weight. The diabetic animals were subcutaneously injected with an intermediate-acting insulin (Neutral Protamine Hagedorn [NPH]) as needed to achieve slow body weight gain without preventing hyperglycemia and glycosuria. Serum glucose and body weight were monitored every other day, and glycosylated hemoglobin was measured using a real-time A1c results kit (A1cNow kit; Bayer, Tarrytown, NY) after 3 months of diabetes. To examine the effect of diabetes on Cx30.2 protein expression, the retinal protein from the diabetic retinas and those of the control nondiabetic retinas were subjected to WB analysis for Cx30.2 protein expression, as described above for RRECs. Retinas from 9-month-old Cx30.2 homozygous KO mice (Nielsen PA, Kumar NM, unpublished data, 2003) and those of age-matched wild-type mice were used in these studies to examine the effect of reduced Cx30.2 expression on the development of AC and PL.
Retinal Capillary Network Isolation: Retinal Trypsin Digest Technique
RTDs were performed to analyze the distribution of Cx30.2 and formation of ACs and PL in retinal vessels of wild-type mice, Cx30.2 KO mice, nondiabetic rats, and diabetic rats. The technique was performed as previously described.26 Briefly, retinas were isolated from enucleated eyes, fixed in formalin, and stored in 0.15 M glycine buffer solution at 4°C for 24 to 48 hours. Retinas were then digested in 3% trypsin buffer solution at 37°C for 1.5 to 3 hours with gentle shaking. After the digestion, nonvascular components were separated from the vascular network under a dissecting microscope (Stereo Zoom Dissecting Microscope; Bausch & Lomb, Rochester, NY) using microtweezers and fine single-haired brushes (Ted Pella Inc., Redding, CA). Once isolated, the capillary networks were mounted on silane-coated slides, and further processed for immunostaining and/or morphologic analysis.
RTD Immunostaining
Analogous to the cell culture protocol, RTDs of wild-type and diabetic animals were fixed with methanol for 30 seconds, blocked with 2% BSA–PBS for 4 hours, and incubated with primary antibody solution containing rabbit anti-Cx30.2T17 at 1:100 dilution, followed by secondary antibody solution containing rhodamine-conjugated anti-rabbit IgG at 1:100 dilution. RTDs were then washed and mounted with an antifade reagent (SlowFade; Molecular Probes/Invitrogen). Representative digital images were captured with a digital camera (Nikon F1; Nikon) attached to a fluorescence microscope (Nikon Diaphot; Nikon). Six to ten random fields were analyzed from each RTD preparation. Experiments were repeated two times.
Assessment of Acellular Capillaries and Pericyte Loss
To analyze the effect of Cx30.2 downregulation on retinal capillaries, RTDs of wild-type and Cx30.2 knockout mice were stained with hematoxylin and examined for ACs and PL. Briefly, RTD slides were rinsed with dH2O and immersed in Harris hematoxylin (Sigma) for 2 minutes. Slides were then rinsed with dH2O and subjected to dehydration via an ethanol gradient followed by clearing with xylene. Mounting was performed using mounting medium (Permount; Fisher Scientific, Pittsburgh, PA) and representative images were photographed with a digital camera (Nikon F1; Nikon), attached to a fluorescence microscope (Nikon Diaphot; Nikon). An average of six random fields were scored for ACs and PL from each RTD preparation. ACs were identified as capillaries devoid of endothelial cells and pericytes. PL was identified as vacant spaces in the capillary basement membrane formerly occupied by pericyte nuclei. Experiments were repeated six times.
Statistical Analysis
Data were expressed as mean ± SD. Control group values represented 100% and all other values were expressed as percentages of the control. Comparisons between groups were performed with the t-test, where P < 0.05 was considered to be statistically significant.
Results
High Glucose Downregulates Cx30.2 Expression In Vitro
To determine if Cx30.2 is expressed in retinal endothelial cells and assess if its expression is altered by high glucose, immunofluorescence microscopy was used to assess the presence and distribution of Cx30.2 in RRECs. Immunostaining showed diffuse Cx30.2 protein localization in the cytoplasm and a brighter “halo-like” distribution around the cell periphery of RRECs grown in N medium (Fig. 1). The overall Cx30.2 immunostaining was reduced in the HG cells compared with N cells (79 ± 11% of control, P < 0.002, n = 6) (Fig. 2). Similarly, Cx43 immunostaining performed as double immunolabeling showed significant reduction (61 ± 15% of control, P < 0.01, n = 4) in Cx43 immunostaining, confirming previous results.5
Figure 1.

