Abstract
Background
The preventive effects of histamine 2 receptor antagonists vs. proton pump inhibitors on low-dose aspirin (LDA)-related gastroduodenal mucosal injury have not been fully investigated. We conducted a cross-sectional study to compare the prevalence of gastroduodenal ulcers or erosions in patients taking LDA with either 40 mg/day of famotidine or 15 mg/day of lansoprazole for at least three months.
Methods
Of 84 eligible patients, two taking 40 mg/day of famotidine and four taking 15 mg/day of lansoprazole refused to undergo upper gastrointestinal endoscopy. Ultimately, we performed upper gastrointestinal endoscopy in 78 patients taking either 40 mg/day of famotidine (group F, n = 31) or 15 mg/day of lansoprazole (group L, n = 47). The prevalence of gastroduodenal ulcers or erosions and the magnitude of gastric mucosal injury evaluated using modified Lanza scores were compared between the two groups.
Results
No patients in either group had gastroduodenal ulcers. Gastroduodenal erosions were more prevalent in group F than in group L (48.4% vs. 17.0%, p = 0.005). The modified Lanza scores (mean ± SD) were significantly higher in group F than in group L (0.9 ± 1.3 vs. 0.3 ± 0.7, p = 0.007). A multivariate logistic regression analysis showed that the use of lansoprazole was negatively associated with gastroduodenal erosions.
Conclusions
This study suggests that 15 mg/day of lansoprazole may be more effective in preventing the development of LDA-related gastroduodenal erosions than 40 mg/day of famotidine. The preventive effects of these two regimens on the development of LDA-related gastroduodenal ulcers require further investigation.
Keywords: Aspirin, Gastroduodenal erosion, Gastroduodenal ulcer, Famotidine, Lansoprazole
Background
Low-dose aspirin (LDA) (75 – 325 mg/day), which is widely used for the primary and secondary prevention of cardiovascular events and the prevention of coronary stent thrombosis [1,2], is associated with a 2- to 4-fold increased risk of upper gastrointestinal complications, such as gastroduodenal ulcers and bleeding [3,4]. Therefore, it is clinically important to prevent gastroduodenal mucosal injury in patients talking LDA. Proton pump inhibitors (PPIs) are considered to be the preferred agents for prophylaxis of LDA-related gastroduodenal mucosal injury [5]. Famotidine, a histamine 2 receptor antagonist (H2RA), has also been reported to suppress the development of LDA-related gastroduodenal ulcers [6]. One prospective, randomized, comparative study [7] examined the effects of H2RAs vs. PPIs on the primary prevention of LDA-related gastric mucosal injury and showed that 15 mg/day of lansoprazole is more effective in preventing LDA-related gastric mucosal injury than 40 mg/day of famotidine. However, in that study, the duration of treatment was only seven days. Another prospective, randomized, comparative study [8] examined the effects of H2RAs vs. PPIs on the secondary prevention of LDA-related dyspeptic or bleeding gastroduodenal ulcers and erosions and showed that 80 mg/day of famotidine is inferior to 20 mg/day of pantoprazole in the secondary prevention of recurrent dyspeptic or bleeding gastroduodenal ulcers and erosions. However, in that study, follow-up endoscopic examinations were performed only in patients with dyspepsia or bleeding (melena or hematemesis). It is well known that LDA-related gastroduodenal mucosal injury is often asymptomatic [9,10]. Therefore, whether there are distinct differences in the effects of H2RAs vs. PPIs on the primary and secondary prevention of LDA-related gastroduodenal mucosal injury has not been fully clarified. We previously demonstrated that PPIs, but not H2RAs, are negatively and independently associated with gastroduodenal ulcers or erosions in asymptomatic patients taking LDA [11]. In the present study, we compared the prevalence of gastroduodenal ulcers or erosions in patients taking LDA with either 40 mg/day of famotidine or 15 mg/day of lansoprazole for at least three months.
