Abstract
Multidrug resistance (MDR) confers agrochemical compatibility to fungal cells-based mycoinsecticdes but mechanisms involved in MDR remain poorly understood for entomopathogenic fungi, which have been widely applied as biocontrol agents against arthropod pests. Here we characterized the functions of five ATP-binding cassette (ABC) transporters, which were classified to the subfamilies ABC-B (Mdr1), ABC-C (Mrp1) and ABC-G (Pdr1, Pdr2 and Pdr5) and selected from 54 full-size ABC proteins of Beauveria bassiana based on their main domain architecture, membrane topology and transcriptional responses to three antifungal inducers. Disruption of each transporter gene resulted in significant reduction in resistance to four to six of eight fungicides or antifungal drugs tested due to their differences in structure and function. Compared with wild-type and complemented (control) strains, disruption mutants of all the five transporter genes became significantly less tolerant to the oxidants menadione and H2O2 based on 22−41% and 10−31% reductions of their effective concentrations required for the suppression of 50% colony growth at 25°C. Under a standardized spray, the killing actions of ΔPdr5 and ΔMrp1 mutants against Spodoptera litura second-instar larvae were delayed by 59% and 33% respectively. However, no significant virulence change was observed in three other delta mutants. Taken together, the examined five ABC transporters contribute differentially to not only the fungal MDR but antioxidant capability, a phenotype rarely associated with ABC efflux pumps in previous reports; at least some of them are required for the full virulence of B. bassiana, thereby affecting the fungal biocontrol potential. Our results indicate that ABC pump-dependent MDR mechanisms exist in entomopathogenic fungi as do in yeasts and human and plant pathogenic fungi.
Introduction
Multidrug resistance (MDR) is a major challenge for the control of human, animal and plant pathogenic fungi by antifungal drugs and fungicides [1], [2], [3] but could be a merit for fungal entomopathogens against arthropod pests [4], [5]. This is because fungal cells, such as conidia produced on solid substrates, are the active ingredients of numerous mycoinsecticids and mycoacaricides [6] and MDR may confer their compatibility with chemical fungicides, herbicides and insecticides. Fungal candidate strains with higher MDR are more tolerant to applied chemical pesticides and thus more potential for commercial development and application.
MDR mechanisms in entomopathogenic fungi remain poorly understood although their compatibility with chemical pesticides has been emphasized as one of the determinants to a success of microbial control [7], [8]. Previously, some of common β-tubulin point mutations that are attributed to benzimidazole resistance in phytopathogenic fungi [9], [10] were found in Beauveria bassiana mutants with extraordinarily high carbendazim resistance [11]. However, none of such point mutations was found in Isaria fumosorosea mutants that showed not only as high carbendazim resistance as in the B. bassiana mutants but also resistance to other compounds different in structure and function [12]. Interestingly, all the I. fumosorosea mutants had three common point mutations occurred at the binding sites of the transcription factors Gal4, Abf1 and Raf in the promoter region of an ATP-binding cassette (ABC) transporter gene (ifT1) and thus their ifT1 transcripts were upregulated by 17- to 137-fold. This implies that ABC transporter-dependent MDR mechanism exists in the fungal entomopathogens.
As a large family, ABC transporter proteins can energize the transport of a huge variety of compounds across biological membranes through ATP hydrolysis and confer cellular resistance to a broad spectrum of drug substrates [i.e., MDR or PDR (pleiotropic drug resistance) phenomenon] or a very limited number of substrates [13]. They are structurally featured with essential nucleotide-binding domain(s) (NBD) and one or two hydrophobic transmembrane domains (TMDs) and usually composed of six K-helical transmembrane segments (TMSs), forming the domain architectures of full-size [(TMS6−NBD)2 or (NBD−TMS6)2], half-size (TMS6−NBD) and TMD-lacking (NBD or NBD2) transporters [14]. Those associated with MDR/PDR are all full-size members classified to the subfamilies ABC-B (MDR type), ABC-C (MRP type, i.e., multidrug resistance-associated proteins) and ABC-G (PDR type) [15]. In human and plant pathogens, MDR/PDR results from drug efflux pumped by ABC transporters to reduce intracellular drug accumulation to toxic level at target sites [16], [17]. For instance, two PDR-type transporters, Cdr1p and Cdr2p, contribute differentially to azole resistance in Candida albicans [18], [19] due to their structural differences associated with substrate specificities and transport mechanism [20], [21]. ABC transporters also mediate cellular tolerance to natural toxic compounds and xenobiotics and/or virulence in many phytopathogenic fungi [22], [23], [24], [25]. Interestingly, the coding gene of a PDR-type ABC transporter in wheat supports durable resistance to wheat pathogenic fungi [26].
