Abstract
Increasing evidence indicates that brain inflammation is involved in the pathogenesis of neuropsychiatric diseases. Autism spectrum disorders (ASD) are characterized by social and learning disabilities that affect as many as 1/80 children in the USA. There is still no definitive pathogenesis or reliable biomarkers for ASD, thus significantly curtailing the development of effective therapies. Many children with ASD regress at about age 3 years, often after a specific event such as reaction to vaccination, infection, stress or trauma implying some epigenetic triggers, and may constitute a distinct phenotype. ASD children respond disproportionally to stress and are also affected by food and skin allergies. Corticotropin-releasing hormone (CRH) is secreted under stress and together with neurotensin (NT) stimulates mast cells and microglia resulting in focal brain inflammation and neurotoxicity. NT is significantly increased in serum of ASD children along with mitochondrial DNA (mtDNA). NT stimulates mast cell secretion of mtDNA that is misconstrued as an innate pathogen triggering an auto-inflammatory response. The phosphatase and tensin homolog (PTEN) gene mutation, associated with the higher risk of ASD, which leads to hyper-active mammalian target of rapamycin (mTOR) signalling that is crucial for cellular homeostasis. CRH, NT and environmental triggers could hyperstimulate the already activated mTOR, as well as stimulate mast cell and microglia activation and proliferation. The natural flavonoid luteolin inhibits mTOR, mast cells and microglia and could have a significant benefit in ASD.
Introduction
Focal brain inflammation
Increasing evidence indicates that brain inflammation is important in the pathogenesis of neuropsychiatric disorders [1,2]. Autism spectrum disorders (ASD) are pervasive neuro-developmental disorders characterized by varying degrees of deficiencies in social interactions, intelligence, and language, as well as the presence of stereotypic behaviors [3-6]. Recent results from the Centers of Disease Control in the USA indicate that as many as 1/80 children have ASD [7]. Many such children regress at about age 3 years, often after a specific event such as reaction to vaccination, infection [8,9], trauma [10,11], toxic exposures [12] or stress [13], implying the importance of some environmental triggers [14,15].
Increasing evidence points to some immune dysfunction/inflammation in ASD [16,17]. The markers of inflammation identified in the brain and cerebrospinal fluid (CSF) of many ASD patients include TNF, IL-6 and monocyte chemotactic protein 1 (MCP-1), the latter of which also is chemotactic for mast cells [18]. Pro-inflammatory cytokine mRNA (IL-1α, IL-1β, IL-6 and TNF-α) is increased in brain inflammation and has been associated with hippocampal and cerebral damage [8]. Mast cells are a rich source of IL-6 and TNF [19]. In fact, mast cells are the only immune cells that store pre-formed TNF and can release it rapidly upon stimulation [20].
Mast cells and cytokines such as IL-6 and TNF are also implicated in disruption of the blood–brain barrier (BBB) [21-23], which may be malfunctioning or leaky in ASD as evidenced by the presence of circulating auto-antibodies directed against the fetal brain proteins [24-27]. We had reported that the cytokine IL-33 synergizes with inflammatory neuropeptides to stimulate mast cells and result in increased vascular permeability [28]. IL-33 has been considered an alarmin, acting through mast cells to alert the innate immune system [29,30], and has recently been linked to brain inflammation [31-33].
We have also reported that neurotensin (NT) and corticotropin-releasing hormone (CRH), secreted under stress, synergistically stimulate mast cells, leading to increase vascular permeability [34] and contribute to BBB disruption [35]. We further showed that NT stimulates mast cell secretion of vascular endothelial growth factor (VEGF) [36], which is also vasodilatory. NT also increases expression of CRH receptor-1 (CRHR-1) [37], activation of which by CRH increases allergic stimulation of human mast cells [38].