Methods
This cross-sectional study was conducted between April 2011 and June 2012 at Oita University Hospital in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the ethics committee of Oita University Hospital, and written informed consent was obtained from all patients before enrollment.
Study population
Eligible inpatients or outpatients of the Cardiology Division of Oita University Hospital who were taking LDA with either 40 mg/day of famotidine or 15 mg/day of lansoprazole for at least three months were prospectively enrolled in this study, unrelated to the presence or absence of gastrointestinal symptoms. The inclusion criteria were as follows: age > 20 years; no history of surgery for esophageal, gastric or duodenal diseases; neither known gastrointestinal bleeding nor gastroduodenal ulcers within the previous 12 months; neither acute coronary syndrome nor stroke within the previous three months; an absence of severe chronic heart failure (New York Heart Association functional class IV); a lack of use of steroids or nonsteroidal anti-inflammatory drugs; and an absence of malignant diseases.
Data collection
The following demographic data were collected: age, gender, body mass index, the presence or absence of current smoking, daily alcohol drinking (unrelated to the amount of alcohol), hypertension, dyslipidemia and diabetes mellitus, a history of cardiovascular disease, gastroduodenal ulcers or eradication of Helicobacter pylori (H. pylori), gastrointestinal symptoms, the H. pylori infection status and current medications. Current smoking was defined as daily or non-daily smoking at the time of study entry. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg or treatment with antihypertensive medications. Dyslipidemia was defined as a serum low-density lipoprotein cholesterol level ≥140 mg/dl, a high-density lipoprotein cholesterol level <40 mg/dl, a triglyceride level ≥150 mg/dl or treatment with lipid-lowering drugs. Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dl, a plasma glucose level ≥200 mg/dl at two hours after a 75-g glucose load or treatment with hypoglycemic agents. Gastrointestinal symptoms (reflux, abdominal pain and indigestion) were evaluated using the gastrointestinal symptom rating scale [12] immediately before esophagogastroduodenal endoscopy. The presence or absence of H. pylori infection was examined using a urine-based enzyme-linked immunosorbent assay (Otsuka Pharmaceutical, Tokyo, Japan) [13,14].
Endoscopic examinations
Esophagogastroduodenal endoscopy was performed without cessation of LDA. An ulcer was defined as a mucosal defect having a significant depth, measuring at least 3 mm over its longest diameter. An erosion was defined as a mucosal defect measuring less than 3 mm. The magnitude of gastric mucosal injury was assessed using the modified Lanza score [15] (Table 1). The evaluations were conducted by experienced endoscopists who were blinded to all clinical data.
Table 1.
Modified Lanza scores
| Score | Findings |
|---|---|
| 0 |
No hemorrhage or erosions observed |
| 1 |
One or two hemorrhages or erosions observed in one gastric area |
| 2 |
Three to five hemorrhages or erosions observed in one gastric area |
| 3 |
Hemorrhages or erosions observed in two gastric areas or six or more hemorrhages or erosions observed in one gastric area, with the total number not exceeding 10 in the entire stomach |
| 4 |
Hemorrhages or erosions observed in three or more gastric areas or 11 or more hemorrhages or erosions observed in the entire stomach |
| 5 | Gastric ulcers |
Statistical analysis
Continuous data are expressed as the mean ± SD. Comparisons of continuous variables between two groups were made using the unpaired t-test or the Mann–Whitney U-test. Comparisons of categorical variables between two groups were made using the Fisher’s exact test or the chi-square test. A multivariate logistic regression analysis was performed to determine whether the use of 15 mg/day of lansoprazole was independently associated with gastroduodenal erosions. A p value of <0.05 was considered to be statistically significant. All analyses were performed using the IBM SPSS Statistics 20 software package (International Business Machines Corp., New York, USA).
