Abstract
The Drosophila humoral innate immune response fights infection by producing antimicrobial peptides (AMPs) through the microbe-specific activation of the Toll or the Imd signaling pathway. Upon systemic infection, the production of AMPs is both positively and negatively regulated to reach a balanced immune response required for survival. Here, we report the function of the dRYBP (drosophila Ring and YY1 Binding Protein) protein, which contains a ubiquitin-binding domain, in the Imd pathway. We have found that dRYBP contributes to the negative regulation of AMP production: upon systemic infection with Gram-negative bacteria, Diptericin expression is up-regulated in the absence of dRYBP and down-regulated in the presence of high levels of dRYBP. Epistatic analyses using gain and loss of function alleles of imd, Relish, or skpA and dRYBP suggest that dRYBP functions upstream or together with SKPA, a member of the SCF-E3-ubiquitin ligase complex, to repress the Imd signaling cascade. We propose that the role of dRYBP in the regulation of the Imd signaling pathway is to function as a ubiquitin adaptor protein together with SKPA to promote SCF-dependent proteasomal degradation of Relish. Beyond the identification of dRYBP as a novel component of Imd pathway regulation, our results also suggest that the evolutionarily conserved RYBP protein may be involved in the human innate immune response.
Introduction
Biological pathways involved in stress responses, like those associated with innate immunity, must quickly and efficiently modulate gene expression to ensure survival of the organism. Drosophila uses the evolutionarily conserved host defense of innate immunity to protect against microbial infection and relies mainly on the Toll and Imd pathways to regulate the expression of different AMP genes (for recent reviews see [1]–[3]). AMPs are constitutively expressed in immuno-competent epithelial tissues to defend the body against infection [4], [5]. Furthermore, upon systemic microbial infection the Toll and Imd pathways up-regulate AMP production by the fat body and blood cells. Once the infection is controlled, AMP expression is down-regulated to avoid deleterious immuno-pathological reactions [6].
The Imd signaling pathway is activated by infection with Gram-negative bacteria and Gram-positive bacilli [7]. The activation is initiated upon detection of peptidoglycan (PGN) by PGRP-LC, a member of the peptidoglycan recognition proteins, at the plasma membrane [7]–[9]. Transduction of this signal requires ligand-induced receptor oligomerization with subsequent assembly of a signaling complex containing IMD, DREDD, and dFADD receptor associated proteins [2], [10]–[12]. The activation of this pathway leads to the post-translational modification of the Drosophila NF-κB factor Relish, and its nuclear translocation [13]. Relish ultimately drives transcription of IMD-specific AMP genes such as Diptericin and Attacin-B as well as several regulatory Imd pathway components.
Regulation of NF-κB pathway activity in both invertebrates and vertebrates is achieved at multiple levels through ubiquitin-mediated post-translational modification of signaling components [14], [15]. In Drosophila, selective linkage of mono- or poly-ubiquitin chains triggering degradation or stabilization of Imd pathway components plays a crucial role in maintaining a balanced immune response. IMD first undergoes cleavage by the caspase DREDD, which itself is activated by poly-ubiquitylation [16], [17]. The cleaved IMD protein is then tagged with K63-linked poly-ubiquitin chains by the E3 ligase dIAP2 in complex with E2 conjugases Uev1a, dUbc13/Bendless and Effete [17], [18], and ubiquitylated IMD acts as an assembly platform for downstream adaptors TAB2/dTAK1 [19]. Subsequent editing of K63- to K48-linked ubiquitin chains through the ubiquitin hydrolase dUsp36 ends signaling by targeting IMD for proteasomal degradation [20]. As in vertebrates, ubiquitylation/deubiquitylation events also regulate stability of the Drosophila IKK complex (ird5/key) [19], [21]–[23]. DREDD-mediated cleavage of Relish is thought to be held in check by Caspar, a protein with multiple ubiquitin-related domains [24], and by DNR1, a RING-domain containing protein which binds to DREDD and has been proposed to target it for proteasomal degradation [25]. Finally, both intact and processed Relish has been suggested to undergo ubiquitin-mediated degradation through SKPA, a member of the E3-ubiquitin ligase SCF complex [26].
The dRYBP (drosophila Ring and YY1 Binding Protein) gene [27] encodes a protein that is evolutionarily conserved in vertebrates (known as RYBP/DEDAF/YAF2) and that contains in its N-terminus a ubiquitin-binding domain of the NZF (Nucleoporin Zinc Finger) type [28]. Studies both in vertebrates and Drosophila have described the phenotypic effects of high and low levels of dRYBP expression and also its interactions with several proteins involved in a range of biological processes, including epigenetic transcriptional regulation mediated by the Polycomb and trithorax groups of proteins [27], [29]–[34]. Human RYBP/DEDAF has been shown to interact with DED (Death Effector Domain) containing proteins [35], [36] that mediate homotypic interactions important for the assembly and activation of apoptotic and inflammatory complexes [37]. In Drosophila, high levels of dRYBP induces apoptosis in imaginal disc cells and this apoptosis is dependent on dFADD and DREDD [31], two DED containing proteins [37] also involved in the IMD-mediated immune response in Drosophila [10]–[12].
In this work we show that dRYBP contributes to the negative regulation of the Imd signaling pathway. Notably, we have found that dRYBP is required for the inhibition of the production of AMPs upon systemic infection. We propose that dRYBP functions in the immune response to promote ubiquitin-dependent degradation of IMD-pathway components, among those the Relish protein.