To explore possible MDR mechanisms in B. bassiana, we characterized the functions of three types of five representative proteins, which were selected from all full-size ABC transporter proteins by analyzing their phylogenetic and structural features and assessing their expressional responses to three different antifungal drugs. We found that the five transporters made differential contributions to the fungal MDR, antioxidation and virulence by multi-phenotypic comparisons of their single-gene disruption mutants with wild-type and complement strains
Results
Features of ABC transporters in B. bassiana
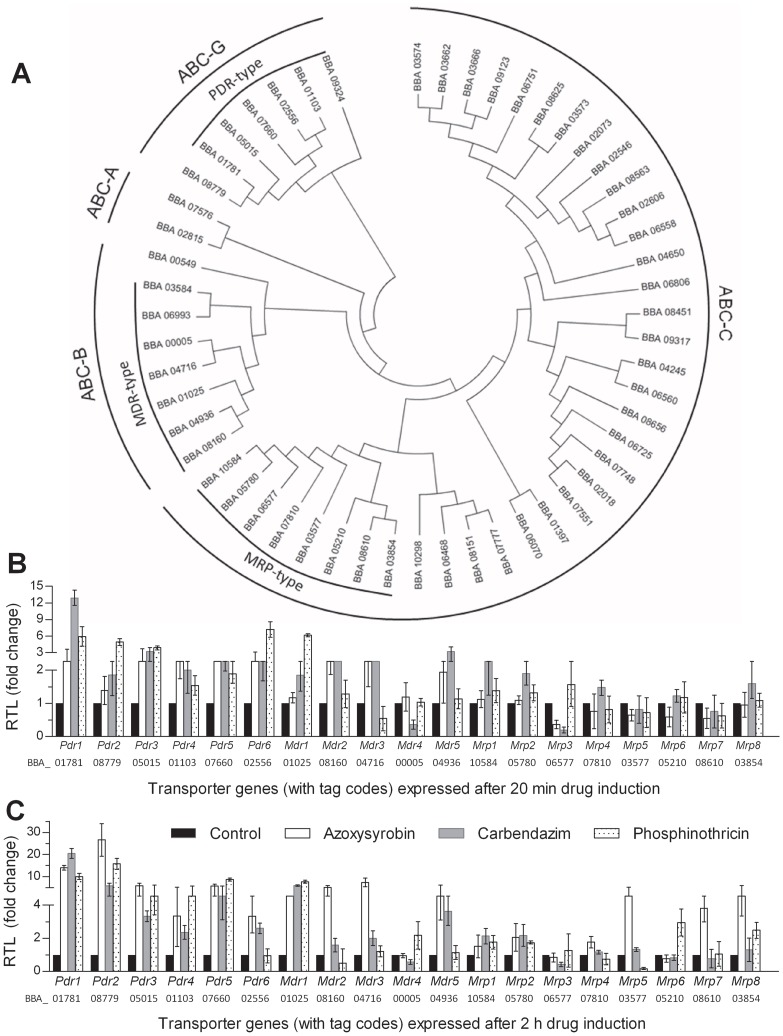
Up to 425 transporter proteins were blasted from the annotated genome of the wild-type strain B. bassiana ARSEF 2860 (Bb2860 or wild type herein) [27], including 54 putative ABC pumps coupled with the queries of conserved NBD and TMD regions of budding yeast Ste6p, Pdr5p or Yor1p in the NCBI protein database. The 54 proteins were classified to four ABC subfamilies (Fig. 1A). The largest ABC-C subfamily includes 37 members, of which eight are likely MRP-type transporters based on their membrane topology. Further comparison of domain architecture led to the recognition of seven ABC-B and six ABC-G proteins as potential MDR- and PDR-type transporters respectively. All the 21 recognized transporters of three types are featured with two NBDs and two TMDs
Figure 1. Screening of full-size ABC transporter proteins associated with multidrug resistance in B. bassiana (Bb2860).
(A) Phylogenetic analysis of 54 full-size ABC proteins. (B), (C) Relative transcript levels (RTL) of 21 ABC transporter genes in the wild-type SDB cultures induced with carbendazim (5 µg/ml), azoxysyrobin (100 µg/ml) and phosphinothricin (100 µg/ml) for 20 min and 2 h at 25°C respectively. Error bars: SD of the mean from three cDNA samples assessed via qRT-PCR with paired primers (Table S1).
The 21 ABC transporters were assessed for the levels of their gene transcripts in wild-type hyphal cells induced with azoxysyrobin, carbendazim and phosphinothricin for 20 min and 2 h at 25°C respectively. As a result of quantitative real-time PCR (qRT-PCR) with paired primers (Table S1), about half of them were upregulated by the drug inducers within 20 min (Fig. 1B). Longer induction enhanced their transcripts to higher levels (Fig. 1C). Consequently, Pdr1, Pdr2, Pdr5, Mdr1 and Mrp1 were chosen as the representatives of the three types because they were inductively upregulated by all the three drugs. Notably, Mdr6 and Mdr7 transcripts were consistently undetectable in the cDNAs from the samples induced or not induced with the drugs (data not shown).