Results
Between April 2011 and June 2012, there were 84 eligible patients taking LDA with either 40 mg/day of famotidine or 15 mg/day of lansoprazole for at least three months. Of the 84 patients, two taking 40 mg/day of famotidine and four taking 15 mg/day of lansoprazole refused to undergo upper gastrointestinal endoscopy. Ultimately, 78 patients taking LDA with either 40 mg/day of famotidine (group F, n = 31) or 15 mg/day of lansoprazole (group L, n = 47) for at least three months were enrolled in the present study (Figure 1).
Figure 1.
Flow chart of the study cohort.
The patient characteristics are shown in Table 2. There were no significant differences in patient characteristics between the two groups.
Table 2.
Patient characteristics
| Group F (n = 31) | Group L (n = 47) | p value | |
|---|---|---|---|
| Age (years) |
72.0 ± 9.3 |
74.6 ± 8.8 |
0.22 |
| Male |
26 (83.9%) |
32 (68.1%) |
0.19 |
| Body mass index (kg/m2) |
23.8 ± 3.4 |
24.3 ± 3.6 |
0.50 |
| Hypertension |
25 (80.6%) |
44 (93.6%) |
0.14 |
| Diabetes mellitus |
9 (29.0%) |
18 (38.3%) |
0.47 |
| Current smoking |
0 (0%) |
2 (4.3%) |
0.52 |
| Daily alcohol drinking |
12 (38.7%) |
13 (27.7%) |
0.33 |
| Coronary artery disease |
26 (83.9%) |
43 (91.5%) |
0.47 |
| Prior percutaneous coronary intervention |
25 (80.6%) |
39 (83.0%) |
1.0 |
| Prior coronary bypass surgery |
1 (3.2%) |
2 (4.3%) |
1.0 |
| Prior stroke |
4 (12.9%) |
4 (8.5%) |
0.71 |
| Atrial fibrillation |
6 (19.4%) |
7 (14.9%) |
0.76 |
| History of gastroduodenal ulcer |
5 (16.1%) |
9 (19.1%) |
0.77 |
| History of eradication of H. pylori |
3 (9.7%) |
5 (10.6%) |
1.0 |
| Gastrointestinal symptom rating scale |
|
|
|
| Reflux symptom score |
1.4 ± 0.6 |
1.3 ± 0.6 |
0.19 |
| Abdominal pain score |
1.4 ± 0.7 |
1.2 ± 0.5 |
0.32 |
| Indigestion score |
1.4 ± 0.6 |
1.6 ± 0.6 |
0.24 |
| Positive urinary H. pylori antibodies |
15 (48.4%) |
22 (46.8%) |
1.0 |
| Duration of lansoprazole or famotidine treatment |
|
|
0.76 |
| <1 year |
7 (14.9%) |
6 (19.4%) |
|
| ≥1 year(s) |
40 (85.1%) |
25 (80.6%) |
|
| Dose of aspirin |
|
|
1.0 |
| 81 mg/day |
1 (3.2%) |
2 (4.3%) |
|
| 100 mg/day |
30 (96.8%) |
45 (95.7%) |
|
| Type of aspirin |
|
|
1.0 |
| Enteric-coated formulation |
30 (96.8%) |
45 (95.7%) |
|
| Buffered formulation |
1 (3.2%) |
2 (4.3%) |
|
| Other antiplatelet agents |
12 (38.7%) |
17 (36.2%) |
1.0 |
| Warfarin |
8 (25.8%) |
8 (17.0%) |
0.40 |
| Dabigatran |
2 (7.4%) |
2 (4.3%) |
0.62 |
| Nitrates |
10 (32.3%) |
16 (34.0%) |
1.0 |
| Calcium antagonists |
14 (45.2%) |
26 (55.3%) |
0.49 |
| Angiotensin-converting enzyme inhibitors |
3 (9.7%) |
4 (8.5%) |
1.0 |
| Angiotensin receptor blockers |
14 (45.2%) |
30 (63.8%) |
0.16 |
| Statins | 27 (87.1%) | 42 (89.4%) | 1.0 |
The data are presented as the mean ± SD or number (%).