Materials And Methods
Fly stocks
CantonS were used as control flies. dRYBP null allele stocks were dRYBP1/CyO GFP and dRYBPΔ55/ CyO GFP [30]. Deficiencies uncovering the dRYBP genomic region were Df(2R)BSC598 and Df(2R)BSC787 (http://flybase.bio.indiana.edu). The GAL4 lines used were c564-Gal4, en-Gal4, hs-Gal4 (http://flybase.bio.indiana.edu). The UAS lines used were: UAS-dRYBP, UAS-dRYBPRNAi [30], UAS-imd [38], UAS-skpARNAi (http://www.flyrnai.org/TRiP-HOME.html), UAS-Relish-His-6 [39], pUASt-Venus-dRYBP (this work). The UAS-VDRCRNAi lines [40] were obtained from http://stockcenter.vdrc.at.
Adult infection
The Gram-negative entomopathogen Erwinia carotovora carotovora 15 (Ecc15), and the Gram-negative Escherichia coli (E. coli) were grown overnight as shaking cultures in LB medium at 29°C (Ecc15) and 37°C (E. coli) and pelleted to an OD600 of 200 (Ecc15) or to an OD600 of 400 (E. coli). Systemic infection of flies was done by pricking a needle dipped in a bacterial pellet into the thorax of 2–5-day old adult females. Where appropriate, pathogens were heat-killed at 95°C during 15 min.
Cloning and generation of transgenic flies
The coding sequence of dRYBP (BDGP DGC clone LD18758) without start codon was cloned into pENTR-D-TOPO (Life Technologies) using the following primers: Forward: 5′-CACCGACAAGAAATCCTCGCCG- 3′, Reverse: 5′-CTAACTCCGGCTGTCGTTG-3′. The Gateway system (Life Technologies) was used to generate the expression vector pUASt-Venus-dRYBP. Transgenic flies were obtained following standard procedures using white1118 flies as host.
Quantitative real-time PCR (qRT-PCR)
RNA was isolated from 10–15 adult female flies of appropriate genotype. RNA extraction and RT reactions were performed as previously described [31]. qRT-PCR was performed using FastStart Universal SYBR Green MasterRox (Roche) in an Applied Biosystems 7900 Sequence Detector System. Quantified mRNA levels were expressed as relative fold change normalized to RpL32. The sequences of the primers and their efficiencies (in brackets) are the following:
RpL32 Forward: 5′-GACGCTTCAAGGGACAGTATCTG-3′, Reverse: 5′-AAACGCGGTTCTGCATGAG-3′ (1,89); dRYBP Forward: 5′-CATGTTGACACCTGGCTCCTG-3′, Reverse: 5′-CGAAGGTGATCGAGGAGAAC-3′ (1,97) ; Dpt: Forward: 5′-GCTGCGCAATCGTTCTACT-3′, Reverse: 5′-TGGTGGAGTGGGCTTCATG-3′ (1,98) ; AttB Forward: 5′-CCTACAACAATGCTGGTCATGGT-3′, Reverse: 5′-CCTACAACAATGCTGGTCATGGT-3′ (2,03); Relish Forward: 5′-TTAGCGTGGCCAACACAATG-3′, Reverse: 5′-GAACTGCCATGTGGAGTGCAT-3′ (1,98); PGRP-LC Forward: 5′-GCATTCAATGGTGGTCCCA-3′ Reverse: 5′-CCGGATCTTCGTGTTTGGAG-3′ (1,97); imd Forward: 5′-TTCGGCTCCGTCTACAACTT-3′, Reverse: 5′-GTGATCGATTATGGCCTGGT-3′ (2,03); Dredd F: 5′-CAAAAGGTGGGCCTCTGCT Reverse: 5′-GTAGGTGGCATCCGAGTGGT-3′ (2,02); Tab-2 Forward: 5′-TGTCATGGAGGAATGCGATC-3′, Reverse: 5′-GCTTCTGACGCTCGATAGTGG-3′ (1,97); TAK1 Forward: 5′-GATCTGAGTCCCAGCGAAAGC-3′, Reverse: 5′-CATCGCTCTTTGCGTTCGT-3′ (1,96); ird-5 Forward: 5′-TAGTGATCCATTGGCGAAACC-3′, Reverse: 5′-GCTTGGTGGCAATTTCACG-3′ (1,96); skpA Forward: 5′-CTCCCGAGGAAATACGCAAG-3′, Reverse: 5′-CGGGCGAAAAGTCCTTCTTA-3′ (1,99); dIAP2 Forward: 5′-ATGCAAGGTATGCTTGGACGA-3′, Reverse: 5′-TGATTGCAGGTGGCCAAGT-3′ (1,90)
Statistical analysis
The data from all the qRT-PCR experiments represent the mean + SEM of three biological repeats with 10–15 individuals per sample. For inter-assay comparability, values within each experiment were routinely normalized to the wild type or relevant other control at a given time point. Data were analyzed using ANOVA with Bonferroni post-test.
Immuno-staining
Fat bodies from adult females were dissected in PBS, fixed in 4% paraformaldehyde and stained with either anti-dRYBP antibody (1∶100) [27] and biotinylated anti-rabbit antibody (1∶200) as previously described [31], or with rabbit-anti-GFP (Interchim) followed by Alexa488-anti-rabbit (Molecular Probes) and DAPI. Images were taken on either a Zeiss CDD microscope or a Zeiss confocal microscope with a 40-fold oil-immersion objective.