Reduced Cx30.2 immunostaining in rat retinal endothelial cells grown in high glucose medium. (A) Representative images show reduced Cx30.2 immunostaining in RRECs grown in high glucose medium compared with cells grown in normal medium. (B) Bar graph represents the cumulative data from six experiments and shows significantly reduced Cx30.2 immunostaining in high glucose. Scale bar: 20 μm, *P < 0.05.
Figure 2.
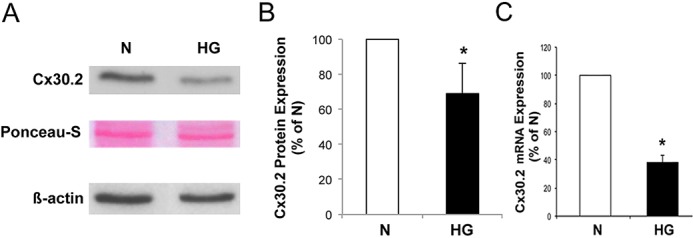
Reduced Cx30.2 expression in rat retinal endothelial cells grown in high glucose medium. (A) Representative Western blot analysis shows decreased Cx30.2 protein expression in RRECs grown in high glucose medium compared with those grown in normal medium. Ponceau-S and β-actin expressions demonstrate equal protein loading. (B) Graph represents cumulative data from four experiments, showing significant downregulation of Cx30.2 protein level in cells grown in high glucose medium. (C) Graph illustrates cumulative data showing reduced Cx30.2 mRNA level in cells grown in high glucose medium. *P < 0.05.
Immunoblotting was performed with total protein isolated from RRECs grown in either N or HG medium (Fig. 2). WB analysis showed Cx30.2 signal at the expected molecular weight size, and consistent with Cx30.2 immunostaining results, Cx30.2 protein level was significantly reduced in RRECs grown in HG medium compared with Cx30.2 protein level in cells grown in N medium (69 ± 17% of control, P < 0.05, n = 4). Ponceau-S staining of the WB membrane and β-actin levels demonstrated equal protein loading (Fig. 2). Cx30.2 mRNA expression was determined in retinal endothelial cells grown in either N or HG medium by RT-PCR. All reactions were performed in duplicate and normalized to the expression of an endogenous housekeeping gene, HPRT. Cx30.2 mRNA levels were significantly reduced in cells grown in HG compared with those grown in N medium (39.2 ± 7% of N, P < 0.05). Although HG decreases Cx30.2 expression, the decrease appears to be more pronounced in the cytoplasm compared with the cell surface (Fig. 1). This could affect Cx30.2 transport to the cell surface and compromise its availability for GJIC in HG condition.
High Glucose–Induced Downregulation of Cx30.2 Expression Significantly Decreases GJIC
SLDT was used to assess the extent of GJIC activity in RRECs grown in either N or HG medium and those transfected with scrambled or Cx30.2 siRNA. The average number of dye-coupled rows of cells on either side of the scrape was reduced in HG medium compared with those grown in N medium (1.6 ± 0.4 vs. 2.7 ± 0.8 rows, respectively, n = 6) by approximately 50%. Similarly, cells grown in N medium and transfected with Cx30.2 siRNA showed significantly reduced dye transfer compared with cells grown in N medium alone (1.5 ± 0.3 vs. 2.7 ± 0.8 rows, respectively, n = 6), and the siRNA-mediated Cx30.2 decrease induced apoptosis of endothelial cells in the absence of HG. The decrease in cell–cell communication seen in the Cx30.2 siRNA-transfected cells was comparable to that of cells grown in HG medium alone (Fig. 3). As control, cells grown in N medium and transfected with scrambled siRNA showed virtually no difference in dye transfer compared with cells grown in N medium alone (3.0 ± 0.6 vs. 2.7 ± 0.8 rows, respectively, n = 6).
Figure 3.