The coding genes of the selected five transporters were disrupted from Bb2860 and complemented into their disruption mutants by integration of the bar- and sur-inclusive plasmids via Agrobacterium-mediated transformation respectively. Putative mutant colonies grown on selective plates were sequentially identified via PCR, reverse transcription PCR (RT-PCR) and Southern blotting with paired primers and amplified probes (Table S2). As a result of the identification, the profiling band or signal for each target gene was consistently present in the wild-type and complement strains (control strains) but absent in the disruption mutant (Fig. S1). Thus, five single-gene disruption mutants were compared with the control strains to differentiate their phenotypic changes below.
Differentiated MDR responses
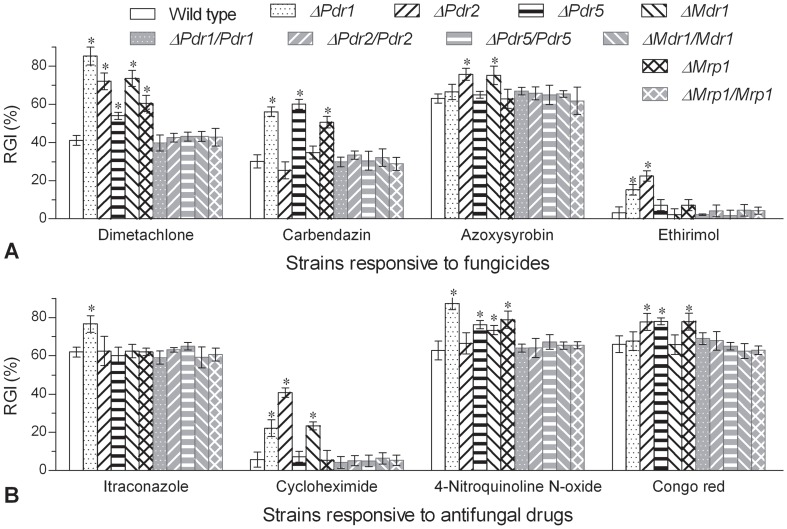
All the five disruption mutants showed differential resistance to different types of four fungicides (Fig. 2A) and four antifungal drugs (Fig. 2B) during 6-day growth on 1/4 SDAY at 25°C but their control strains responded equally to each drug (Tukey's HSD, P>0.1). Compared with the means of relative growth inhibition (RGI) values observed in the control strains, six, five and four of the tested chemicals were significantly more inhibitory to ΔPdr1 (12−43%), ΔPdr2 (11−35%) and three other delta mutants (9−31%), respectively. During the colony growth, dimetachlone exerted inhibitory effect on all the delta mutants while itraconazole, azoxysyrobin and ethirimol were influential only on one or two of them. Null responses were observed in ΔPdr1 to Congo red and azoxysyrobin and in ΔPdr2 to carbendazim, itraconazole and 4-nitroquinoline-N-oxide. These data indicated that the spectra and preference of drug substrates were partially different among the five ABC transporters.
Figure 2. Changes in multidrug resistance of five single-gene disruption mutants of B. bassiana.
(A) Relative growth inhibition (RGI) of fungal colonies after 6-day incubation at 25°C on 1/4 SDAY supplemented with the fungicides dimetachlone (0.1 mg/ml), carbendazim (0.5 µg/ml), azoxysyrobin (0.1 mg/ml) and ethirimol (1 mg/ml) respectively. (B) RGI values of fungal colonies after 6-day incubation at 25°C on 1/4 SDAY supplemented with the antifungal drugs itraconazole (5 µg/ml), cyclonheximide (20 µg/ml), 4-nitroquinoline-N-oxide (5 µg/ml) and Congo red (0.5 mg/ml) respectively. The bars of each group marked with asterisks differed significantly from those unmarked (Tukey's HSD, P<0.05). Error bars: SD of the mean from three repeated assays.
Additionally, all the disruption mutants and the control strains grew equally well on drug-free SDAY or 1/4 SDAY at 25°C (P>0.15 in F tests) and responded equally to hyperosmotic (NaCl) stress (F 10,22 = 1.25, P = 0.32; data not shown).