NT is a vasoactive peptide originally isolated from the brain [39], but also found in the gut where it has been implicated in inflammation [40], and in increased intestinal permeability in rodents [41]. NT is also increased in the skin following acute stress, stimulates skin mast cells and increases vascular permeability in rodents [42]. NT stimulates rodent peritoneal mast cells to secrete histamine and elevates histamine plasma levels through activation of specific NT receptors (NTR) [43-45]. Moreover, NT is rapidly degraded by mast cell proteases [34,46] implying tight regulation of its activity.
Mast cells are hemopoietic-derived tissue immune cells responsible for allergies, but also implicated in immunity [47] and inflammation [18]. Mast cells can produce both pro- and anti-inflammatory mediators [48] and may have immuno-modulatory functions [47,49-51]. It is, therefore, of interest that allergic-like reactions are common in ASD children [52,53] implying activation of mast cells by non-allergic triggers [17]. The richest source of mast cells in the brain is the diencephalon [54] that regulates behavior, while the highest concentration of NTR is in the Broca area [55], which regulates language, known to be lost in many children with ASD. Mast cells are responsible for eliciting neutrophil infiltration that promotes inflammation [56]. Mast cell-microglial interactions are important in neuroinflammatory diseases [57,58]. Microglia are the innate brain immune cells that are increasingly implicated in a number of neuropsychiatric diseases [59]. In fact, abnormal microglial growth and activation was recently reported in the brain of ASD patients [60,61]. Microglia express NTR3, activation of which leads to their proliferation [62].
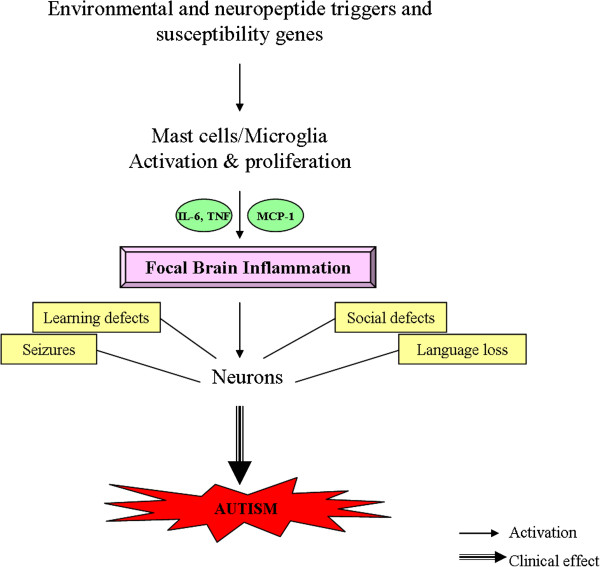
NT has additional actions that are relevant to ASD (Table 1): it induces intestinal secretion and mobility [63], stimulates glial cell proliferation [64], and can facilitate seizures through activation of glutamate receptors [65]. In fact, the glutamate receptor mGluR5 was reported to be overactive in fragile X mice [66,67], a condition associated with high risk of ASD. In other words, NT could contribute to ASD pathogenesis through different mechanisms (Figure 1).
Table 1.
Neurotensin actions relevant to autism spectrum disorder (ASD) pathogenesis
| Effect | Result |
|---|---|
| Activation and proliferation of microglia |
Brain inflammation |
| Activation of mast cells |
Blood–brain-barrier disruption and inflammation |
| Disruption of gut-blood barrier |
Leaky gut and inflammation |
| Mast cell stimulation, especially in the subgroup of ASD patients with allergic symptoms |
Augmentation of allergic symptoms |
| Extracellular secretion of mitochondrial components that act as innate pathogens |
Inflammation |
| Stimulation of glutamate receptors |
Neuronal damage |
| Direct neurotoxicity | Neuronal damage |
Figure 1.
Diagrammatic representation of how stimulation of mast cells and microglia could lead to multiple effects that contribute brain inflammation and the pathogenesis and symptoms of autism. MCP, monocyte chemotactic protein.