Results
Loss of dRYBP results in over-activation of the Imd pathway
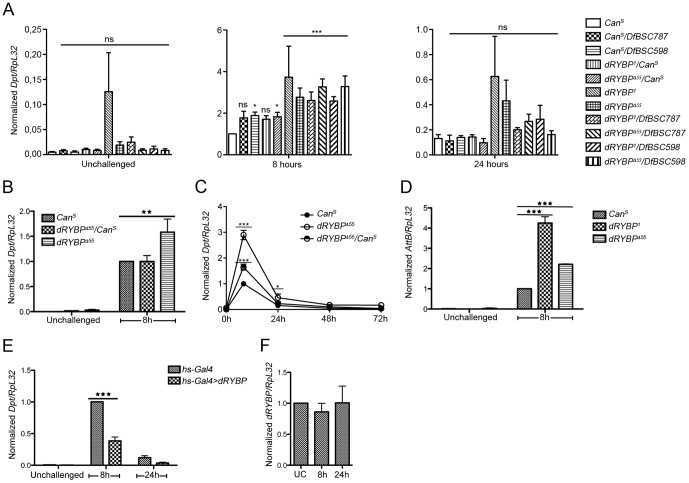
The phenotypes associated with null alleles of dRYBP indicate that this gene plays a role in diverse biological processes [30]. We performed a genetic screen to search for dRYBP interacting genes and found a number of components of the Imd pathway (Fig. S1). Therefore, we chose to analyze the possible involvement of dRYBP in modulating the immune response to Gram-negative infection. We investigated whether the dRYBP gene could be regulating the expression of antimicrobial peptides (AMPs), which are always induced upon activation of the immune response. mRNA expression levels of Diptericin (Dpt), an AMP normally induced upon activation of the Imd pathway [41], were measured by qRT-PCR in dRYBP null adult flies [30]. In uninfected adult dRYBP mutant flies, Dpt expression was not significantly altered compared to the wild-type control (Fig. 1A, unchallenged). However, upon infection with the Gram-negative bacterium Erwinia carotovora carotovora 15 (Ecc15), Dpt expression was significantly increased beyond wild type levels after 8 h of infection in dRYBP1and dRYBPΔ55 homozygous mutant flies, as well as in flies of dRYBP1 or dRYBPΔ55 genotype over dRYBP genomic deficiencies excluding background effects (Fig. 1A, 8 hours). dRYBP mutants showed a normal return to baseline at 24h after challenge (Fig 1A, 24 hours). Infection with another Gram-negative pathogen, E.coli, gave similar results to Ecc15 (Fig. 1B). Moreover, the increase in Dpt expression was dose dependent since dRYBP heterozygous mutant flies showed an intermediate phenotype, suggesting that one dose of dRYBP is insufficient to ensure wild type regulation (Fig. 1A and C). Furthermore, expression of AttB, another IMD-dependent AMP [42], was similarly affected by loss of dRYBP (Fig. 1D). Taken together, these results indicate that dRYBP functions as a negative modulator of the IMD-mediated immune response.
Figure 1. Genetic modulation of dRYBP expression levels affects AMP production.
(A, B, C, D, E). (A) All tested dRYBP mutants show an excessive immune response to Gram-negative infection. Adult females of indicated genotypes were left unchallenged (left panel) or infected by pricking with Ecc15 (heat-killed at 95°C during 15 min) and collected at 8 h (middle panel) and 24 h (right panel) after infection for Diptericin (Dpt) mRNA quantification. The complete data set (unchallenged, 8 h, 24 h) was analysed by two-way ANOVA with Bonferroni post-tests, using CanS as control. (B) dRYBP mutants also show excessive Imd activation after infection with another Gram-negative pathogen, E. coli. Adult females of indicated genotypes were treated as in (A). (C) Infection time-course in wild-type, heterozygous and homozygous dRYBPΔ55 mutants. Flies were treated as in (A). (D) Loss of dRYBP affects at least two Imd-dependent antimicrobial peptides. mRNA levels of Attacin-B (AttB) were quantified after infection as described in (A). (E) Overexpression of dRYBP reduces the Imd response to Gram-negative infection. Adult females were infected as in (A), then were heat-shocked for 1 h at 37°C and collected at 8 h and 24 h after infection for quantification of Dpt mRNA levels. (F) dRYBP expression levels are not affected by infection. Wild-type adult females were infected as in (A) and dRYBP mRNA levels were monitored over time. For all graphs, data represent mean + SEM of at least 3 biological repeats, and asterisks denote the following p values: *, 0.01<p<0.05; **, 0.001<p<0.01; *** p<0.001; ns, not significant.
The up-regulation of the Dpt expression observed in dRYBP mutants prompted us to study whether high levels of dRYBP expression were capable of repressing Dpt production. mRNA levels of Dpt were measured in hs-Gal4;UAS dRYBP flies in the presence or absence of Ecc15 infection. Fig. 1E shows that dRYBP overexpression significantly reduced Dpt expression following 8 h of infection. The reduction in Dpt expression in the presence of high levels of dRYBP further supports the notion that dRYBP is contributing to the negative regulation of the IMD-pathway mediated immune response.
Several negative regulators of the Imd pathway, including PGRP-LF, Pirk and PGRP-LB, are induced upon Gram-negative infection and form a negative feedback loop [43]–[47]. We therefore tested whether dRYBP expression levels in adult flies were affected by infection with Ecc15. mRNA levels of dRYBP, as measured by qRT-PCR, were not significantly changed after either 8 h or 24 h of infection (Fig. 1F). This result is in agreement with microarray studies [48], [49] and suggests that dRYBP-mediated regulation of Dpt expression is not controlled at the level of its own transcription.
Expression of Imd pathway components in dRYBP mutant flies
Because dRYBP has been suggested to function as a transcriptional regulator together with the Polycomb and trithorax proteins [27], [30] we analyzed whether dRYBP could be involved in the transcriptional regulation of canonical Imd pathway components. For this, we quantified the mRNA levels of selected Imd pathway components in homozygous dRYBP1 and dRYBPΔ55 mutant flies both in unchallenged conditions and after 8 h of infection with Ecc15. Expression levels of all Imd pathway components studied were unaffected in dRYBP mutant flies compared to control CanS flies, whether in unchallenged conditions (Fig. 2A) or after 8 h of infection (Fig. 2B). Moreover, we also studied whether over-expression of dRYBP affects the expression of the most downstream component, Relish. Fig. 2C shows that high levels of dRYBP do not influence Relish expression. These results indicate that dRYBP-mediated repression of Dpt expression is not due to dRYBP acting directly as a transcriptional repressor on canonical Imd pathway genes.
