Abstract
Purpose.
VEGF production by RPE cells has been shown to be important in regulating aberrant angiogenesis in the retina, which is responsible for multiple types of ocular pathology. EMP2 is highly expressed in the RPE and has been shown to regulate FAK activation, which is implicated in VEGF expression in other cell lines. The purpose of this study was to determine whether EMP2 regulates VEGF expression in the RPE cell line, ARPE-19.
Methods.
ARPE-19 cells were engineered to overexpress EMP2. EMP2 siRNA was used to decrease EMP2 expression. The small molecule inhibitor PP2 was used to inhibit FAK activation. VEGF levels were measured by Western blot and ELISA. Functional differences in secreted VEGF were assayed using HUVEC migration.
Results.
VEGF expression levels correlated with levels of EMP2. An increase of VEGF by 150% was observed in EMP2 overexpressing cells as compared with ARPE-19 cells. Concordantly, EMP2 knockdown resulted in a 57% decrease in VEGF expression. HUVEC migration (P = 0.01) and vessel tube formation (P < 0.01) were significantly increased when exposed to cell culture supernatants from EMP2 overexpressing cells.
Conclusions.
This study establishes a novel connection between EMP2 and VEGF and may reflect either a direct effect through the tetraspan web or an indirect change through FAK activation. This connection is functionally significant. In addition to the direct use of anti-VEGF antibodies, modulation of EMP2 with impact on VEGF is potentially a distinct therapeutic target for the treatment of neovascularization associated with retinal diseases that involve pathologic angiogenesis.
EMP2 increased VEGF protein expression. Both PP2 and Dasatinib decrease VEGF in ARPE-19 cells. Increased VEGF from EMP2 modulation has functional consequences in vitro.
Introduction
The quest to identify novel therapeutic targets for intervention of retinal diseases is important in retinal cell biology. A key cell type that is critical in maintaining retinal homeostasis is RPE. RPE is a monolayer of cuboidal, polarized epithelium with close contact to the neurosensory retina at the apical membrane. RPE is essential for normal retinal functions and is implicated in many blinding retinal diseases, including AMD.1 RPE plays major roles in photoreceptor health, in particular through the provision of nutrients and phagocytosis of the shed tips of photoreceptor outer segments.2,3
Perhaps the most important disease associated with RPE is AMD, which is characterized by global changes, including deposition of subretinal deposits called drusen, loss of the normal RPE and associated vascular changes of the choriocapillaris, and neovascularization of the retina.4,5 It is believed that RPEs are one of the major cell types responsible for the production of VEGF in the eye, which is a critical step in neovascularization and vascular leakage in AMD disease pathogenesis.6–9 It is also hypothesized that some VEGF is critical for the normal homeostasis of the eye, and as such, it might be important to decrease VEGF in these pathologies without eliminating its expression.10,11
Epithelial membrane protein 2 (EMP2), highly expressed in ARPE-19,12 is a member of the growth arrest specific gene 3/peripheral myelin protein 22 (GAS3/PMP22) group of the tetraspan protein superfamily.13–18 In this respect, modulation of EMP2 expression levels or its location on the plasma membrane alters the surface repertoire of several classes of molecules, including integrins, focal adhesion kinase, class I major histocompatibility molecules, and other immunoglobulin superfamily members, such as CD54 and GPI-linked proteins.13–15,17,19 EMP2 has also been shown to physically associate with and regulate activity of integrin–focal adhesion kinase (FAK) signaling complexes.13,14,19–21 FAK has been implicated as an important regulator of VEGF expression.22
There is precedence for tetraspan control of VEGF in tumorigenesis.23 In hepatocytes, ectopic expression of TM4SF5 (homolog of tumor-associated antigen L6) led to increased surface expression of integrin α5 with activation of FAK, c-Src, and signal transducer activator of transcription 3 (STAT3). This activation resulted in increased VEGF secretion and angiogenesis using human umbilical vein endothelial cells (HUVECs).23 TM4SF5 was shown to regulate FAK and RhoA activity leading to epithelial mesenchymal transition, multilayer growth, and actin reorganization, similar to our previous observations studying the tetraspan protein EMP2 in the RPE ARPE-19 cell line and in primary RPE cells (Morales SA, et al. IOVS 2010;51:ARVO E-Abstract 4102).14,20,21
In this article, in vitro studies suggest that EMP2 controls VEGF expression and secretion in the cell line ARPE-19.This study establishes a novel connection between levels of EMP2 and VEGF and may reflect either a direct effect through the tetraspan web or an indirect change through FAK activation. This connection is functionally active, despite the modest changes in VEGF levels. In addition to the direct use of anti-VEGF antibodies, control of EMP2 expression could potentially provide a new approach to control pathologic neovascularization.