There is also support for increased oxidative stress [68] and some mitochondrial (mt) defects at least in subgroups of patients with ASD [69]. We showed that mtDNA is significantly increased in the serum of young autistic children [70], who also had significantly increased serum level of NT [71]; this triggers mast cells to secrete mtDNA [38] that acts as innate pathogen to stimulate mast cells [72] and other immune cells, leading to auto-inflammation [73]. Moreover, mtDNA can cause neuronal degeneration and altered behavior [74]. We believe that ASD originate from immune perinatal insults [75,76] that activate ASD susceptibility genes leading to focal encephalitis (Table 2).
Table 2.
Key pathologic processes in ASD*
| Change | Pathologic processes |
|---|---|
| ↑ |
Allergic-like symptoms |
| ↑ |
Anti-brain protein auto-antibodies |
| ↑ |
Food intolerance |
| ↑ |
Brain and gut inflammatory markers |
| ↑ |
High anxiety and response to stress |
| ↑ |
Oxidative stress |
| ↓ |
Glutathione |
| ↓ | Methylation, sulfation |
*Not present all ASD children.
Epigenetic activation of ASD susceptibility genes
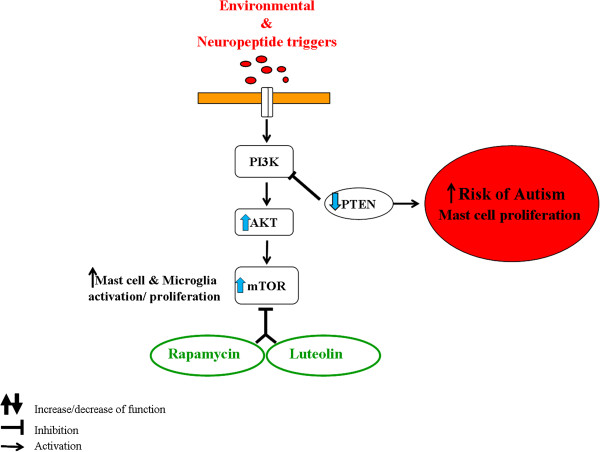
In spite of the fact that almost 100 gene mutations have been identified in patients with ASD [77,78], they do not explain more than a few percent of ASD cases [6]. High risk for developing ASD has been associated with mutations leading to decreased phosphatase and tensin homolog (PTEN) and tuberous sclerosis protein 1 and 2 (TSC1/2) [77]. These proteins are upstream inhibitors of the mammalian target of rapamycin (mTOR) [77,79], which leads to microglia and mast cell proliferation [80,81]. Activation of susceptibility genes is being increasingly invoked to explain ASD [7,82]. A recent paper reported that offspring of maternal immune activation in mice led to increased IL-6 and IL-17, and contributed to ASD-related behaviors [9]; repopulation of control irradiated mice with bone marrow derived from affected mothers did not induce those effects suggesting the contribution of some epigenetic environmental influences. Stimulation of mTOR in subjects with overactive mTOR due to gene mutations, leading to low PTEN, would contribute to a form of epigenetic signal.
Novel treatments
Behavioral interventions are the most common treatment approaches [83], but do not address the core ASD symptoms [84,85]. Psychotropic drugs are used much too often in ASD [86-88]. Such drugs include antipsychotic medications [89], the newer atypical compounds [90,91] risperidone [92,93] and aripiprazole [94] for obsessive-compulsive symptoms, aggression and self-injury, as well as methylphenidate for hyperactivity [95]. However, two recent reviews concluded that there is insufficient evidence to support any benefit of psychotropic drugs [96] or selective serotonin re-uptake inhibitor (SSRIs) [97] in ASD. In fact, the SSRI citalopram may actually be detrimental [98], especially in children [99]. Moreover, a recent paper reported that citalopram administration perinataly altered cortical network function and led to ASD-like behaviors in rodents [100].