Differentiated antioxidation responses
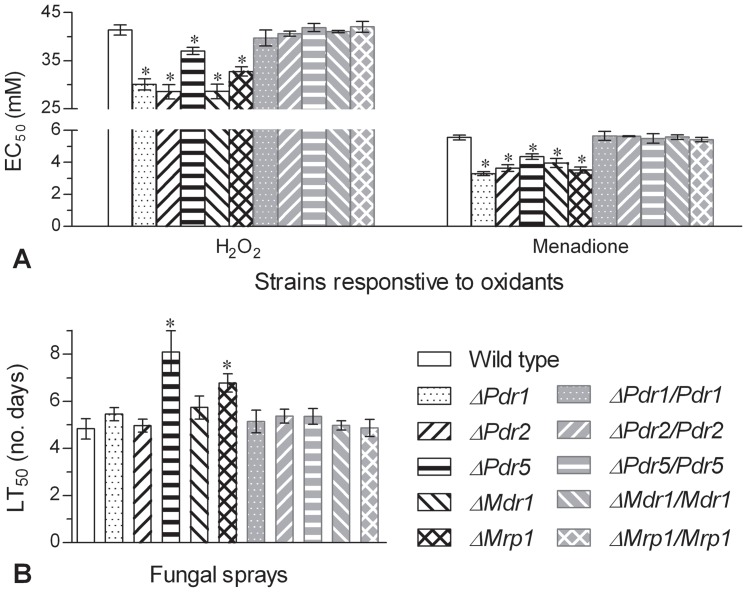
Two oxidants, H2O2 and menadione, were assayed for their effective concentrations (EC50s) to suppress 50% colony growth of each strain by modeling analysis of relative growth trend over the gradient concentrations of each oxidant after 6-day incubation at 25°C. Compared with the EC50 estimates of menadione [5.6 (±0.09) mM] and H2O2 [41.1 (±0.87) mM] towards the control strains (Fig. 3A), all the five delta mutants were 22−41% less tolerant to menadione and 10−31% less tolerant to H2O2 (Tukey's HSD, P<0.01).
Figure 3. Changes in antioxidant capability and virulence of five single-gene disruption mutants of B. bassiana.
(A) Effective concentrations (EC50s) estimated for H2O2 and menadione to suppress 50% colony growth by modeling analysis of relative growth trends over the concentrations of 0−80 mM H2O2 or 0−8 mM menadione added to 1/4 SDAY. (B) Median lethal times (LT50s) of wild-type and mutant strains against the second-instar larvae of S. litura under a standardized spray. The bars of each group marked with asterisks differed significantly from those unmarked (Tukey's HSD, P<0.05). Error bars: SD of the mean from three repeated assays.
Differentiated virulence
Time-mortality trends of all the tested strains against the second-instar larvae of Spodoptera litura in the bioassays standardized by a uniform spray of conidial suspension in an automatic spray tower were differentiated by probit analysis. Median lethal time (LT50) estimates fell in a narrow range of 4.8−5.3 (average 5.1) days for all the control strains (Tukey's HSD, P≥0.15) but significantly increased to 8.1 and 6.8 days for ΔPdr5 and ΔMrp1 respectively (Fig. 3D). However, no significant LT50 differences were found between other delta mutants and the control strains.
Discussion
B. bassiana harbors 21 full-size ABC transporter genes that may act as potential MDR regulators due to their classification, membrane topology and domain architecture. Of those, however, Mdr6 and Mdr7 had no detectable transcriptional signals in the cDNAs from the total RNAs of the wild-type cultures induced with drugs or not induced, suggesting a likelihood of their pseudogene status. Five representatives selected by their transcript levels inductively upregulated by azoxysyrobin, carbendazim and phosphinothricin were confirmed contributing differentially to the fungal MDR, antioxidation and virulence but not involving in osmoregulation, as discussed below.
First of all, up to 425 transporter proteins can be blasted from the genome of B. bassiana [27] and the counts of the counterparts in the genomes of Metarhizium robertsii (previously in M anisopliae sensu lato) and M. acridum [28] are 304 and 307 respectively. Despite a remarkable diversity, only a small proportion of them are full-size ABC pumps of B. bassiana (Fig. 1A) and an even smaller proportion are likely associated with the fungal MDR/PDR in terms of their membrane topology and main domain architecture (14,15,21). The five transporters more responsive to the three inducers (Fig. 1) were all proven to regulate MDR in B. bassiana because their single-gene disruption mutants showed less resistance to four to six of the eight antifungal drugs (Fig. 2), which were used in MDR assays due to differences in structure and function. Apparently, ABC pump-dependent MDR mechanisms exist in entomopathogenic fungi as do in yeasts and human and plant pathogenic fungi [13], [16].
Despite partially overlapping drug spectra, the examined five transporters showed some degree of substrate preference based on the MDR changes in their delta mutants. The preferred substrate was dimetachlone for Pdr1 and Mdr1, carbendazim for Pdr5 and Mrp1, and cycloheximide for Pdr2. As a broad-spectrum fungicide, dimetachlone was a mere common substrate for the five transporters but itraconazole was specific to only Pdr1 among the tested drugs. Moreover, the drug spectrum was broadest for Pdr1, followed by Pdr2 and three others. Regardless of broader or narrower substrate spectrum, Pdr1, Pdr2, Pdr5, Mdr1 and Mrp1 are functionally very close to other fungal ABC transporters, such as those mediating Aspergillus nidulans resistance to all major classes of fungicides [29], Botrytis cinerea sensitivity to phenylpyrrole fungicides [30], and Mycosphaerella graminicola responses to azole fungicides [25]. Their drug preferences, substrate spectra and MDR levels altered by single-gene disruption are partially different from one to another. This is in accordance with those of documented fungal ABC pumps between different types [13] or within a type [18], [19] and likely due to low primary sequence similarity between their TMDs [21]. Thus, the five transporters of B. bassiana regulate differentially the fungal MDR/PDR.