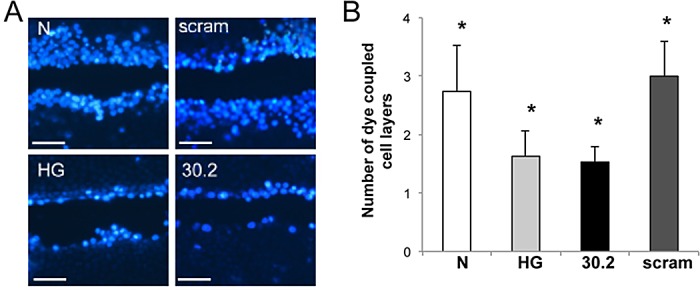
Reduced gap junction intercellular communication in rat retinal endothelial cells transfected with Cx30.2 siRNA. (A) Representative images showing reduced dye transfer in RRECs grown in high glucose medium or transfected with Cx30.2 siRNA compared with cells grown in normal medium or transfected with scrambled siRNA. (B) Bar graph represents the cumulative data from three experiments showing significant reduction in the number of dye-coupled cells grown in high glucose compared with those transfected with Cx30.2 siRNA. Scale bar: 100 μm, *P < 0.05.
Reduced Cx30.2 Expression in Retinas of Diabetic Rats
To determine if Cx30.2 is expressed in retinal blood vessels of rat and mouse retina, retinal capillary networks isolated by the RTD technique (as described in the Materials and Methods section) were subjected to Cx30.2 immunostaining. Capillaries from both wild-type rat and mouse retinas showed a discrete pattern of fine punctate Cx30.2 plaques throughout the capillary network (n = 4) (Fig. 4). Distribution of the Cx30.2 signal indicates that Cx30.2 is expressed both in the pericytes and endothelial cells of the retinal capillaries. Additionally, Cx30.2 immunostaining seen as punctate dots and plaques was significantly reduced in the retinal capillary network of diabetic rats (Fig. 5A). WB analysis confirmed a significant reduction in Cx30.2 protein level in retinas of diabetic rats compared with those of normal nondiabetic rats (64 ± 21% of control, P < 0.02, n = 4) (Fig. 5B).
Figure 4.
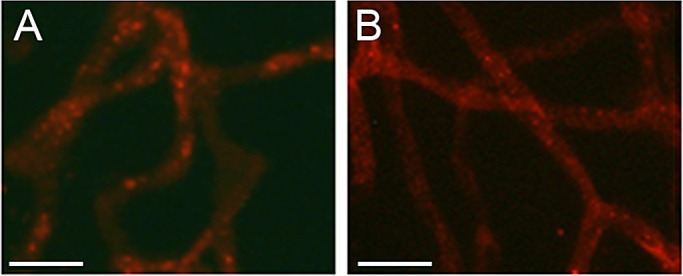
Cx30.2 immunostaining in (A) rat and (B) mouse retinal capillaries. Representative image shows Cx30.2 immunostaining as punctate dots or plaques in the retinal capillary network. Scale bar: 50 μm.
Figure 5.
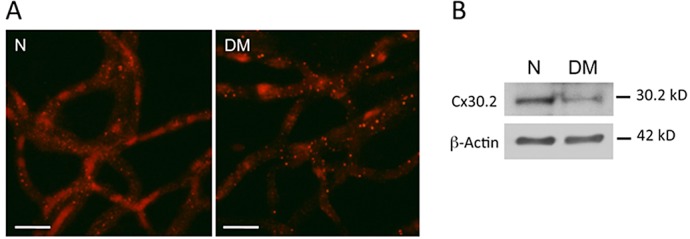
Reduced Cx30.2 immunostaining in retinal capillaries of diabetic rats. (A) Representative images show reduced Cx30.2 immunostaining seen as punctate dots and plaques in the retinal capillary network of diabetic rats. (B) A representative Western blot shows reduced Cx30.2 protein level in retinas of diabetic rats compared with those of normal nondiabetic rats. Scale bar: 50 μm.
Increased Number of ACs and PL in Retinas of Cx30.2 Knockout Mice
To determine the consequence of reduced Cx30.2 expression in the retinal vasculature, retinas of Cx30.2 knockout mice were analyzed for ACs and PL and compared with those of wild-type mice. Retinas of Cx30.2 knockout mice showed a significant increase in the number of ACs and PL compared with those of wild-type mice (198 ± 57% of control, P < 0.002, n = 6; 206 ± 50% of control, P < 0.001, n = 6, respectively) (Fig. 6).