Rapamycin and its analogs are mTOR inhibitors [101] and are being tried for treatment of ASD [102-105] (Figure 2). Our preliminary results (not shown) indicate for the first time that the natural flavonoid luteolin [106] is more potent that rapamycin in its ability to inhibit human mast cell TNF release (Figure 2). A previous report also indicated that flavonoid-related epigallocatechin gallate (EGCG) is an mTOR inhibitor [107]. Luteolin may not only inhibit mTOR, but also has additional beneficial effects in brain inflammation. It inhibits oxidative stress [106], inflammation [106], mast cell degranulation [108], mast cell cytokine release [38], thimerosal-induced inflammatory mediator release [109], microglial activation and proliferation [110-112], and auto-immune T cell activation [113,114]. Luteolin is also protective against methylmercury-induced mitochondrial damage [115], is neuroprotective [116] and mimics brain-derived neurotrophic factor (BDNF) [117], which was recently associated with autistic-like-behavior in mice [118]. Finally, luteolin could reverse ASD-like behavior in mice [53], and was recently shown to have significant benefit in children with ASD [38,119].
Figure 2.
Diagrammatic representation of the mTOR pathway, how it may lead to increased risk of autism and the inhibitory effect of luteolin. mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homolog; AKT, protein kinase B.
Conclusions
The prevalence of ASD continues to rise, but there is no clinically effective drug for the core ASD symptoms. Unfortunately, the lack of distinct pathogenesis and biomarkers makes it difficult to develop effective treatments. Stimulation of mTOR, which is already activated due to PTEN mutations, by NT, CRH and/or IL-33, could serve as novel targets for drug development. NTR and CRHR-1 antagonists could, therefore, be used in ASD, along with luteolin.
Abbreviations
ASD: autism spectrum disorders; BBB: blood–brain barrier; BDNF: brain-derived neurotrophic factor; CSF: cerebrospinal fluid; CRH: corticotropin-releasing hormone; EGCG: epigallocatechin gallate; IL: interleukin; MCP: monocyte chemotactic protein; mTOR: mammalian target of rapamycin; mt: mitochondrial; NT: neurotensin; NTR: neurotensin receptor; PTEN: phosphatase and tensin homolog; SSRI: selective serotonin re-uptake inhibitor; TNF: tumor necrosis factor; TSC1/2: tuberous sclerosis protein 1 and 2; VEGF: vascular endothelial growth factor.
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
TCT, SA, and AP conceived and wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Theoharis C Theoharides, Email: theoharis.theoharides@tufts.edu.
Shahrzad Asadi, Email: shahrzad.asadi@tufts.edu.
Arti B Patel, Email: arti.patel@tufts.edu.
Acknowledgements
Aspects of the work discussed were funded in part by the Autism Research Institute, the National Autism Association, Safe Minds, and Theta Biomedical Consulting and Development Co. Inc. (Brookline, MA).