No patients had gastroduodenal ulcers. Gastroduodenal erosions were more prevalent in group F than in group L (48.4% vs. 17.0%, p = 0.005) (Figure 2).
Figure 2.
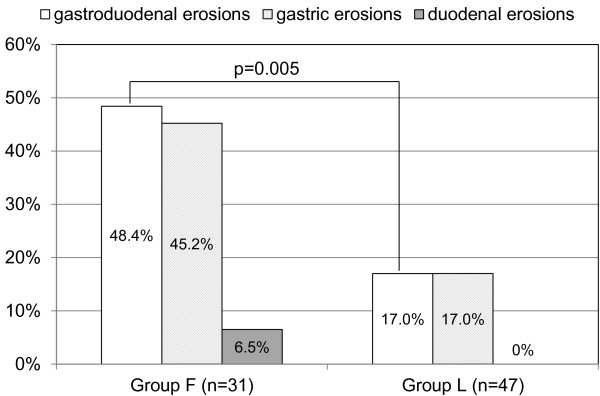
Comparison of the prevalence of gastroduodenal erosions between patients taking 40 mg/day of famotidine (group F) and those taking 15 mg/day of lansoprazole (group L).
The modified Lanza scores (mean ± SD) were significantly higher in group F than in group L (0.9 ± 1.3 vs. 0.3 ± 0.7, p = 0.007). After adjusting for confounding factors, including age, male gender, body mass index, current smoking, daily alcohol drinking, a history of gastroduodenal ulcers and positive urinary H. pylori antibodies, the use of lansoprazole was found to be negatively associated with gastroduodenal erosions (odds ratio 0.18, 95% confidence interval 0.06-0.59, p = 0.005).
Discussion
Primary findings
The primary findings of the present study are as follows: (1) gastroduodenal erosions were less prevalent in patients taking LDA with 15 mg/day of lansoprazole than in those taking LDA with 40 mg/day of famotidine; (2) no patients in either group had gastroduodenal ulcers; and (3) the multivariate logistic regression analysis showed that the use of 15 mg/day of lansoprazole was negatively associated with gastroduodenal erosions.
Previous studies
Although PPIs are considered to be the preferred agents for prophylaxis of LDA-related gastroduodenal mucosal injury [5], there are only two prospective, randomized, comparative studies examining the effects of H2RAs vs. PPIs on the prevention of LDA-related gastric mucosal injury [7,8]. Nishio et al. [7] randomly administered 100 mg/day of aspirin, 100 mg/day of aspirin plus 40 mg/day of famotidine or 100 mg/day of aspirin plus 15 mg/day of lansoprazole for seven days in a crossover fashion in 15 H. pylori-negative healthy volunteers and compared the magnitude of gastric mucosal injury among the three regimens using modified Lanza scores. The results showed that the modified Lanza scores were significantly lower in the 100 mg/day of aspirin plus 15 mg/day of lansoprazole group than in the other two groups, suggesting that 15 mg/day of lansoprazole may be more effective in preventing LDA-related gastric mucosal injury than 40 mg/day of famotidine. However, in that study, the subjects were H. pylori-negative healthy young volunteers, and the duration of treatment was only seven days. Ng et al. [8] compared the recurrence rate of LDA-related dyspeptic or bleeding gastroduodenal ulcers and erosions occurring within 48 weeks between 65 patients receiving famotidine (80 mg/day) treatment and 65 patients receiving pantoprazole (20 mg/day) treatment and showed that the recurrence rate of dyspeptic or bleeding gastroduodenal ulcers and erosions was lower in the latter group than in the former group (0% vs. 9.2%, p = 0.03). However, in that study, follow-up endoscopic examinations were performed only in patients with dyspepsia or bleeding (melena or hematemesis). Therefore, the exact effects of H2RAs vs. PPIs on the primary and secondary prevention of LDA-related gastroduodenal mucosal injury have not been fully clarified.