Figure 2. Loss of dRYBP does not affect expression of canonical Imd pathway components.
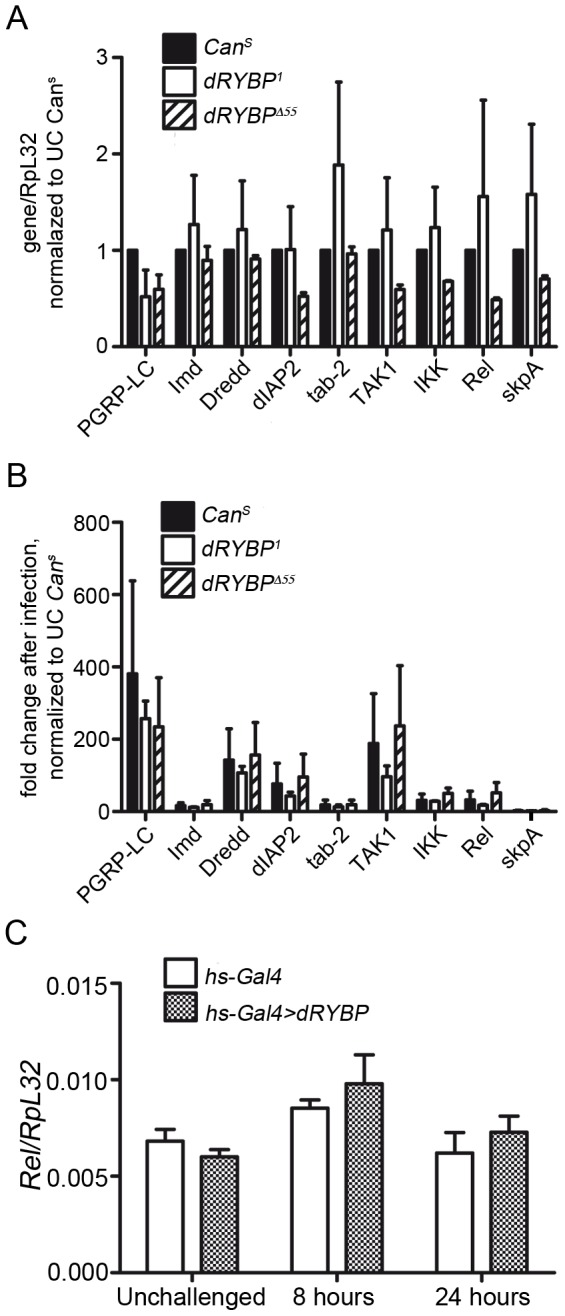
(A) mRNA levels of indicated Imd pathway components were quantified in wild-type and dRYBP mutant flies under unchallenged conditions (A) or (B), 8 h after infection by pricking with Ecc15. Data in (A) and (B) represent mean + SEM of two pooled biological repeats. For each gene, expression is normalized to expression in unchallenged (uc) CanS. No significant difference in gene expression between wild-type and dRYBP mutants, by two-way ANOVA with Bonferroni post-test. (C) Overexpression of dRYBP does not affect Relish expression. Adult females were infected as in (B), then were heat-shocked for 1 h at 37°C and collected at 8 h and 24 h after infection for quantification of mRNA levels of the IMD-dependent transcription factor Relish. Data represent mean + SEM of 3 biological repeats.
dRYBP is expressed in the nucleus of fat body cells
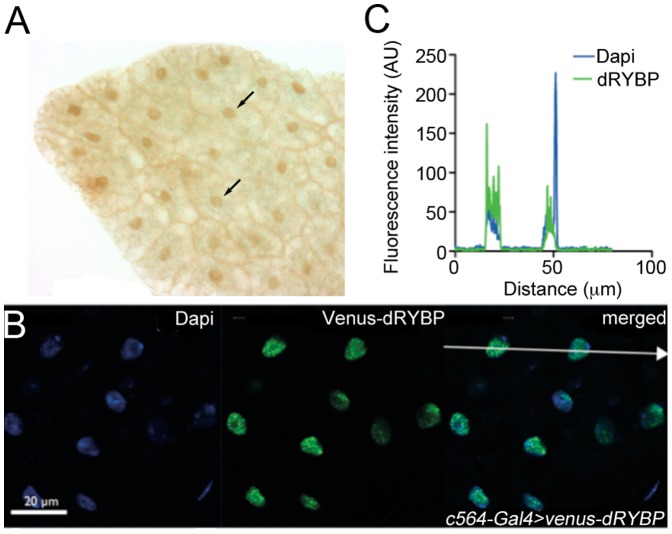
We have previously shown that dRYBP is ubiquitously expressed in the embryo and the imaginal discs of the larva and localizes to the nuclei of cells [27]. We performed immuno-staining with anti-dRYBP antibody (Fig. 3A) in fat body cells and found that dRYBP is also present in the nuclei of this immuno-competent tissue in wild type conditions, which is in agreement with FlyAtlas data [50] (CG12190, http://www.flyatlas.org). Since the Imd pathway includes both cytosolic and nuclear steps, we asked whether dRYBP localized to any cellular compartment in particular during infection. To this aim we constructed UAS-Venus-dRYBP flies and used the c564-Gal4 line to drive Venus-dRYBP expression specifically in the fat body where AMPs are highly up-regulated in response to systemic infection [2]. As shown in Fig. 3B and C, the subcellular localization of dRYBP in adult female fat body cells was exclusively nuclear at 3 h after infection with Ecc15, as shown by fluorescence intensity profiles across cells. This suggests that either the putative dRYBP expression in the cytoplasm is too low to be detected using this approach or that dRYBP acts on nuclear Imd pathway components rather than upstream cytosolic adaptors.