Materials and Methods
Cell Line
ARPE-19, a spontaneously arising RPE cell line that expresses the RPE-specific markers CRALBP and RPE-65, was obtained from the American Type Culture Collection (CRL-2302; ATCC, Manassas, VA). The EMP2 overexpressing cells ARPE-19/EMP2 have been previously reported and were produced through stable infection of an EMP2 overexpressing retrovirus construct.14 An additional control ARPE-19 cell line, a retrovirally infected cell line without the EMP2 construct, did not alter cellular functional activity as compared with the wild-type control ARPE-19 cell line (data not shown).14 ARPE-19 cells were cultured in Dulbecco's modified Eagle's medium-F12, supplemented with 10% fetal bovine serum (ATCC) at 37°C in a humidified chamber with 5% CO2.
EMP2 levels were decreased by transiently transfecting ARPE-19 cells with 150 pmol EMP2 small interfering RNA (siRNA) (L-016226-00; Dharmacon, Lafayette, CO) and lipophilic transfection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA) and analyzed after 48 hours. As a negative control, the cells were transfected with 150 pmol of scramble control siRNA (D-001206-13-05; Dharmacon). The EMP2 siRNA and control siRNA are a pool of four siRNAs targeting EMP2 or a pool of four nontargeting siRNAs, respectively. The level of EMP2 expression was quantified by Western blot.
Antibodies
A rabbit antibody specific for human VEGF (clone A-20; Santa Cruz Biotechnology, Santa Cruz, CA) and a mouse antibody specific for human β-actin (clone 2A2.1; US Biological, Swampscott, MA) were used in Western blot analysis. Horseradish peroxidase–conjugated goat antirabbit and goat antimouse antibodies were purchased from the same manufacturer (Southern Biotech, Birmingham, AL). Diabody construction was performed as previously described.24,25 Briefly, anti-EMP2 diabodies binding to the second extracellular loop of human EMP2 were isolated from a human tonsil antibody phage display library. Preparations of diabody were expressed and purified according to published protocol.26
Western Blot Analysis
Western blots were performed as previously described.20 Protein from cultured cells was isolated and protein concentration was determined with a BCA Protein Assay (Bio Rad, Hercules, CA). Each cell lysate was examined in triplicate. The membrane was then blocked with nonfat milk in TBS Tween (TBST; Upstate, Charlottesville, VA). Blots were incubated overnight with primary antibody at a dilution of 1:200 for VEGF and 1:5000 for β-actin. Horseradish peroxidase–conjugated goat antirabbit or horseradish peroxidase–conjugated goat antimouse was exposed to the blots at a 1:2000 dilution. Blots were then developed with ECL to visualize bound antibody (Pierce, Rockford, IL) and quantified. β-actin served as an internal control. Following digitization using a flatbed scanner, the band density was measured using Image J (National Institutes of Health [NIH], Bethesda, MD) and quantified using the NIH program Image J. To account for loading variability, β-actin was used to normalize each sample. At least three independent experiments were performed and, where indicated, the results were evaluated for statistical significance using a Student's t-test (unpaired, two-tailed). A level of P less than 0.05 was considered to be statistically significant. When comparing more than two groups, an ANOVA test was used. A level of P less than 0.05 was considered to be statistically significant.
VEGF ELISA
ARPE-19 cells were seeded onto a six-well plate at a density of 1 × 106 cells per well in serum-free medium. The medium was collected after 24 hours. Secreted VEGF was measured with Quantikine Human VEGF Immunoassay (R&D Systems, Minneapolis, MN). The VEGF ELISA assay is a colorimetric procedure. The reaction product was quantified with the microplate reader (Model 550; Bio-Rad, Hercules, CA) at a wavelength of 540 nm. Each experiment included at least six replicates, and at least three independent experiments were performed. The results were evaluated for statistical significance using a Student's t-test (unpaired, two-tailed). A level of P less than 0.05 was considered to be statistically significant.