References
- Theoharides TC, Zhang B, Conti P. Decreased mitochondrial function and increased brain inflammation in bipolar disorder and other neuropsychiatric diseases. J Clin Psychopharmacol. 2011;31:685–687. doi: 10.1097/JCP.0b013e318239c190. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- McPartland J, Volkmar FR. Autism and related disorders. Handb Clin Neurol. 2012;106:407–418. doi: 10.1016/B978-0-444-52002-9.00023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC. Genetics: searching for answers. Nature. 2012;491:S4–S6. doi: 10.1038/491S4a. [DOI] [PubMed] [Google Scholar]
- Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenner S, Reddy A, Augustyn M. Diagnosis and management of autism in childhood. BMJ. 2011;343:d6238. doi: 10.1136/bmj.d6238. [DOI] [PubMed] [Google Scholar]
- Rapin I, Tuchman RF. What is new in autism? Curr Opin Neurol. 2008;21:143–149. doi: 10.1097/WCO.0b013e3282f49579. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Lanni KE, Schupp CW, Simon D, Corbett BA. Verbal ability, social stress, and anxiety in children with autistic disorder. Autism. 2012;16:123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicol Teratol. 2012. [DOI] [PMC free article] [PubMed]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, Toniato E, Kalogeromitros D. Mast cell activation and autism. Biochim Biophys Acta. 1822;2012:34–41. doi: 10.1016/j.bbadis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 1822;2010:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby K. Mast cells and de novo angiogenesis: angiogenic capability of individual mast-cell mediators such as histamine, TNF, IL-8 and bFGF. Inflamm Res. 1997;46(Suppl. 1):S7–S8. [PubMed] [Google Scholar]
- Zhang B, Weng Z, Sismanopoulos N, Asadi S, Therianou A, Alysandratos KD, Angelidou A, Shirihai O, Theoharides TC. Mitochondria distinguish granule-stored from de novo synthesized tumor necrosis factor secretion in human mast cells. Int Arch Allergy Immunol. 2012;159(1):23–32. doi: 10.1159/000335178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Doyle R. Autism, gut-blood–brain barrier and mast cells. J Clin Psychopharm. 2008;28:479–483. doi: 10.1097/JCP.0b013e3181845f48. [DOI] [PubMed] [Google Scholar]
- Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood–brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- Rossi CC, Van de Water J, Rogers SJ, Amaral DG. Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain Behav Immun. 2011;25:1123–1135. doi: 10.1016/j.bbi.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord. 2012;42(7):1435–1445. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69:693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Weir MA, Manno M, Cordy P, Gomes T, Hackam DG, Juurlink DN, Mamdani M, Moist L, Parikh CR, Paterson JM, Wald R, Yao Z, Garg AX. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med. 2012;156:560–569. doi: 10.7326/0003-4819-156-8-201204170-00003. [DOI] [PubMed] [Google Scholar]
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Kaushik DK, Gupta M, Basu A. Inflammasome signaling at the heart of central nervous system pathology. J Neurosci Res. 2010;88:1615–1631. doi: 10.1002/jnr.22343. [DOI] [PubMed] [Google Scholar]
- Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Konstantinidou A. Corticotropin-releasing hormone and the blood–brain-barrier. Front Biosci. 2007;12:1615–1628. doi: 10.2741/2174. [DOI] [PubMed] [Google Scholar]
- Vasiadi M, Therianou A, Alysandratos KD, Katsarou-Katsari A, Petrakopoulou T, Theoharides A, Papadavid E, Stavrianeas N, Antoniou C, Kalogeromitros D, Theoharides TC. Serum neurotensin (NT) is increased in psoriasis and NT induces VEGF release from human mast cells. Br J Dermatol. 2012;166:1349–1352. doi: 10.1111/j.1365-2133.2012.10843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alysandratos K-D, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, Critchfield A, Theoharides TC. Neurotensin and CRH interactions augment human mast cell activation. PLoS ONE. 2012;7(11):e48934. doi: 10.1371/journal.pone.0048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S, Theoharides TC. Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J Neuroinflam. 2012;9:85. doi: 10.1186/1742-2094-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93:125–136. doi: 10.1016/S0167-0115(00)00183-X. [DOI] [PubMed] [Google Scholar]