Figure 6.
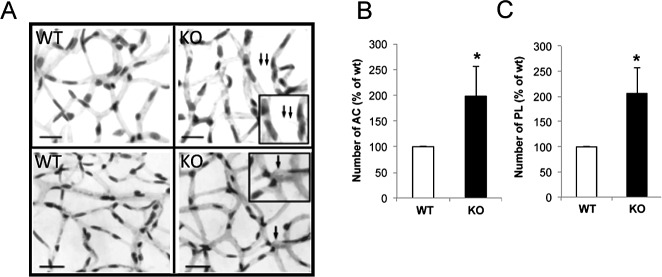
Downregulation of Cx30.2 expression increases pericyte loss and acellular capillaries. (A) Representative images of retinal capillary vessels showing an increased number of ACs and PL in Cx30.2 knockout mice compared with those of WT mice. Bar graphs (B, C) represent the cumulative data from six separate retinas showing a significant increase in the number of ACs and PL, respectively, in Cx30.2 knockout mice. Scale bar: 20 μm. Arrow, PL, *P < 0.05; double arrows, AC, *P < 0.05. Inset: enlarged view of the area indicated by arrow(s) showing ACs or PL.
Discussion
In this study we have determined that the gap junction protein, Cx30.2, is expressed in rat retinal endothelial cells, and that high glucose significantly downregulates Cx30.2 expression with concomitant reduction in GJIC. Furthermore, Cx30.2 expression is decreased in retinal vascular cells of diabetic rats and reduced Cx30.2 expression can contribute to the development of ACs and PL, characteristic lesions of DR. These results, derived from an in vitro cell culture model of hyperglycemia, an animal model of diabetes, and a Cx30.2 knockout mouse, suggest that downregulation of Cx30.2 may play an essential role in promoting development of retinal vascular lesions characteristic of DR and disruption of retinal vascular homeostasis.
Cell-to-cell communication is necessary for retinal vascular cell survival.5,12,18 Previous studies have determined that rat retinal endothelial cells express Cx43 and that it is downregulated by high glucose.5 Furthermore, a previous study has demonstrated that rat pericytes express at least three connexins, Cx37, Cx40, and Cx43, which are differently regulated by high glucose in a culture system.18 We have extended these studies to demonstrate that Cx30.2, like Cx43, is downregulated by high glucose in RRECs as well as in the retinal vascular network in a rat model of diabetes. In previous studies the downregulation of expression of Cx43 protein by high glucose was determined to be approximately 59%,5,27 similar to the value of 69% determined for Cx30.2 in cultured RRECs in the present study. These values for downregulation of connexins in RRECs are similar to those found in the diabetic rodent retina for Cx43 (60.7%)12 and for Cx30.2 (64%). The mechanisms by which diabetes reduces gap junctions are not known but may involve phosphorylation and a proteasome-dependent mechanism.27
DR is one of the most common complications in diabetic patients. The retinopathy is associated with degenerative changes, which likely involve apoptosis of a variety of vascular and neural cells. Thus, in the retina, the loss of vascular cells leads to the appearance of acellular capillaries and pericyte “ghosts” and increased apoptosis of ganglion and amacrine cells.28 We have previously demonstrated that a decrease in gap junctions can trigger apoptosis in retinal endothelial cells as well as in the retina of diabetic mice and in heterozygous Cx43 KO mice and that this leads to increases in pericyte loss and development of acellular capillaries.5,12 In the present study, we extend these observations to demonstrate that loss of Cx30.2 also results in loss of pericytes and increases in acellular capillaries. Since loss of either Cx30.2 or Cx43 leads to these vascular changes, the findings suggest that reduction in total levels of gap junctions contributes to the observed phenotypic changes. This would also be consistent with Cx43 and Cx30.2 forming heteromeric and/or heterotypic gap junctions, as previously demonstrated.29 In the inner retina, the neuronal cells are associated with the vasculature, likely via astrocytes. Since both express connexins, an intriguing possibility is that the changes in connexin expression in one cell type, such as endothelial cells, may lead to apoptotic signals to be generated in neighboring cells, such as astrocytes or ganglion cells, in the absence of GJIC. This would be consistent with a previous study that indicated the antiapoptotic effect of endogenous Cx43 in rat primary astrocytes.30 Another important role for such heterocellular and homocellular gap junctions may be in providing nutrients and removing metabolites from the poorly vascularized inner retina.