The present study
In the present study, we compared the prevalence of gastroduodenal ulcers or erosions in patients taking LDA with either 40 mg/day of famotidine or 15 mg/day of lansoprazole for at least three months and found that gastroduodenal erosions were less prevalent in patients taking LDA with 15 mg/day of lansoprazole than in those taking LDA with 40 mg/day of famotidine. This result suggests that 15 mg/day of lansoprazole may be more effective in preventing the development of LDA-related gastroduodenal erosions than 40 mg/day of famotidine. Gastroduodenal erosions are the most readily observable finding of initial gastroduodenal mucosal injury following aspirin administration [16]. Gastric acid plays an important role in the development of LDA-related gastroduodenal mucosal injury, and the severity of LDA-related gastric mucosal injury is associated with gastric acidity [7,17]. Because PPIs are more effective in suppressing the secretion of gastric acid than H2RAs [7,17,18], the findings of the present study are reasonable. However, it should be noted that, in the present study, the mean modified Lanza score among the patients taking 40 mg/day of famotidine was low and that the difference in the scores between the two groups was small. In the present study, none of the patients taking either 40 mg/day of famotidine or 15 mg/day of lansoprazole had gastroduodenal ulcers. Yeomans et al. [10] reported that gastroduodenal ulcers were observed in 10.7% of 187 patients taking LDA without gastroprotective medications. In the OBERON trial, which compared the occurrence of peptic ulcers over 26 weeks among patients taking LDA with those taking a placebo, 20 mg/day of esomeprazole or 40 mg/day of esomeprazole, peptic ulcers developed in 7.4% of 805 placebo recipients (an intention-to-treat analysis) [19]. Given that, in the present study, 82.1% of the patients had no history of gastroduodenal ulcers and that none of the patients in the famotidine group had gastroduodenal ulcers, 40 mg/day of famotidine may be as effective as 15 mg/day of lansoprazole in the primary prevention of LDA-related gastroduodenal ulcers. The preventive effects of 40 mg/day of famotidine vs. 15 mg/day of lansoprazole on the development of LDA-related gastroduodenal ulcers require further investigation.
The clinical significance of gastroduodenal erosions in patients taking LDA
The prevention of gastroduodenal erosions is considered to be clinically important in patients taking LDA for the following reasons. First, in patients taking LDA, some gastroduodenal erosions may bleed. It has been shown that in 16% to 23% of patients with upper gastrointestinal bleeding, gastroduodenal erosions are the cause of the bleeding [20,21]. Furthermore, gastric erosions have been reported to be the second most common source of gastrointestinal bleeding after acute myocardial infarction [22] in which the use of LDA is essential for secondary prevention. Second, some gastroduodenal erosions may develop into gastroduodenal ulcers in patients taking LDA. It has been reported that one-sixth of gastroduodenal erosions become gastroduodenal ulcers after 17 years [23]. Furthermore, the presence of gastroduodenal erosions has been reported to increase the risk of developing gastroduodenal ulcers in nonsteroidal anti-inflammatory drug users [24].
The strength and limitations of the present study
The strength of the present study is the sufficient sample size to detect significant differences in the prevalence of gastroduodenal erosions between the two groups. A post hoc power determinant analysis showed that the present study has an estimated power of 85% with a two-sided alpha risk of 0.05. On the other hand, the present study has a few limitations. First, this study had a cross-sectional design, which could potentially carry a risk of reverse causation. The duration of lansoprazole and famotidine treatment was not identical between the two groups. In addition, we did not investigate why either 40 mg/day of famotidine or 15 mg/day of lansoprazole was selected for each patient. Therefore, prospective, randomized, longitudinal studies are needed to clarify whether 15 mg/day of lansoprazole is indeed more effective in preventing LDA-related gastroduodenal erosions than 40 mg/day of famotidine. Second, we did not investigate the mechanisms of the lower prevalence of gastroduodenal erosions observed in the lansoprazole group. Third, larger doses of famotidine and lansoprazole may be more effective in preventing LDA-related gastroduodenal erosions. Further investigations are required to clarify the associations between the doses of famotidine and lansoprazole and their preventive effects on the development of LDA-related gastroduodenal erosions.