- Carraway RE, Singer EA, Ferris CF, Mitra SP. Generation of immunoreactive neurotensin(s) and enkephalin(s) by pepsin-treatment of plasma. Adv Exp Med Biol. 1986;198 Pt B:169–179. doi: 10.1007/978-1-4757-0154-8_21. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin and substance P: a link to neurogenic skin disorders. Brain Behav Immunity. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Carraway R, Cochrane DE, Lansman JB, Leeman SE, Paterson BM, Welch HJ. Neurotensin stimulates exocytotic histamine secretion from rat mast cells and elevates plasma histamine levels. J Physiol. 1982;323:403–414. doi: 10.1113/jphysiol.1982.sp014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg RS, Cochrane DE, Carraway RE, Brown EB, Sawyer R, Hartunian M, Wentworth D. Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm Res. 1998;47:245–250. doi: 10.1007/s000110050325. [DOI] [PubMed] [Google Scholar]
- Barrocas AM, Cochrane DE, Carraway RE, Feldberg RS. Neurotensin stimulation of mast cell secretion is receptor-mediated, pertussis-toxin sensitive and requires activation of phospholipase C. Immunopharmacology. 1999;41:131–137. doi: 10.1016/S0162-3109(98)00064-2. [DOI] [PubMed] [Google Scholar]
- Cochrane DE, Carraway RE, Boucher W, Feldberg RS. Rapid degradation of neutotensin by stimulated rat mast cells. Peptides. 1991;12:1187–1194. doi: 10.1016/0196-9781(91)90193-S. [DOI] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Ishizuka T, Okayama Y. Human mast cells and basophils as sources of cytokines. Clin Exp Allergy. 2000;30:1205–1212. doi: 10.1046/j.1365-2222.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T. A crucial door to the mast cell mystery knocked in. J Immunol. 2009;183:6861–6862. doi: 10.4049/jimmunol.0990101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S, Costanza M, Musio S, Tripodo C, Poliani PL, Gri G, Burocchi A, Pittoni P, Gorzanelli A, Colombo MP, Pedotti R. Exacerbated experimental autoimmune encephalomyelitis in mast-cell-deficient Kit W-sh/W-sh mice. Lab Invest. 2011;91:627–641. doi: 10.1038/labinvest.2011.3. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;5:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Angelidou A, Alysandratos KD, Asadi S, Zhang B, Francis K, Vasiadi M, Kalogeromitros D, Theoharides TC. Brief report: “allergic symptoms” in children with autism spectrum disorders. More than meets the eye? J Autism Dev Disord. 2011;41:1579–1585. doi: 10.1007/s10803-010-1171-z. [DOI] [PubMed] [Google Scholar]
- Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC. Definitive characterization of rat hypothalamic mast cells. Neuroscience. 1996;73:889–902. doi: 10.1016/0306-4522(95)00606-0. [DOI] [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–1442. doi: 10.1016/S0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 1822;2012:57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc Lond B Biol Sci. 2012;367:3312–3325. doi: 10.1098/rstb.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Hatori K, Choi HB, McLarnon JG, Lee MA, Kim SU. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiol Dis. 2001;8:1057–1068. doi: 10.1006/nbdi.2001.0437. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 2012;1456:72–81. doi: 10.1016/j.brainres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Rodriguez JI, Kern JK. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol. 2011;7:205–213. doi: 10.1017/S1740925X12000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Dicou E, Vincent JP, Mazella J. Neurotensin and the neurotensin receptor-3 in microglial cells. J Neurosci Res. 2005;81:322–326. doi: 10.1002/jnr.20477. [DOI] [PubMed] [Google Scholar]
- Riegler M, Castagliuolo I, Wang C, Wlk M, Sogukoglu T, Wenzl E, Matthews JB, Pothoulakis C. Neurotensin stimulates Cl(−) secretion in human colonic mucosa In vitro: role of adenosine. Gastroenterology. 2000;119:348–357. doi: 10.1053/gast.2000.9310. [DOI] [PubMed] [Google Scholar]