Figure 3. dRYBP localizes to the nuclei of adult fat body cells.
(A) dRYBP protein is expressed in the nuclei of fat body cells (arrows). (B) Flies overexpressing Venus-dRYBP under the fat body specific driver c564-Gal4 were infected by pricking with Ecc15. Fat bodies were dissected 3 h after infection, fixed and stained with anti-GFP antibody and DAPI. Images are representative of several UAS-Venus-dRYBP insertion lines. (C) Fluorescence profile along arrow in (B) shows dRYBP is exclusively nuclear and excluded from nucleoli. AU (Arbitrary Units). Scale bar denotes 20 µm.
Epistatic relationships between dRYBP and components of the Imd pathway
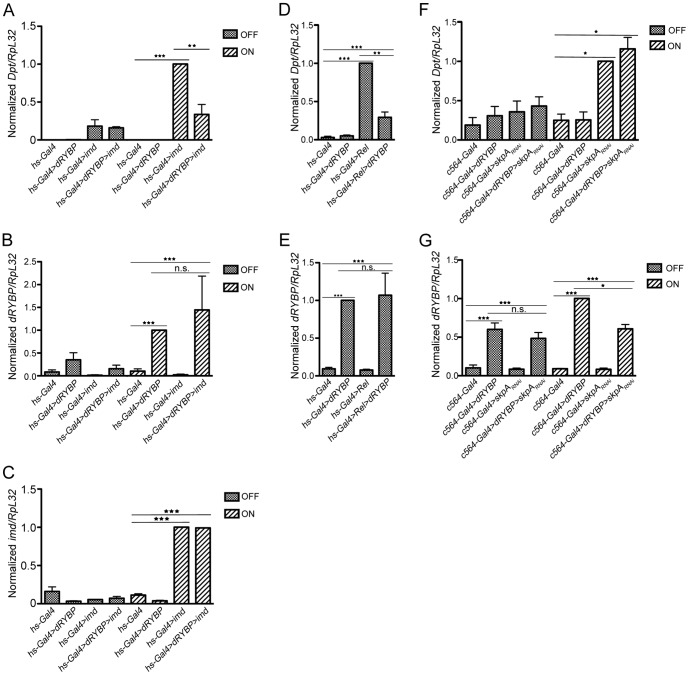
To delineate the hierarchical positioning of dRYBP in the Imd signaling cascade, we studied whether overexpression of dRYBP could repress Dpt expression when the pathway was activated by forced expression of IMD or Relish, both of them activators of the Imd cascade [13], [38], or by decreasing the expression of skpA, a known repressor of the Imd cascade [26]. The latter two seemed particularly relevant based on their proven nuclear localization and hence accessibility to dRYBP.
Activation of the Imd cascade by overexpression of IMD in hs-Gal4,UAS-imd flies resulted in increased production of Dpt (Fig. 4A, compare Dpt expression in hs-Gal4 vs hs-Gal4,UAS-imd flies) [38]. Furthermore, in hs-Gal4,UAS-imd/UAS-dRYBP flies, Dpt expression was repressed (Fig. 4A, compare Dpt expression in hs-Gal4,UAS-imd vs hs-Gal4,UAS-imd/UAS-dRYBP). This inhibition is not due to dilution of the GAL4 protein as the expression levels of both dRYBP and imd were very similar in flies overexpressing a single gene versus flies overexpressing both genes concomitantly (Fig. 4B, C for controls of the dilution of the GAL4 protein). Therefore, these results indicate that dRYBP functions either together with the IMD protein or downstream of the IMD protein in the regulation of the Imd pathway.
Figure 4. Epistatic relationships between dRYBP and components of the Imd pathway.
(A) dRYBP acts downstream of IMD. Flies overexpressing dRYBP, imd, or both under the control of a heat-shock-inducible promoter were kept at 18°C (OFF) or exposed to 37°C for 1 h, then shifted to 29°C for 7 h (ON), at which point Dpt mRNA levels were assessed to measure Imd pathway activity. (B, C) Overexpression of multiple UAS constructs does not lead to Gal4 dilution. (B) shows qRT-PCR quantification of mRNA levels of dRYBP expression. dRYBP expression is increased under induced conditions (compare hs-Gal4 to hs-Gal4;UAS-dRYBP) and does not significantly change when two UAS constructs are concomitantly expressed (compare hs-Gal4;UAS-dRYBP to hs-Gal4,UAS-imd/UAS-dRYBP). (C) shows qRT-PCR quantification of mRNA levels of imd expression. imd expression is increased under induced conditions (compare hs-Gal4 to hs-Gal4,UAS-imd) and does not significantly change when two UAS constructs are concomitantly expressed (compare hs-Gal4,UAS-imd to hs-Gal4,UAS-imd/UAS-dRYBP). (D) dRYBP acts downstream of Relish. Flies overexpressing dRYBP, Rel (Relish-His-6), or both under the control of a heat-shock-inducible promoter were kept at 18°C, which induces low overexpression of all constructs. Dpt mRNA levels were assessed as a measure of Relish-dependent transcriptional activation. (E) qRT-PCR quantification of mRNA levels of dRYBP expression in the same crosses. dRYBP expression is increased when overexpressed with hs-Gal4 in flies kept at 18°C (compare hs-Gal4 to hs-Gal4;UAS-dRYBP) and does not significantly change when two UAS constructs are concomitantly expressed (compare hs-Gal4;UAS-dRYBP to hs-Gal4/UAS-Relish-His-6;UAS-dRYBP). (F) dRYBP acts upstream of SKPA. Flies overexpressing dRYBP, RNAi against skpA, or both under the control of the fat-body specific driver c564-Gal4 were either kept at 18°C (OFF) or shifted to 29°C for 24 h (ON), at which point Dpt mRNA levels were assessed to measure Imd pathway activity. (G) qRT-PCR quantification of mRNA levels of dRYBP expression. dRYBP expression is increased under induced conditions (compare c564-Gal4 to c564-Gal4;UAS-dRYBP) and does not significantly change when two UAS constructs are concomitantly expressed (compare c564-Gal4;UAS-dRYBP to c564-Gal4;UAS-dRYBP/UAS-skpARNAi). Asterisks denote the following p values: *, 0.01<p<0.05; **, 0.001<p<0.01; *** p<0.001; ns, not significant. Data represent mean + SEM of 3 biological repeats.