HUVEC Migration
HUVEC cells were grown in VEC complete media (VEC technologies, Rensselaer, NY), and were used between passages four and nine. Cells were harvested and placed in the top chamber of a 24-well Transwell plate. Conditioned media from ARPE/EMP2, siRNA transfected cells, or control treated cells was added to the bottom of the Transwell. Cells were incubated for 4 hours. Wells were washed, fixed, and stained with crystal violet. Cells that migrated through the Transwell were manually counted using three random fields on an Olympus microscope (Olympus BX51; Olympus, Center Valley, PA).
HUVEC Capillary Tube Formation
Capillary tube formation was measured after 18 hours. The cells were stained with calcein AM and analyzed using an Olympus BX51 light microscope (Olympus). Tubes were counted using a ×10 objective connected to a DP72 digital camera (Olympus). Three random fields were manually counted and measured at each culture condition. Each experiment was repeated at least three times. The results were evaluated for statistical significance using an ANOVA test. A level of P less than 0.05 was considered to be statistically significant.
Results
EMP2 Modulates VEGF Protein Levels
To examine whether EMP2 expression alters VEGF production, ARPE-19 cells and ARPE-19/EMP2 cells were examined for VEGF expression. Total VEGF protein expression was examined by Western blot analysis. An increase in VEGF expression by 1.5-fold (P = 0.003) was observed in the EMP2 overexpressing cell line ARPE-19/EMP2 as compared with ARPE-19 cells (Fig. 1a). Band density was normalized to β-actin and quantitated using NIH Image J software (Fig. 1b).
Figure 1.
EMP2 increases VEGF protein expression. Steady-state protein levels of EMP2 were measured by Western blot analysis in ARPE-19 cells and ARPE-19/EMP2 cells. EMP2 overexpression increased VEGF expression by 1.5-fold (***P = 0.003). Cell extracts were fractionated by 4% to 20% SDS-PAGE gradient gel in reducing conditions. (a) Representative immunoblots. (b) Band density, normalized to the β-actin loading control, was quantitated. (c) ARPE-19 cells were treated with 20 μg/mL of anti-EMP2 diabody for 24 hours. The level of VEGF expression was quantified by Western blot. Anti-EMP2 diabody reduced total VEGF expression by 70% (*P = 0.01). (d) EMP2 levels were decreased by transiently transfecting ARPE-19 cells with 150 pmol EMP2 siRNA and analyzed after 48 hours. As a negative control, the cells were transfected with 150 pmol of scramble control siRNA. The EMP2 siRNA and control siRNA are a pool of four siRNAs targeting EMP2 or a pool of four nontargeting siRNAs, respectively. The level of VEGF expression was quantified by Western blot. EMP2 siRNA reduced total VEGF expression by 57% (**P = 0.005) as compared with control siRNA.
The previous results demonstrate a positive correlation between EMP2 expression and VEGF expression. To further examine this observation, ARPE-19 cells were treated with an anti-EMP2 diabody. We have previously shown that anti-EMP2 diabody treatment significantly reduced EMP2 expression, whereas control diabody had no effect on EMP2 expression.24 Anti-EMP2 diabody reduced VEGF expression in ARPE-19 cells by 70% (P = 0.01) as compared with control diabody (Fig. 1c). To confirm the EMP2 effect on VEGF expression, a second method was used to reduce EMP2 expression. ARPE-19 cells were treated with siRNA specifically targeting EMP2 or control siRNA. EMP2 protein levels were significantly decreased in the EMP2 siRNA–treated cells but not the control siRNA–treated cells, as described in previous articles.14 Total VEGF expression was measured by Western blot. EMP2 siRNA reduced total VEGF expression by 57% (P = 0.005) as compared with control siRNA–treated cells (Fig. 1d).