- Martin S, Vincent JP, Mazella J. Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J Neurosci. 2003;23:1198–1205. doi: 10.1523/JNEUROSCI.23-04-01198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A. Targeting neurotensin as a potential novel approach for the treatment of autism. J Neuroinflammation. 2010;7:58. doi: 10.1186/1742-2094-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33:84–87. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17(4):389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Angelidou A, Alysandratos KD, Vasiadi M, Francis K, Asadi S, Theoharides A, Sideri K, Lykouras L, Kalogeromitros D, Theoharides TC. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J Neuroinflammation. 2010;7:80. doi: 10.1186/1742-2094-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidou A, Francis K, Vasiadi M, Alysandratos K-D, Zhang B, Theoharides A, Lykouras L, Kalogeromitros D, Theoharides TC. Neurotensin is increased in serum of young children with autistic disorder. J Neuroinflam. 2010;7:48. doi: 10.1186/1742-2094-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, Weng Z, Miniati A, Vasiadi M, Katsarou-Katsari A, Miao B, Leeman SE, Kalogeromitros D, Theoharides TC. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: relevance to atopic dermatitis. J Allergy Clin Immunol. 2011;127:1522–1531. doi: 10.1016/j.jaci.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS ONE. 2012;7(12):e49767. doi: 10.1371/journal.pone.0049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen KH, Moldestad O, Eide L, Carlsen H, Nesse G, Storm JF, Mansuy IM, Bergersen LH, Klungland A. Mitochondrial DNA toxicity in forebrain neurons causes apoptosis, neurodegeneration, and impaired behavior. Mol Cell Biol. 2010;30:1357–1367. doi: 10.1128/MCB.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidou A, Asadi S, Alysandratos KD, Karagkouni A, Kourembanas S, Theoharides TC. Perinatal stress, brain inflammation and risk of autism-Review and proposal. BMC Pediatr. 2012;12:89. doi: 10.1186/1471-2431-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Redwood L. Autism: an emerging ‘neuroimmune disorder’ in search of therapy. Exp Opin Pharmacother. 2009;10:2127–2143. doi: 10.1517/14656560903107789. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Plummer JT, Qiu S, Levitt P. SnapShot: genetics of autism. Neuron. 2011;72:418. doi: 10.1016/j.neuron.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Zhou J, Parada LF. PTEN signaling in autism spectrum disorders. Curr Opin Neurobiol. 2012;22(5):873–879. doi: 10.1016/j.conb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Smrz D, Kim MS, Zhang S, Mock BA, Smrzova S, DuBois W, Simakova O, Maric I, Wilson TM, Metcalfe DD, Gilfillan AM. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and non-neoplastic human mast cells. Blood. 2011;118:6803–6813. doi: 10.1182/blood-2011-06-359984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard T, Chavez B, Olfson M, Crystal S. National patterns in the outpatient pharmacological management of children and adolescents with autism spectrum disorder. J Clin Psychopharmacol. 2009;29:307–310. doi: 10.1097/JCP.0b013e3181a20c8a. [DOI] [PubMed] [Google Scholar]
- Lang R, Mahoney R, El Zein F, Delaune E, Amidon M. Evidence to practice: treatment of anxiety in individuals with autism spectrum disorders. Neuropsychiatr Dis Treat. 2011;7:27–30. doi: 10.2147/NDT.S10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-Vanderweele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303–e1311. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- Munshi KR, Gonzalez-Heydrich J, Augenstein T, D’Angelo EJ. Evidence-based treatment approach to autism spectrum disorders. Pediatr Ann. 2011;40:569–574. doi: 10.3928/00904481-20111007-08. [DOI] [PubMed] [Google Scholar]
- Nazeer A. Psychopharmacology of autistic spectrum disorders in children and adolescents. Pediatr Clin North Am. 2011;58:85–97. doi: 10.1016/j.pcl.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Parikh MS, Kolevzon A, Hollander E. Psychopharmacology of aggression in children and adolescents with autism: a critical review of efficacy and tolerability. J Child Adolesc Psychopharmacol. 2008;18:157–178. doi: 10.1089/cap.2007.0041. [DOI] [PubMed] [Google Scholar]