Activation of the Imd cascade by overexpression of Relish in hs-Gal4/UAS-Relish flies increased the expression of Dpt (Fig. 4D, compare Dpt expression in hs-Gal4 flies to hs-Gal4/UAS-Relish) [39]. However, Dpt expression was still diminished in hs-Gal4/UAS-Relish;UAS-dRYBP flies (Fig. 4D, compare Dpt expression in hs-Gal4/UAS-Relish to hs-Gal4/UAS-Relish;UAS-dRYBP and Fig. 4E for Gal4 dilution). These results indicate that dRYBP functions either together with Relish or downstream of Relish in the repression of the Imdpathway.
Finally, inactivation of skpA increased Dpt expression (Fig. 4F, compare Dpt expression in c564-Gal4 flies to c564-Gal4;UAS-skpARNAi) [26]. However, overexpression of dRYBP in these conditions did not affect the expression of Dpt (Fig. 4F, compare Dpt expression in c564-Gal4;UAS-skpARNAi to c564-Gal4;UAS-skpA RNAi /UAS-dRYBP and Fig. 4G for controls of the dilution of the GAL4 protein). These results indicate that dRYBP functions either together with SKPA or upstream of SKPA to repress the activation of the Imdpathway.
Taken together, our investigation into the epistatic relationships between dRYBP and imd, Relish and skpA indicates that dRYBP functions at the level of the ubiquitin E3-ligase SKPA to repress the activation of the Imd pathway and suggest that the ubiquitin-binding dRYBP may function together with SKPA in the degradation of Relish to inhibit the immune response.
Discussion
A balanced response to infection requires the control of both positive and negative regulation of the Toll and Imd immune signaling pathways [2], [3], [6]. Activation of the pathways to combat the infection through the production of AMPs is as important as their repression since flies rapidly die when several negative regulators of the Imd pathway are simultaneously deleted [51]. In the last decades, investigations into the mechanisms of immune responses in Drosophila have mainly focused on deciphering the activation of AMP production. However, more recently research interest has switched to inhibitors of the pathway, and with the identification of multiple candidates a picture of the mechanisms controlling negative regulation is emerging [6], [20], [23]–[25], [43]–[46], [52]. Here we have introduced the dRYBP gene as a novel player in the negative regulation of the Drosophila Imdpathway.
The dRYBP protein and its vertebrate ortholog RYBP/DEDAF/YAF2 have been shown to interact with a diverse range of proteins [27]–[30], [33], [34], [53]. Both the fly and the vertebrate proteins have been found to interact genetically and molecularly [27]–[30], [34] with the Polycomb group of proteins that maintain the repressed gene transcriptional states by epigenetic mechanisms (for recent reviews [54], [55]). Our investigation into the mechanisms by which dRYBP is acting on the Imd pathway indicates that dRYBP does not control the expression of the canonical Imd pathway genes transcriptionally (Fig. 2) suggesting that dRYBP does not function as a Polycomb group protein in the regulation of the immune response.
Notably, the dRYBP protein contains a ubiquitin binding domain and vertebrate RYBP/DEDAF/YAF2 has been shown to bind ubiquitylated proteins [28]. An increasing number of both activating and regulatory Imd pathway components involve ubiquitin-dependent modifications [16], [19], [20], [26]. It seemed therefore likely that dRYBP might exert its regulatory role through interaction with proteins that are either ubiquitylated themselves or play a role in ubiquitylation. A search for dRYBP-interacting proteins by mass spectrometry (Simón et al., manuscript in preparation and Fig. S2A) has shown that in unchallenged conditions the dRYBP protein physically interacts with CULLIN-1 and SKPA proteins, both members of the E3-ubiquitin ligase SCF complex that targets substrates to the 26S proteasome and plays a pivotal role in regulating diverse developmental events [56]–[59]. Members of the SCF-E3 ubiquitin ligase complex, in particular SKPA, CULLIN-1 and SLIMB, have been previously reported to function as repressors of AMP production in uninfected flies [26] and proposed to repress the Imd pathway by promoting the ubiquitylation and subsequent degradation of a constitutively active Relish protein, as well as down-regulating AMP production after infection in vivo [26]. Our epistatic genetic interaction analysis (Fig. 4) demonstrates that dRYBP functions either together with or downstream of Relish, and together with or upstream of SKPA to dampen the activated immune response. Based on the epistatic and proteomic data, it is likely that dRYBP associates with the SCF-E3 ubiquitin ligase complex to inhibit the activated immune response at the level of Relish degradation.