FAK Inhibition Reduces VEGF Expression
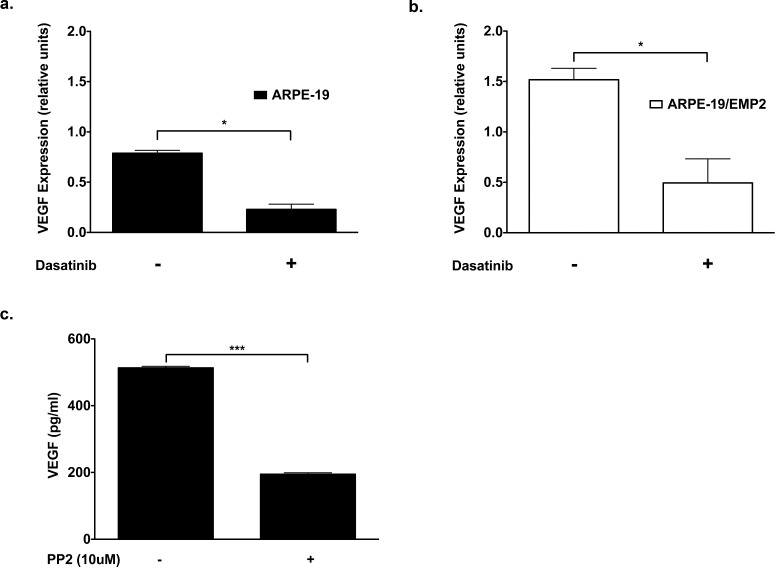
VEGF expression is regulated by a variety of mechanisms. One such mechanism involves FAK.22,27 We have previously published that EMP2 expression regulates FAK activation.14,20 EMP2-regulated VEGF expression may involve the FAK signal transduction pathway. To address this issue, ARPE-19 and APRE-19/EMP2 cells were treated with 300 nM dasatinib, a FAK inhibitor, for 24 hours. The level of VEGF expression was quantified by Western blot. Dasatinib treatment reduced VEGF expression by 72% (P = 0.01) and 68% (P = 0.03) in the ARPE-19 and ARPE-19/EMP2 cells respectively as compared with vehicle control (Figs. 2a, 2b). To confirm these results ARPE-19 cells were treated with a second FAK inhibitor, PP2, and cellular VEGF secretion was measured by ELISA. ARPE-19 cells treated with 10 μM of PP2 for 24 hours showed a significant reduction in VEGF secretion (Fig. 2) (P < 0.0001) as compared with vehicle-treated cells.
Figure 2. .
FAK inhibition reduces VEGF expression. (a) ARPE-19 cells were treated with 300 nM of dasatinib or DMSO vehicle control for 24 hours. The level of VEGF expression was quantified by Western blot. Dasatinib treatment reduced VEGF expression by 72% (*P = 0.01) in the ARPE-19 cells as compared with vehicle control. (b) ARPE-19/EMP2 cells were treated with 300 nM of dasatinib or DMSO vehicle control for 24 hours. The level of VEGF expression was quantified by Western blot. Dasatinib treatment reduced VEGF expression by 68% (*P = 0.03) in the ARPE-19 cells as compared with vehicle control. (c) ARPE-19 cells were treated with 10 μM of PP2 for 24 hours, a FAK inhibitor. VEGF secretion was measured following PP2 treatment by ELISA. ARPE-19 cells treated with PP2 showed a significant reduction in VEGF secretion. PP2-treated cells showed a 62% (***P < 0.0001) reduction as compared to untreated cells.
EMP2 Alters HUVEC Migration
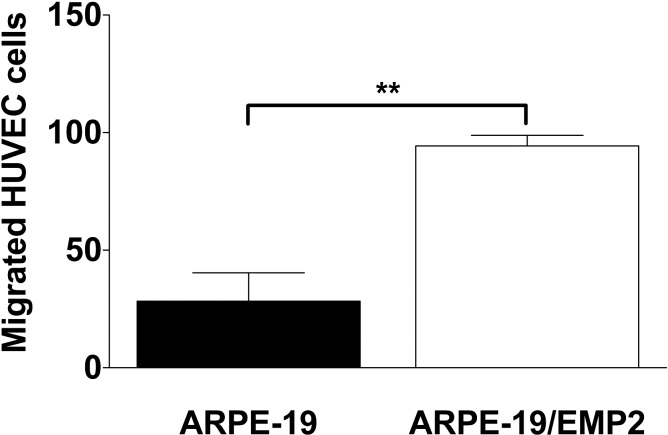
EMP2 regulation of VEGF expression is a novel finding, but prompts the question of whether this is accompanied by a functional paracrine cellular response. To address this issue, HUVEC migration was measured. HUVEC migration through a Transwell filter following incubation with conditioned media from ARPE-19 or ARPE-19/EMP2 cells was assessed. Increased expression of EMP2 resulted in a 3-fold increase in HUVEC migration (P = 0.01) as compared with wild-type cells (Fig. 3).