- Posey DJ, Stigler KA, Erickson CA, McDougle CJ. Antipsychotics in the treatment of autism. J Clin Invest. 2008;118:6–14. doi: 10.1172/JCI32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez B, Chavez-Brown M, Sopko MA Jr, Rey JA. Atypical antipsychotics in children with pervasive developmental disorders. Paediatr Drugs. 2007;9:249–266. doi: 10.2165/00148581-200709040-00006. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Stigler KA, Erickson CA, Posey DJ. Atypical antipsychotics in children and adolescents with autistic and other pervasive developmental disorders. J Clin Psychiatry. 2008;69(Suppl 4):15–20. [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Dhillon S. Risperidone: a review of its use in the treatment of irritability associated with autistic disorder in children and adolescents. Paediatr Drugs. 2007;9:343–354. doi: 10.2165/00148581-200709050-00006. [DOI] [PubMed] [Google Scholar]
- Curran MP. Aripiprazole: in the treatment of irritability associated with autistic disorder in pediatric patients. Paediatr Drugs. 2011;13:197–204. doi: 10.2165/11207230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychparmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Williams K, Wheeler DM, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2010;8:CD004677. doi: 10.1002/14651858.CD004677.pub2. [DOI] [PubMed] [Google Scholar]
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Donnelly CL, Anagnostou E, Dukes K, Sullivan L, Hirtz D, Wagner A, Ritz L. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR. Citalopram treatment in children with autism spectrum disorders and high levels of repetitive behavior. Arch Gen Psychiatry. 2009;66:581–582. doi: 10.1001/archgenpsychiatry.2009.42. [DOI] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March JS. Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry. 2011;72:655–670. doi: 10.4088/JCP.11r07064. [DOI] [PubMed] [Google Scholar]
- Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10:571–581. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med. 2011;17:78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol. 2012;22(5):895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ. Targeted treatments for cognitive and neurodevelopmental disorders in tuberous sclerosis complex. Neurotherapeutics. 2010;7:275–282. doi: 10.1016/j.nurt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DR, Gholizadeh S, Pacey LK. Pathways to drug development for autism spectrum disorders. Clin Pharmacol Ther. 2012;91:189–200. doi: 10.1038/clpt.2011.245. [DOI] [PubMed] [Google Scholar]
- Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Van Aller GS, Carson JD, Tang W, Peng H, Zhao L, Copeland RA, Tummino PJ, Luo L. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res Commun. 2011;406:194–199. doi: 10.1016/j.bbrc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Asadi S, Zhang B, Weng Z, Angelidou A, Kempuraj D, Alysandratos KD, Theoharides TC. Luteolin and thiosalicylate inhibit HgCl(2) and thimerosal-induced VEGF release from human mast cells. Int J Immunopathol Pharmacol. 2010;23:1015–1020. doi: 10.1177/039463201002300406. [DOI] [PubMed] [Google Scholar]
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation. 2010;7:3. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TK, Ou YC, Lin SY, Pan HC, Song PJ, Raung SL, Lai CY, Liao SL, Lu HC, Chen CJ. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J Nutr Biochem. 2011;22:612–624. doi: 10.1016/j.jnutbio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolferg A, Theoharides TC. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Missau F, Pizzolatti MG, Dos Santos AR, Souza DO, Aschner M, Rocha JB, Dafre AL, Farina M. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol. 2010;30:272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett. 2008;448:175–179. doi: 10.1016/j.neulet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakata T, Shinoda Y, Oka M, Sekine Y, Sato Y, Saruta C, Miwa H, Tanaka M, Itohara S, Furuichi T. Reduced axonal localization of a Caps2 splice variant impairs axonal release of BDNF and causes autistic-like behavior in mice. Proc Natl Acad Sci USA. 2012;109(51):21104–21109. doi: 10.1073/pnas.1210055109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Asadi S, Panagiotidou S. A case series of a luteolin formulation (Neuroprotek®) in children with autism spectrum disorders. Intl J Immunopathol Pharmacol. 2012;25:317–323. doi: 10.1177/039463201202500201. [DOI] [PubMed] [Google Scholar]