We propose that dRYBP functions as a ubiquitin adaptor protein in the regulation of the Imd pathway. dRYBP may contribute to the termination of Imd pathway activation by promoting assembly of the SCF complex, which is known to ensure degradation of Relish [26]. Of note, the observed haploinsufficiency of dRYBP (Fig. 1) would suggest that complex assembly is sensitive to the stoichiometry of its components. However, the fact that dRYBP null mutants show a normal return to baseline suggests that dRYBP is non-essential in shutting off signaling, and contributes to the amplitude rather than the duration of signaling output. In the complete absence of dRYBP, failure to assemble the SCF complex correctly and timely would lead to transient accumulation of activated nuclear Relish and to excessive transcription of Relish-dependent genes.
The vertebrate homolog of Relish, the NF-κB subunit p105 protein, has been demonstrated both to be ubiquitylated and to belong to the superfamily of DD (Death Domain) containing proteins [60]. The latter plays important roles in the assembly and activation of apoptotic and inflammatory complexes [36]. Curiously, the Drosophila RYBP protein and the vertebrate RYBP/DEDAF/YAF2 protein have been shown to interact with Death Effector Domain (DED) proteins, a subfamily of the DD proteins [31], [32], [36]. A search for a DD domain in the Relish protein using the Death Domain Database (www.deathdomain.org) [61] returned that indeed Relish contains a putative DD domain with a relevant percentage of similarity to the DD in mammalian p105 (Fig. S2B, C) [62]. Possibly, the interaction between Relish and dRYBP involves both the DD binding domain and ubiquitin signatures on Relish to promote its proteasomal degradation through the SKPA/Cullin complex. It now remains to be clarified whether and how dRYBP interacts with Relish upon infection and whether dRYBP has an effect on the degradation of Relish. At this stage, we cannot exclude that dRYBP might also function at other steps in this pathway, including in the process of ubiquitylation of the IMD protein [17], [19], to negatively regulate the immune response.
The present work has shown that dRYBP contributes to the regulation of the fly immune response, a fundamental systemic organism reaction to overcome external harm or stress. The decision to repress the activated immune response likely depends on the nature of the stress signal. Further investigations will shed light on the contribution of dRYBP in reaching a balanced immune response and will reveal whether the evolutionarily conserved RYBP protein may have a novel function in the control of the human innate immune response.
Supporting Information
The dRYBP loss of function phenotype is modulated by mutant alleles of the Imd pathway. (A) Crossing scheme. The engrailed-Gal4 (en-Gal4) driver was used to express UAS-dRYBPRNAi and UAS-VDRCRNAi in the wing. Female virgins en-Gal4/CyO;UAS-dRYBPRNAi /MKRS were crossed with males UAS-VDRCRNAi and maintained at 29°C. From these crosses, the en-Gal4;UAS-VDRCRNAi progeny were analyzed for wing phenotypes associated with the particular VDRCRNAi line under study and the en-Gal4;UAS-dRYBPRNAi,/UAS-VDRCRNAi progeny were analyzed for the penetrance of the wing blister phenotype. (B) Wild-type wing. (C) en-Gal4>dRYBPRNAi wing showing a blister (arrow) in the posterior compartment. (D) Quantification of flies with wing blisters of the indicated genotypes. en-Gal4>dRYBPRNAi/UAS-GFP was used as a control. Importantly, this screen merely shows genetic interaction between dRYBP and Imd pathway components. It is not yet clear why the penetrance of blister phenotype in our screen can be modulated by mutations in genes involved in the innate immune response or why the penetrance is modulated by mutations in both activators and repressors of the Imd pathway. The wing phenotypes are probably due to these factors involved in other biological processes [38], [63].
(TIF)
Mass spectrometry data and localization of a Death Domain in the Relish protein. (A) Mass spectrometry results showing the scores for the indicated dRYBP interacting proteins. (B) Alignment of Human p105 and Drosophila Relish protein sequence. Indicated in green is the predicted Death Domain (DD) sequence (www.deathdomain.org). (C) Magnification of the predicted DD domain. (D) Alignment of other DD containing proteins with the predicted DD domain in the Relish proteins.
(TIF)
Acknowledgments
We thank our colleagues Sol Fereres, Olga Redondo, Carolina Simoes da Silva and Rocío Simón for fruitful discussions; the Bloomington Stock Center and the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), the Vienna Drosophila RNAi Center (VDRC) for providing transgenic RNAi fly stocks; the Consolider transgenic Drosophila facility for embryo injections, and the Death Domain Database (www.deathdomain.org). We are indebted to Rocío Simón, C. Peter Verrijzer and members of his laboratory for sharing the mass spectrometry data.