It would be important to validate the role of Cx30.2 and its association with Cx43 in retinal vascular cell loss associated with DR. Other potential mechanisms involved in high glucose–induced downregulation of GJIC activity such as Cx30.2 phosphorylation and internalization of Cx30.2 have yet to be determined. An investigation into the relationship between Cx30.2 and Cx43 may shed light on an approach for protection against retinal vascular cell loss in DR.
Footnotes
Supported by National Eye Institute (NEI)/National Institutes of Health Grant EY018218 (SR) and NEI Core Grant EY001792, Research to Prevent Blindness (NMK), and in part by a departmental grant from the Massachusetts Lions Organization (SR).
Disclosure: J. Manasson, None; T. Tien, None; C. Moore, None; N.M. Kumar, None; S. Roy, None
References
- 1. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010; 376: 124–136 [DOI] [PubMed] [Google Scholar]
- 2. Frank RN. Diabetic retinopathy. N Engl J Med. 2004; 350: 48–58 [DOI] [PubMed] [Google Scholar]
- 3. Porta M, Allione A. Current approaches and perspectives in the medical treatment of diabetic retinopathy. Pharmacol Ther. 2004; 103: 167–177 [DOI] [PubMed] [Google Scholar]
- 4. Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011; 11: 253–264 [DOI] [PubMed] [Google Scholar]
- 5. Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009; 50: 1400–1407 [DOI] [PubMed] [Google Scholar]
- 6. Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996; 84: 381–388 [DOI] [PubMed] [Google Scholar]
- 7. Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004; 62: 228–232 [DOI] [PubMed] [Google Scholar]
- 8. Bedner P, Steinhauser C, Theis M. Functional redundancy and compensation among members of gap junction protein families? Biochim Biophys Acta. 2011; 1818: 1971–1984 [DOI] [PubMed] [Google Scholar]
- 9. Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001; 42: 1915–1920 [PubMed] [Google Scholar]
- 10. Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009; 11: 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol. 2002; 29: 620–625 [DOI] [PubMed] [Google Scholar]
- 12. Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010; 51: 3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol. 1998; 396: 310–321 [PubMed] [Google Scholar]
- 14. Roy S, Trudeau K, Behl Y, Dhar S, Chronopoulos A. New insights into hyperglycemia-induced molecular changes in microvascular cells. J Dent Res. 2010; 89: 116–127 [DOI] [PubMed] [Google Scholar]
- 15. Sohl G, Guldenagel M, Traub O, Willecke K. Connexin expression in the retina. Brain Res Brain Res Rev. 2000; 32: 138–145 [DOI] [PubMed] [Google Scholar]
- 16. Muller LP, Dedek K, Janssen-Bienhold U, et al. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010; 27: 91–101 [DOI] [PubMed] [Google Scholar]
- 17. Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 2003; 540: 151–156 [DOI] [PubMed] [Google Scholar]
- 18. Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci. 2003; 44: 5376–5382 [DOI] [PubMed] [Google Scholar]
- 19. Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002; 51: 1565–1571 [DOI] [PubMed] [Google Scholar]
- 20. Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int. 2009; 103: 1686–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. J Neurosci Res. 2011; 89: 2052–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanetta L, Marcus SG, Vasile J, et al. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000; 85: 281–288 [DOI] [PubMed] [Google Scholar]
- 23. Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res. 2011; 36: 747–753 [DOI] [PubMed] [Google Scholar]
- 24. Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006; 55: 86–92 [PubMed] [Google Scholar]
- 25. el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987; 168: 422–430 [DOI] [PubMed] [Google Scholar]
- 26. Roy S, Nasser S, Yee M, Graves DT. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis. 2011; 17: 3166–3174 [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandes R, Girao H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem. 2004; 279: 27219–27224 [DOI] [PubMed] [Google Scholar]
- 28. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gemel J, Lin X, Collins R, Veenstra RD, Beyer EC. Cx30.2 can form heteromeric gap junction channels with other cardiac connexins. Biochem Biophys Res Commun. 2008; 369: 388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giardina SF, Mikami M, Goubaeva F, Yang J. Connexin 43 confers resistance to hydrogen peroxide-mediated apoptosis. Biochem Biophys Res Commun. 2007; 362: 747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]