Apart from differential responses to the tested antifungal drugs, all five delta mutants showed significantly less, but differential, resistance to the oxidants menadione and H2O2 (Fig. 3A). Fungal antioxidant capability has rarely been associated with ABC transporters in previous studies but is important for the success of B. bassiana infection. This capability usually depends on the activities of antioxidative enzymes, such as catalases [31] and superoxide dismutases [32], [33], and can be regulated by cellular signaling pathways, such as the mitogen-activated protein kinase cascades of Hog1 [34] and Slt2 [35], P-type calcium ATPase [36] and Ras1/Ras2 GTPases [37]. Particularly, fungal tolerance to oxidation is linearly correlated with B. bassiana UV resistance and virulence [33], [37], two parameters important for the fungal biocontrol potential. Thus, the antioxidant capability reduced by the disruption of each ABC transporter gene implies that the fungal pathogen is less capable of scavenging harmful superoxide anions often generated from infected host cells. We consider that the five transporters could pump both oxidants as they usually pump xenobiotic efflux although they are not antioxidant enzymes.
Finally, fungal virulence has been infrequently associated with the effects of ABC transporters but this association has been found in some phytopathogenic fungi. For instance, three ABC pumps, namely ABC1 in Magnaporthe grisea [24], NhABC1 in Nectria haematococca [22] and BcatrB in Botrytis cinerea [38], have proved to influence the fungal virulence due to their pumping action of cytotoxic compound efflux. In this study, only Pdr5 and Mrp1 were found contributing significantly to the virulence of B. bassiana to S. litura larvae because the killing actions of their delta mutants under a standardized spray were 59% and 33% slower than those of the control strains (Fig. 3B). However, three other transporters we examined showed null effect on the fungal virulence. Taken together with previous reports and our results, not all ABC transporters are contributors to fungal virulence but at least some of them are necessary for the full virulence of a fungal pathogen, thereby affecting the biocontrol potential of B. bassiana.
Materials and Methods
Microbial strains and culture conditions
The wild-type strain Bb2860 was cultured on Sabouraud dextrose agar plus 1% yeast extract (SDAY) at 25°C and used as a recipient of gene manipulation and expression. Escherichia coli Top10 and E. coli DH5α from Invitrogen (Shanghai, China) used for vector propagation were cultured at 37°C in LB medium plus kanamycin (100 µg/ml). For fungal transformation, A. tumefaciens AGL-1 was cultured in YEB medium [39] at 28°C.
Phylogenetic, structural and transcriptional analyses of B. bassiana ABC transporters
The conserved NBD and TMD regions of the typical ABC transporters Ste6p, Pdr5p and Yor1p in Saccharomyces cerevisiae (NCBI accession codes: NC_001143.9, NC_00147.6 and NC_00113.9 respectively) were used as queries to locate PDR-, MDR- and MRP-type transporters respectively in the sequenced genome of Bb2860 under the NCBI accession ADAH00000000 [27] via blastp (http://blast.ncbi.nlm.nih.gov/blast.cgi) and BioEdit analysis (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The resultant protein sequences were screened to remove half-size transporters not associated with MDR in terms of their domain architecture and then classified to ABC subfamilies following a documented system [15] via phylogenetic analysis with MEGA 4.0 software [40]. Their membrane topological features were further analyzed to assess the likelihood of their involvements in fungal MDR/PDR [13] via CD search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), generating 21 full-size ABC transporters (Mdr1−7, Mrp1−8 and Pdr1−6) for study.
To assess the transcriptional expression levels of the selected transporters in response to different antifungal chemicals, Bb2860 was grown in 50 ml aliquots of Sabouraud dextrose broth (SDB) inoculated to 1×106 conidia/ml. After 2-day shaking by 120 rpm at 25°C, hyphal cells were harvested from the cultures and transferred to the same volume of fresh SDB supplemented with carbendazim (benzimidazole fungicide, 5 µg/ml), azoxysyrobin (broad-spectrum fungicide, 100 µg/ml) or phosphinothricin (herbicide, 100 µg/ml) for the induction of 20 min and 2 h at 25°C respectively. Total RNAs were extracted from the drug-induced and drug-free (control) cultures. Three samples of 5 µg RNA from each extract was reversely transcribed with PrimeScript™ RT kit (Takara, Dalian, China). The cDNA samples (diluted to 10 µg/ml) synthesized with the kit were assessed for the transcript levels of all the 21 transporter genes via qRT-PCR with paired primers (Table S1) using B. bassiana 18S rRNA as internal standard. The relative transcript level of each gene in a drug-induced sample versus control was calculated as its transcript ratio using the method 2−ΔΔCt [41]. Five transporter genes whose transcript levels were inductively upregulated by all the three drugs were selected for further study, including Pdr1, Pdr2, Pdr5, Mdr1 and Mrp1.