Figure 3. .
EMP2 alters HUVEC migration. HUVEC cells were grown on a 24-well Transwell plate in normal HUVEC media. Conditioned media was added to the bottom of the Transwell. Cells were incubated for 4 hours. Cells were fixed and stained with crystal violet. Cells that migrated through the Transwell were counted. Increased expression of EMP2 resulted in a 3-fold increase in HUVEC migration (**P = 0.01) as compared with wild-type cells.
EMP2 Increased HUVEC Tube Formation by Increasing Tube Length and Number
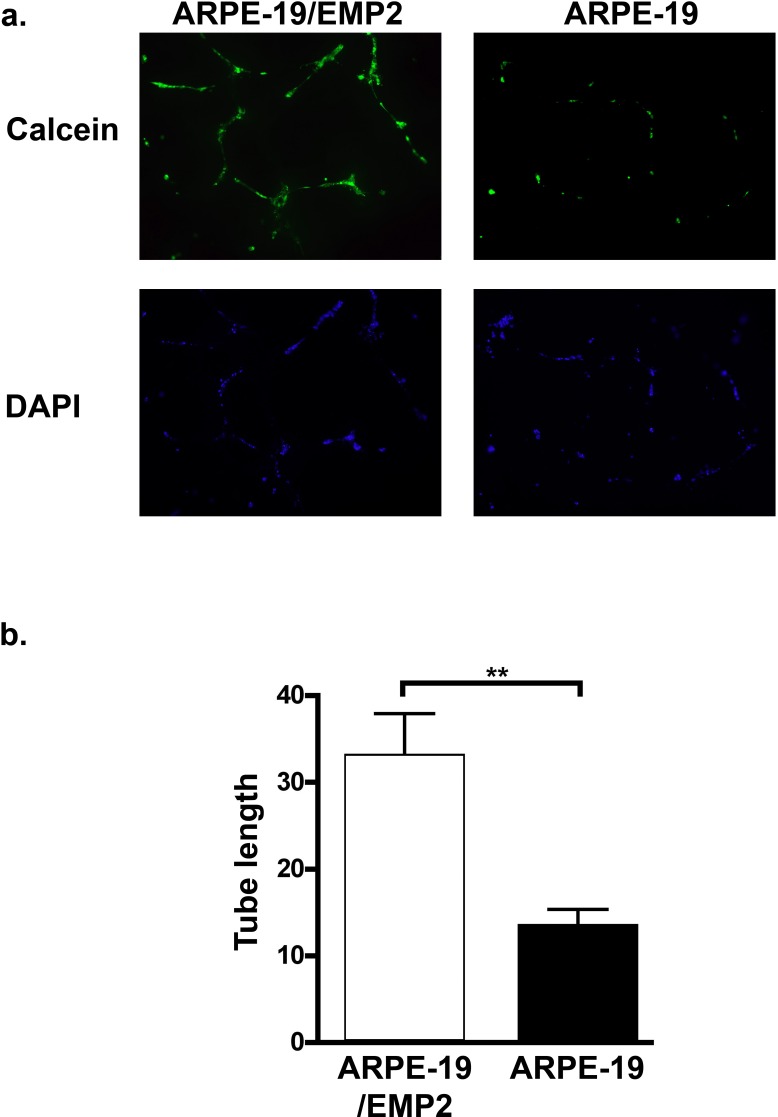
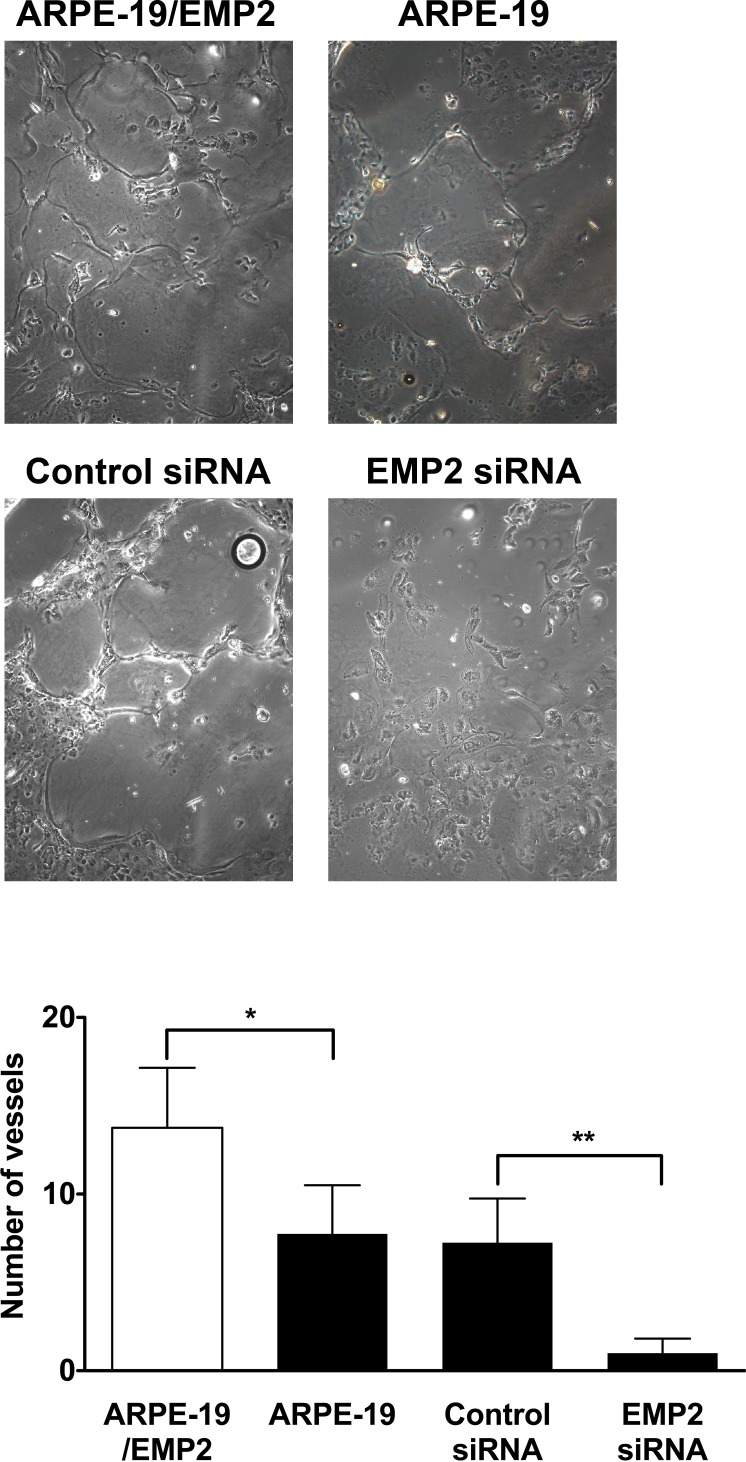
To further examine the functional significance of EMP2-regulated VEGF expression, capillary tube formation was examined. The number of tubes and the average length of the tubes formed from HUVEC cells were measured following incubation in conditioned media from ARPE-19/EMP2 or ARPE-19 cells (Fig. 4). EMP2 expression significantly increased the number of capillary tubes as well as the average length of each tube. The number of vessels formed from HUVEC cells grown in conditioned media from ARPE-19/EMP2, ARPE-19, and ARPE-19 cells treated with control or EMP2 siRNA was counted and an average was calculated from at least three fields. Increased expression of EMP2 significantly increased vessel tube number (P = 0.03) as compared with ARPE-19 cells (Fig. 5). Decreased EMP2 expression by EMP2 siRNA significantly decreased vessel tube number (P = 0.003) as compared with control siRNA–treated cells (Fig. 5). Moreover, increased expression of EMP2 significantly increased vessel tube length (P < 0.01) as compared with ARPE-19 cells with basal levels of EMP2 (Fig. 4).
Figure 4. .
EMP2 increases HUVEC tube length. Vessel tube length of HUVEC cells was measured following incubation in conditioned media from ARPE-19/EMP2 or ARPE-19 cells. (a) HUVEC cells stained with Calcein or 4′,6-diamidino-2-phenylindole (DAPI). (b) Tube length was measured and an average was calculated from several fields. Increased expression of EMP2 significantly increased vessel tube formation (**P < 0.01).
Figure 5. .