Conclusion
The results of the present study suggest that 15 mg/day of lansoprazole may be more effective in preventing LDA-related gastroduodenal erosions than 40 mg/day of famotidine. Elucidating the preventive effects of these two regimens on the development of LDA-related gastroduodenal ulcers, however, still requires further investigation.
Appendix
OITA-GF Study 2 investigators: Y. Goto, K. Torigoe, Y. Kawano, K. Shinozaki, M. Kotoku, Internal Medicine 2, Oita University; Y. Hirashita, R. Ogawa, O. Matsunari, T. Abe, S. Shiota, K. Mizukami, Y. Nakagawa, M. Uchida, T. Okimoto, M. Kodama, General Medicine, Oita University.
Abbreviations
H. pylori: Helicobacter pylori; H2RA: Histamine 2 receptor antagonist; LDA: Low-dose aspirin; PPI: Proton pump inhibitor
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AT, KM and JK were involved in the design of the study, the review of the literature and the writing of the manuscript. The OITA-GF2 investigators were involved in the data collection. All authors read and approved the manuscript.
Authors’ information
The investigators of the OITA-GF study 2 are listed in Appendix.
Contributor Information
Akira Tamura, Email: akira@oita-u.ac.jp.
Kazunari Murakami, Email: Murakam@oita-u.ac.jp.
Junichi Kadota, Email: kadota@oita-u.ac.jp.
References
- Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC Jr, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2004 Writing Committee Members. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on practice guidelines: developed in collaboration with the Canadian cardiovascular society endorsed by the American academy of family physicians: 2007 writing group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevationmyocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;2008(117):296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons, endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, Rawlins M, Vessey M, Wainwright P. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ. 1995;310:827–830. doi: 10.1136/bmj.310.6983.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, Olsen JH. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95:2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American college of cardiology foundation task force on clinical expert consensus documents. Circulation. 2008;118:1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119–125. doi: 10.1016/S0140-6736(09)61246-0. [DOI] [PubMed] [Google Scholar]
- Nishino M, Sugimoto M, Kodaira C, Yamade M, Uotani T, Shirai N, Ikuma M, Tanaka T, Sugimura H, Hishida A, Furuta T. Preventive effects of lansoprazole and famotidine on gastric mucosal injury induced by low-dose aspirin in Helicobacter pylori-negative healthy volunteers. J Clin Pharmacol. 2011;51:1079–1086. doi: 10.1177/0091270010376194. [DOI] [PubMed] [Google Scholar]
- Ng FH, Wong SY, Lam KF, Chu WM, Chan P, Ling YH, Kng C, Yuen WC, Lau YK, Kwan A, Wong BC. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138:82–88. doi: 10.1053/j.gastro.2009.09.063. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Gschossmann J, Buenger L, Gerken G, Talley NJ. Do changes in visceral sensory function determine the development of dyspepsia during treatment with aspirin? Gastroenterology. 2002;123:1451–1458. doi: 10.1053/gast.2002.36556. [DOI] [PubMed] [Google Scholar]
- Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B, Appelman-Eszczuk S, Långström G, Naesdal J, Serrano P, Singh M, Skelly MM, Hawkey CJ. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005;22:795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- Tamura A, Murakami K, Kadota J. OITA-GF Study Investigators. Prevalence and independent factors for gastroduodenal ulcers/erosions in asymptomatic patients taking low-dose aspirin and gastroprotective agents: the OITA-GF study. QJM. 2011;104:133–139. doi: 10.1093/qjmed/hcq169. [DOI] [PubMed] [Google Scholar]
- Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–687. doi: 10.3109/00365529309098272. [DOI] [PubMed] [Google Scholar]
- Katsuragi K, Noda A, Tachikawa T, Azuma A, Mukai F, Murakami K, Fujioka T, Kato M, Asaka M. Highly sensitive urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori. Helicobacter. 1998;3:289–295. doi: 10.1046/j.1523-5378.1998.08045.x. [DOI] [PubMed] [Google Scholar]
- Kato M, Asaka M, Saito M, Sekine H, Ohara S, Toyota T, Akamatsu T, Kaneko T, Kiyosawa K, Nishizawa O, Kumagai T, Katsuyama T, Abe M, Kosaka M, Hariya S, Minami K, Sanai Y, Sawamura M, Tachikawa T. Clinical usefulness of urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori: a collaborative study in nine medical institutions in Japan. Helicobacter. 2000;5:109–119. doi: 10.1046/j.1523-5378.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- Yamao J, Kikuchi E, Matsumoto M, Nakayama M, Ann T, Kojima H, Mitoro A, Yoshida M, Yoshikawa M, Yajima H, Miyauchi Y, Ono H, Akiyama K, Sakurai G, Kinoshita Y, Haruma K, Takakura Y, Fukui H. Assessing the efficacy of famotidine and rebamipide in the treatment of gastric mucosal lesions in patients receiving long-term NSAID therapy (FORCE–famotidine or rebamipide in comparison by endoscopy) J Gastroenterol. 2006;41:1178–1185. doi: 10.1007/s00535-006-1952-5. [DOI] [PubMed] [Google Scholar]
- Brodie DA, Chase BJ. Role of gastric acid in aspirin-induced gastric irritation in the rat. Gastroenterology. 1967;53:604–610. [PubMed] [Google Scholar]
- Nishino M, Sugimoto M, Kodaira C, Yamade M, Shirai N, Ikuma M, Tanaka T, Sugimura H, Hishida A, Furuta T. Relationship between low-dose aspirin-induced gastric mucosal injury and intragastric pH in healthy volunteers. Dig Dis Sci. 2010;55:1627–1636. doi: 10.1007/s10620-009-0920-3. [DOI] [PubMed] [Google Scholar]
- Bell NJ, Hunt RH. Progress with proton pump inhibition. Yale J Biol Med. 1992;65:649–657. [PMC free article] [PubMed] [Google Scholar]
- Scheiman JM, Devereaux PJ, Herlitz J, Katelaris PH, Lanas A, Veldhuyzen van Zanten S, Nauclér E, Svedberg LE. Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON) Heart. 2011;97:797–802. doi: 10.1136/hrt.2010.217547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L, Weinstein WM. Subepithelial hemorrhages and erosions of human stomach. Dig Dis Sci. 1988;33:490–503. doi: 10.1007/BF01536037. [DOI] [PubMed] [Google Scholar]
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding. II. Clinical prognostic factors. Gastrointest Endosc. 1981;27:80–93. doi: 10.1016/S0016-5107(81)73156-0. [DOI] [PubMed] [Google Scholar]
- Cappell MS. The safety and clinical utility of esophagogastroduodenoscopy for acute gastrointestinal bleeding after myocardial infarction: a six-year study of 42 endoscopies in 34 consecutive patients at two university teaching hospitals. Am J Gastroenterol. 1993;88:344–350. [PubMed] [Google Scholar]
- Toljamo KT, Niemelä SE, Karttunen TJ, Karvonen AL, Lehtola JK. Clinical significance and outcome of gastric mucosal erosions: a long-term follow-up study. Dig Dis Sci. 2006;51:543–537. doi: 10.1007/s10620-006-3168-1. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Laine L, Harper SE, Quan HU, Bolognese JA, Mortensen E. Rofecoxib Osteoarthritis Endoscopy Multinational Study Group. Influence of risk factors on endoscopic and clinical ulcers in patients taking rofecoxib or ibuprofen in two randomized controlled trials. Aliment Pharmacol Ther. 2001;15:1593–1601. doi: 10.1046/j.1365-2036.2001.01007.x. [DOI] [PubMed] [Google Scholar]