Single-gene disruption and complementation
The plasmid p0380-bar vectoring the bar marker and PtrpC promoter [33] was used as backbone to construct the disruption plasmids of all selected genes except Pdr1. The 5′ and 3′ fragments of Pdr2 (1261 and 1874 bp), Pdr5 (1560 and 1666 bp), Mdr1 (1480 and 1147 bp) and Mrp1 (1531 and 1320 bp) were separately amplified from Bb2860 via PCR with paired primers (Table S2), digested with specific restriction enzymes, and inserted into p0380-bar, generating p0380-5′x-bar-3′x for the disruption of each target gene (x). To delete Pdr1, alternatively, its ORF fragment (2500 bp) was amplified from Bb2860 with Pdr1-F/R (Table S2) and inserted into p0380-bar linearized with BamHI/HindIII. After digestion with XhoI/SacI, the plasmid released a fragment of ∼200 bp to separate the ORF into two fragments and a PtrpC-bar cassette amplified from p0380-bar with the Insert-F/R primers was inserted between the separated fragments, yielding p0380-pdr1D for Pdr1 disruption.
To rescue each of the disrupted genes, p0380-sur-gateway [33] vectoring the sur marker gene was used as backbone. The full-length sequences with flanking regions of Pdr1 (7899 bp), Pdr2 (7926 bp), Pdr5 (7754 bp), Mdr1 (7850 bp) and Mrp1 (7389 bp) were separately amplified from Bb2860 with paired primers (Table S1) under the action of LATaq polymerase (TaKaRa) and ligated into the backbone to replace the gateway fragment under the action of Gateway® BP ClonaseTM II Enzyme Mix (Invitrogen), forming p0380-sur-x, where x denotes one of the rescued target genes.
All the disruption and complement plasmids were individually transformed into A. tumefaciens AGL-1 for further transformation into Bb2860 or the corresponding disruption mutants using a documented protocol [39] with slight modification. Briefly, the recipient strain (wild type or delta mutant) was co-cultivated with the vector-integrated AGL-1 on induced medium for 48 h at 25°C in dark, followed by washing with ∼5 ml of 0.02% Tween 80. The suspension was spread onto M-100 plates [39] supplemented with cefotaxime (300 µg/ml for suppressing AGL-1 growth) and phosphinothricin (200 µg/ml for the selective growth of disruption mutants) or chorimuron ethyl (10 µg/ml for the selective growth of rescued mutants). All the plates were incubated for 6 days at 25°C and 12∶12 h (light:dark cycle). Colonies grown on the selective plates were identified via PCR, RT-PCR and Southern blotting with paired primers or amplified probes (Table S2). For Southern blotting, 30 µg genomic DNA extracted from the monoclonal culture of each putative mutant on SDAY was digested with XbaI/HindIII, separated via electrophoresis in 0.7% agarose gel, and then transferred to Biodyne B nylon membrane (Gelman Laboratory, Shelton, WA, USA) in Trans-Blot SD Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA). Probe preparation, membrane hybridization and visualization were carried out using DIG High Prime DNA Labeling and Detection Starter Kit II (Roche, Mannheim, Germany). Positive disruption and complement mutants of each target gene were assayed together with wild type for their phenotypic changes in triplicate experiments below.
Assays of multidrug responses
The aliquots of 200 µl conidial suspension (2×107 conidia/ml) were evenly spread onto cellophane-overlaid SDAY plates. After 3-day incubation at 25°C and 12∶12 h, cellophane discs (5 mm diameter) with growing mycelia were cut from the culture of each strain and attached centrally onto the plates (9 cm diameter) of 1/4 SDAY (SDAY nutrients diluted to 1/4) supplemented with the antifungal drugs azoxysyrobin (100 µg/ml), carbendazim (0.5 µg/ml), dimetachlone (pyrrole fungicide, 100 µg/ml), ethirimol (pyrimidine fungicide, 1 mg/ml), itraconazole (triazole agent, 5 µg/ml), cyclonheximide (protein biosynthesis inhibitor, 20 µg/ml), 4-Nitroquinoline N-oxide (potent mutagenic agent, 10 µg/ml) and Congo red (cell wall biosynthesis inhibitor, 500 µg/ml) for MDR assays and NaCl (40 mg/ml) for osmosensitivity assay respectively. All the plates were incubated for 6 days at the same regime, followed by cross-measuring the diameters of their colonies. For each strain stressed with a given drug, relative growth inhibition (RGI) was calculated as (C–N)/(C–19.6)×100, where the constant is the area of inoculated disc, and C and N denote the measurements of colony area (mm2) from the control (free of drug) and drug treatment respectively.
To quantify antioxidant capability of each strain, the plates of the same medium supplemented with the gradient concentrations of menadione (0−8 mM) or H2O2 (0−80 mM) for varying intensity of oxidative stress were inoculated with the culture discs as above. After 6-day incubation at the same regime, colony diameters were cross-measured and the ratio of a stressed colony size over the size of the control colony was defined as relative growth rate (R g). The R g trend of each strain over the concentrations (C) of menadione or H2O2 was fitted to the equation R g = 1/[1−exp(a+bC)]. Solving the fitted equation gave an effective concentration of each oxidant (EC50) to suppress 50% colony growth when R g = 0.5.