EMP2 increases HUVEC tube formation. Vessel tube number was measured following incubation of HUVEC cells in conditioned media from ARPE-19/EMP2, ARPE-19, and ARPE-19 cells treated with control siRNA or EMP2 siRNA was counted and an average was calculated from several fields. Increased expression of EMP2 significantly increased vessel tube number (*P = 0.03) as compared with ARPE-19 cells. Decreased EMP2 expression by EMP2 siRNA significantly decreased vessel tube number (**P = 0.003) as compared with control siRNA treated cells.
Discussion
In this study, we described a novel connection between levels of EMP2 and VEGF in the cell line ARPE-19. These findings demonstrate a positive correlation between the levels of EMP2 protein expression and VEGF expression. The reduction of EMP2 expression leads to a significant decrease of VEGF to approximately 30% of the baseline level, which may reflect either a direct effect through the tetraspan web or an indirect change through FAK activation.
The tetraspan web is a dynamic multiprotein complex regulating diverse cellular functions based on the interaction of integrins, tyrosine kinases, and tetraspan proteins.28–31 The mechanisms by which tetraspan proteins regulate VEGF production are not fully delineated, but a more detailed understanding is beginning to emerge. An important question is whether VEGF regulation is a direct effect of the tetraspan web or an indirect change through FAK. These alternatives may in fact be reflecting a single mechanism viewed at either upstream or downstream points in the process. In 2006, Mitra and colleagues22 demonstrated that FAK catalytic activity promotes tumor progression through phosphorylation and activation of the FAK signal transduction pathway regulating VEGF expression in breast carcinoma cells. Three years later, Choi and colleagues23 reported that TM4SF5, a tetraspan family member, facilitates angiogenesis through VEGF induction in malignant epithelial cells. This involved cooperation between TM4SF5 and integrin α5 to transduce signaling through the FAK/Src complex, leading to STAT3 phosphorylation and consequent induction of VEGF. In the present study, we show that EMP2, a tetraspan protein, regulates VEGF protein expression. Our prior studies have shown that EMP2 expression positively correlates with FAK activation. By specifically targeting EMP2 with an anti-EMP2 diabody or siRNA, or FAK with small molecule inhibitors, VEGF expression is significantly reduced in the ARPE-19 and ARPE-19/EMP2 cell lines. This report adds additional evidence linking tetraspan regulation of VEGF.
AMD is increasing in prevalence with the increased aging of the population.32,33 AMD is the primary cause of blindness in people 50 and older in developed countries. Currently, control of VEGF is the only effective therapy for neovascular AMD that results in visual improvements. However, there are real and potential limitations in the use of antibodies against VEGF in patients with AMD. First, these antibodies are not universally effective and clinical benefit is found in only 30% to 40% of patients.34 Second, the long-term effectiveness, beyond 24 months, has not yet been described. Third, there is some evidence that some endogenous VEGF is required for normal visual function, in particular for maintenance of the choriocapillaris.35 This suggests that optimal modulation of VEGF should achieve a sufficient reduction for therapeutic effect, but preserve a basal VEGF level sufficient to avert pathology caused by excessive VEGF depletion. This study demonstrates that targeting EMP2 can affect such graded control of VEGF expression. However, the work presented here does not address whether modifying EMP2 expression can lead to an effective physiologic decrease in VEGF in retinal pathology. Therefore, additional investigations, both with primary RPE and with in vivo models of pathological angiogenesis, are required to determine if this approach could potentially be therapeutically beneficial in the future.
Footnotes
Supported by National Institutes of Health Grant RO1 EY019909 Grant (LKG), the American Health Assistance Foundation (LKG), and the A. P. Giannini Foundation (SAM).