Virulence bioassay
All the fungal strains were bioassayed for their changes in virulence to the second-instar larvae of S. litura using a standardized method described elsewhere (36,37). Briefly, batches of 30−40 larvae on cabbage leaf discs (∼10 cm diameter) were separately sprayed with 1 ml of conidial suspension (2×107 conidia/ml) as treatment or 0.02% Tween 80 (used for suspending conidia) as control in automatic Potter Spray Tower (Burkard Scientific Ltd, Uxbridge, UK). After spray, all larvae were reared on the leaf discs in large Petri dishes (15 cm diameter) for 7 days at 25°C and 12∶12 h and fresh leaf discs were supplied daily for their feeding. Mortality in each plate was daily examined during the period. The resultant time-mortality trends were subjected to probit analysis, generating an estimate of medial lethal time (LT50) for each fungal strain against the pest species.
Supporting Information
Paired primers used for assessing the transcript levels of 21 full-size ABC transporter genes in B. bassiana via qRT-PCR.
(DOC)
Paired primers used for the manipulation of five ABC transporter genes in B. bassiana .
(DOC)
Disruption and complementation of five selected ABC transporter genes in B. bassiana wild-type strain (Bb2860).
(JPG)
Funding Statement
Funding for this study was provided by the Natural Science Foundation of China (Grant Nos: 30930018 and 30971960) and the Ministry of Science and Technology of China (Grant No: 2011AA10A204). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bardas GA, Veloukas T, Koutita O, Karaoglanidis GS (2010) Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag Sci 66: 967–973. [DOI] [PubMed] [Google Scholar]
- 2. Cowen LE, Steinbach WJ (2008) Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell 7: 747–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Del Sorbo G, Schoonbeek HJ, De Waard MA (2000) Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet Biol 30: 1–15. [DOI] [PubMed] [Google Scholar]
- 4. Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol 4: 3–34. [Google Scholar]
- 5. Roberts DW, St Leger RJ (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54: 1–70. [DOI] [PubMed] [Google Scholar]
- 6. de Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43: 237–256. [Google Scholar]
- 7. Majchrowicz I, Poprawski TJ (1993) Effects in vitro of nine fungicides on growth of entomopathogenic fungi. Biocontrol Sci Technol 3: 321–336. [Google Scholar]
- 8. Shi WB, Jiang Y, Feng MG (2005) Compatibility of ten acaricides with Beauveria bassiana and Enhancement of fungal infection to Tetranychus cinnabarinus (Acari: Tetranychidae) eggs by sublethal application rates of pyridaben. Appld Entomol Zool 40: 659–666. [Google Scholar]
- 9. Ma ZH, Yoshimura MA, Michailides TJ (2003) Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Appl Environ Microbiol 69: 7145–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma ZH, Yoshimura MA, Michailides TJ (2005) Characterization and PCR-based detection of benzimidazole-resistant isolates of Monilinia laxa in California. Pest Manag Sci 61: 449–457. [DOI] [PubMed] [Google Scholar]
- 11. Zou G, Ying SH, Shen ZC, Feng MG (2006) Multi-sited mutations of beta-tubulin are involved in benzimidazole resistence and thermotolerance of fungal biocontrol agent Beauveria bassiana . Environ Microbiol 8: 2096–2105. [DOI] [PubMed] [Google Scholar]
- 12. Song TT, Ying SH, Feng MG (2012) High resistance of Isaria fumosorosea to carbendazim arises from the overexpression of an ABC transporter (ifT1) rather than tubulin mutation. J App Microbiol 112: 175–184. [DOI] [PubMed] [Google Scholar]
- 13. Klein C, Kuchler K, Valachovic M (2011) ABC proteins in yeast and fungal pathogens. Essays Biochem 50: 101–119. [DOI] [PubMed] [Google Scholar]
- 14. Lamping E, Baret PV, Holmes AR, Monk BC, Goffeau A, et al. (2010) Fungal PDR transporters: Phylogeny, topology, motifs and function. Fungal Genet Biol 47: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kovalchuk A, Driessen AJM (2010) Phylogenetic analysis of fungal ABC transporters. BMC Genomics 11: 177 doi:10.1186/1471-2164-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ernst R, Kueppers P, Stindt J, Kuchler K, Schmitt L (2010) Multidrug efflux pumps: Substrate selection in ATP-binding cassette multidrug efflux pumps – first come, first served? FEBS Journal 277: 540–549. [DOI] [PubMed] [Google Scholar]
- 17. Morschhäser J (2010) Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 47: 94–106. [DOI] [PubMed] [Google Scholar]
- 18. Holmes AR, Lin YH, Niimi K, Lamping E, Keniya M, et al. (2008) ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother 52: 3851–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsao S, Rahkhoodaee F, Raymond M (2009) Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance. Antimicrob Agents Chemother 53: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanabe K, Lamping E, Nagi M, Okawada A, Holmes AR, et al. (2011) Chimeras of Candida albicans Cdr1p and Cdr2p reveal features of pleiotropic drug resistance transporter structure and function. Mol Microbiol 82: 416–433. [DOI] [PubMed] [Google Scholar]
- 21. Zolnerciks JK, Andress EJ, Nicolaou M, Linton KJ (2011) Structure of ABC transporters. Essays Biochem 50: 43–61. [DOI] [PubMed] [Google Scholar]
- 22. Coleman JJ, White GJ, Rodriguez-Carres M, VanEtten HD (2011) An ABC transporter and a cytochrome P450 of Nectria haematococca MPVI are virulence factors on pea and are the major tolerance mechanisms to the phytoalexin pisatin. Mol Plant-Microbe Interact 24: 368–376. [DOI] [PubMed] [Google Scholar]
- 23. Schoonbeek HJ, Del Sorbo G, De Waard MA (2001) The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol Plant-Microbe Interact 14: 562–571. [DOI] [PubMed] [Google Scholar]
- 24. Urban M, Bhargava T, Hamer JE (1999) An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J 18: 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zwiers LH, De Waard MA (2000) Characterization of the ABC transporter genes MgAtr1 and MgAtr2 from the wheat pathogen Mycosphaerella graminicola . Fungal Genet Biol 30: 115–125. [DOI] [PubMed] [Google Scholar]
- 26. Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323: 1360–1363. [DOI] [PubMed] [Google Scholar]
- 27. Xiao GH, Ying SH, Zheng P, Wang ZL, Zhang SW, et al. (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana . Sci Rep 2: 483 doi:10.1038.srep00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao Q, Jin K, Ying SH, Zhang YJ, Xiao GH, et al. (2011) Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum . PLoS Genet 7: e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andrade AC, Del Sorbo G, Van Nistelrooy JGM, De Waard MA (2000) The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology-(UK) 146: 1987–1997. [DOI] [PubMed] [Google Scholar]
- 30. Vermeulen T, Schoonbeek H, De Waard MA (2001) The ABC transporter BcatrB from Botrytis cinerea is a determinant of the activity of the phenylpyrrole fungicide fludioxonil. Pest Manag Sci 57: 393–402. [DOI] [PubMed] [Google Scholar]
- 31. Wang ZL, Zhang LB, Ying SH, Feng MG (2013) Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ Microbiol 15: 409–418. [DOI] [PubMed] [Google Scholar]
- 32. Xie XQ, Wang J, Ying SH, Feng MG (2010) A new manganese superoxide dismutase identified from Beauveria bassiana enhances virulence and stress tolerance when overexpressed in the fungal pathogen. Appl Microbiol Biotechnol 86: 1543–1553. [DOI] [PubMed] [Google Scholar]
- 33. Xie XQ, Li F, Ying SH, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana . PLoS One 7: e30298 doi:10.1371/journal.pone.00302988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang YJ, Zhao JH, Fang WG, Zhang JQ, Luo ZB, et al. (2009) Mitogen-acticated protein kinase hog1 in the entomopathogenic fungus Beauveria bassiana regulates environmental stress responses and virulence to insects. Appl Environ Microbiol 75: 3787–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo X, Keyhani NO, Yu X, He Z, Luo Z, et al. (2012) The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana . Fungal Genet Biol 49: 544–555. [DOI] [PubMed] [Google Scholar]
- 36. Wang J, Zhou G, Ying SH, Feng MG (2013) P-type calcium ATPase functions as a core regulator of Beauveria bassiana growth, conidiation and responses to multiple stressful stimuli through cross-talk with signaling networks. Environ Microbiol 15: 967–979. [DOI] [PubMed] [Google Scholar]
- 37. Xie XQ, Guan Y, Ying SH, Feng MG (2013) Differentiated functions of Ras1 and Ras2 proteins in regulating the germination, growth, conidiation, multi-stress tolerance and virulence of Beauveria bassiana . Environ Microbiol 15: 447–462. [DOI] [PubMed] [Google Scholar]
- 38. Stefanato FL, Abou-Mansour E, Buchala A, Kretschmer M, Mosbach A, et al. (2009) The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana . Plant J 58: 499–510. [DOI] [PubMed] [Google Scholar]
- 39. Fang WG, Zhang YJ, Yang XY, Zheng XL, Duan H, et al. (2004) Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marks. J Invertebr Pathol 85: 18–24. [DOI] [PubMed] [Google Scholar]
- 40. Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT . Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Paired primers used for assessing the transcript levels of 21 full-size ABC transporter genes in B. bassiana via qRT-PCR.
(DOC)
Paired primers used for the manipulation of five ABC transporter genes in B. bassiana .
(DOC)
Disruption and complementation of five selected ABC transporter genes in B. bassiana wild-type strain (Bb2860).
(JPG)