Disclosure: S.A. Morales, P; D.G. Telander, P; D. Leon, None; K. Forward, None; J. Braun, P; M. Wadehra, None; L.K. Gordon, P
References
- 1. Zarbin MA. Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol. 1998; 8: 199–206 [DOI] [PubMed] [Google Scholar]
- 2. Chang Y, Finnemann SC. Tetraspanin CD81 is required for the alpha v beta5- integrin-dependent particle-binding step of RPE phagocytosis. J Cell Sci. 2007; 120: 3053–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayerson PL, Hall MO. Rat retinal pigment epithelial cells show specificity of phagocytosis in vitro. J Cell Biol. 1986; 103: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008; 358: 2606–2617 [DOI] [PubMed] [Google Scholar]
- 5. Andrews A, Balciunaite E, Leong FL, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999; 40: 2683–2689 [PubMed] [Google Scholar]
- 6. Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002; 8: 119–126 [PubMed] [Google Scholar]
- 7. Ohno-Matsui K, Morita I, Tombran-Tink J, et al. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001; 189: 323–333 [DOI] [PubMed] [Google Scholar]
- 8. Schwesinger C, Yee C, Rohan RM, et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001; 158: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sall JW, Klisovic DD, O'Dorisio MS, Katz SE. Somatostatin inhibits IGF-1 mediated induction of VEGF in human retinal pigment epithelial cells. Exp Eye Res. 2004; 79: 465–476 [DOI] [PubMed] [Google Scholar]
- 10. Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002; 29: 10–14 [DOI] [PubMed] [Google Scholar]
- 11. Ford KM, D'Amore PA. Molecular regulation of vascular endothelial growth factor expression in the retinal pigment epithelium. Mol Vis. 18: 519–527 [PMC free article] [PubMed] [Google Scholar]
- 12. Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003; 74: 106–112 [DOI] [PubMed] [Google Scholar]
- 13. Forbes A, Wadehra M, Mareninov S, et al. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem. 2007; 282: 26542–26551 [DOI] [PubMed] [Google Scholar]
- 14. Morales SA, Mareninov S, Wadehra M, et al. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009; 50: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wadehra M, Goodglick L, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004; 15: 2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002; 277: 41094–41100 [DOI] [PubMed] [Google Scholar]
- 17. Wadehra M, Su H, Gordon LK, Goodglick L, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003; 107: 129–136 [DOI] [PubMed] [Google Scholar]
- 18. Wang CX, Wadehra M, Fisk BC, Goodglick L, Braun J. Epithelial membrane protein 2, a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001; 97: 3890–3895 [DOI] [PubMed] [Google Scholar]
- 19. Wadehra M, Forbes A, Pushkarna N, et al. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005; 287: 336–345 [DOI] [PubMed] [Google Scholar]
- 20. Morales SA, Mareninov S, Coulam P, et al. Functional consequences of interactions between FAK and epithelial membrane protein 2 (EMP2). Invest Ophthalmol Vis Sci. 2009; 50: 4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morales SA, Mareninov S, Prasad P, Wadehra M, Braun J, Gordon LK. Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway. Exp Eye Res. 2007; 85: 790–798 [DOI] [PubMed] [Google Scholar]
- 22. Mitra SK, Mikolon D, Molina JE, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006; 25: 5969–5984 [DOI] [PubMed] [Google Scholar]
- 23. Choi S, Lee SA, Kwak TK, et al. Cooperation between integrin alpha5 and tetraspan TM4SF5 regulates VEGF-mediated angiogenic activity. Blood. 2009; 113: 1845–1855 [DOI] [PubMed] [Google Scholar]
- 24. Morales SA, Telander DG, Mareninov S, et al. Anti-EMP2 diabody blocks epithelial membrane protein 2 (EMP2) and FAK mediated collagen gel contraction in ARPE-19 cells. Exp Eye Res. 2012; 102C: 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimazaki K, Lepin EJ, Wei B, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008; 14: 7367–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marks JD, Bradbury A. Selection of human antibodies from phage display libraries. Methods Mol Biol. 2004; 248: 161–176 [DOI] [PubMed] [Google Scholar]
- 27. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006; 18: 516–523 [DOI] [PubMed] [Google Scholar]
- 28. Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001; 58: 1189–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda). 2005; 20: 218–224 [DOI] [PubMed] [Google Scholar]
- 30. Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005; 5: 136–148 [DOI] [PubMed] [Google Scholar]
- 31. Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci. 2003; 28: 106–112 [DOI] [PubMed] [Google Scholar]
- 32. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572 [DOI] [PubMed] [Google Scholar]
- 33. Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002; 109: 1767–1779 [DOI] [PubMed] [Google Scholar]
- 34. Tao Y, Jonas JB. Intravitreal bevacizumab combined with intravitreal triamcinolone for therapy-resistant exudative age-related macular degeneration. J Ocul Pharmacol Ther. 26: 207–212 [DOI] [PubMed] [Google Scholar]
- 35. Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on Muller cells and photoreceptors. PLoS One. 2008; 3: e3554 [DOI] [PMC free article] [PubMed] [Google Scholar]