Funding Statement
This work was supported by grants from the Dirección General de Investigación (BFU2008-01154) to A.B, the Consolider Ingenio 2010 Program of the Ministerio de Ciencia e Innovación (CSD 2007-00008) to A.B., by an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa, by the National Research Fund Luxembourg (AFR Postdoctoral Grant 08/037) to C.N., by the Bettencourt-Schueller Foundation to B.L., by the ERC Advanced Grant to C.N. and B.L., and the Swiss National Fund (3100A0-12079/1) to B.L. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Charroux B, Royet J (2010) Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly (Austin) 4: 40–47. [DOI] [PubMed] [Google Scholar]
- 2. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743. [DOI] [PubMed] [Google Scholar]
- 3. Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7: 862–874. [DOI] [PubMed] [Google Scholar]
- 4. Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, et al. (2000) Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13: 737–748. [DOI] [PubMed] [Google Scholar]
- 5. Uvell H, Engstrom Y (2007) A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet 23: 342–349. [DOI] [PubMed] [Google Scholar]
- 6. Aggarwal K, Silverman N (2008) Positive and negative regulation of the Drosophila immune response. BMB Rep 41: 267–277. [DOI] [PubMed] [Google Scholar]
- 7. Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, et al. (2003) The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol 4: 478–484. [DOI] [PubMed] [Google Scholar]
- 8. Gottar M, Gobert V, Michel T, Belvin M, Duyk G, et al. (2002) The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416: 640–644. [DOI] [PubMed] [Google Scholar]
- 9. Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV (2002) Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296: 359–362. [DOI] [PubMed] [Google Scholar]
- 10. Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B (2000) The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep 1: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B (2002) Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol 12: 996–1000. [DOI] [PubMed] [Google Scholar]
- 12. Naitza S, Rosse C, Kappler C, Georgel P, Belvin M, et al. (2002) The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity 17: 575–581. [DOI] [PubMed] [Google Scholar]
- 13. Stoven S, Ando I, Kadalayil L, Engstrom Y, Hultmark D (2000) Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep 1: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW (2009) The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. Embo J 28: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18: 621–663. [DOI] [PubMed] [Google Scholar]
- 16. Meinander A, Runchel C, Tenev T, Chen L, Kim CH, et al. (2012) Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. Embo J 31: 2770–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, et al. (2010) Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol Cell 37: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leulier F, Lhocine N, Lemaitre B, Meier P (2006) The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol Cell Biol 26: 7821–7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou R, Silverman N, Hong M, Liao DS, Chung Y, et al. (2005) The role of ubiquitination in Drosophila innate immunity. J Biol Chem 280: 34048–34055. [DOI] [PubMed] [Google Scholar]
- 20. Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, et al. (2009) The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe 6: 309–320. [DOI] [PubMed] [Google Scholar]
- 21. Lu Y, Wu LP, Anderson KV (2001) The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev 15: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, et al. (2000) A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14: 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsichritzis T, Gaentzsch PC, Kosmidis S, Brown AE, Skoulakis EM, et al. (2007) A Drosophila ortholog of the human cylindromatosis tumor suppressor gene regulates triglyceride content and antibacterial defense. Development 134: 2605–2614. [DOI] [PubMed] [Google Scholar]
- 24. Kim M, Lee JH, Lee SY, Kim E, Chung J (2006) Caspar, a suppressor of antibacterial immunity in Drosophila. Proc Natl Acad Sci U S A 103: 16358–16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guntermann S, Primrose DA, Foley E (2009) Dnr1-dependent regulation of the Drosophila immune deficiency signaling pathway. Dev Comp Immunol 33: 127–134. [DOI] [PubMed] [Google Scholar]
- 26. Khush RS, Cornwell WD, Uram JN, Lemaitre B (2002) A ubiquitin-proteasome pathway represses the Drosophila immune deficiency signaling cascade. Curr Biol 12: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 27. Bejarano F, Gonzalez I, Vidal M, Busturia A (2005) The Drosophila RYBP gene functions as a Polycomb-dependent transcriptional repressor. Mech Dev 122: 1118–1129. [DOI] [PubMed] [Google Scholar]
- 28. Arrigoni R, Alam SL, Wamstad JA, Bardwell VJ, Sundquist WI, et al. (2006) The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett 580: 6233–6241. [DOI] [PubMed] [Google Scholar]
- 29. Garcia E, Marcos-Gutierrez C, del Mar Lorente M, Moreno JC, Vidal M (1999) RYBP, a new repressor protein that interacts with components of the mammalian Polycomb complex, and with the transcription factor YY1. Embo J 18: 3404–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez I, Aparicio R, Busturia A (2008) Functional characterization of the dRYBP gene in Drosophila. Genetics 179: 1373–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez I, Busturia A (2009) High levels of dRYBP induce apoptosis in Drosophila imaginal cells through the activation of reaper and the requirement of trithorax, dredd and dFADD. Cell Res 19: 747–757. [DOI] [PubMed] [Google Scholar]
- 32. Novak RL, Phillips AC (2008) Adenoviral-mediated Rybp expression promotes tumor cell-specific apoptosis. Cancer Gene Ther 15: 713–722. [DOI] [PubMed] [Google Scholar]
- 33. Pirity MK, Locker J, Schreiber-Agus N (2005) Rybp/DEDAF is required for early postimplantation and for central nervous system development. Mol Cell Biol 25: 7193–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlisio S, Halperin T, Vidal M, Nevins JR (2002) Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. Embo J 21: 5775–5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schickling O, Stegh AH, Byrd J, Peter ME (2001) Nuclear localization of DEDD leads to caspase-6 activation through its death effector domain and inhibition of RNA polymerase I dependent transcription. Cell Death Differ 8: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 36. Zheng L, Schickling O, Peter ME, Lenardo MJ (2001) The death effector domain-associated factor plays distinct regulatory roles in the nucleus and cytoplasm. J Biol Chem 276: 31945–31952. [DOI] [PubMed] [Google Scholar]
- 37. Park HH, Lo YC, Lin SC, Wang L, Yang JK, et al. (2007) The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol 25: 561–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, et al. (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell 1: 503–514. [DOI] [PubMed] [Google Scholar]
- 39. Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, et al. (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell 4: 827–837. [DOI] [PubMed] [Google Scholar]
- 40. Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- 41. Wicker C, Reichhart JM, Hoffmann D, Hultmark D, Samakovlis C, et al. (1990) Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem 265: 22493–22498. [PubMed] [Google Scholar]
- 42. Dushay MS, Roethele JB, Chaverri JM, Dulek DE, Syed SK, et al. (2000) Two attacin antibacterial genes of Drosophila melanogaster. Gene 246: 49–57. [DOI] [PubMed] [Google Scholar]
- 43. Aggarwal K, Rus F, Vriesema-Magnuson C, Erturk-Hasdemir D, Paquette N, et al. (2008) Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog 4: e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleino A, Myllymaki H, Kallio J, Vanha-aho LM, Oksanen K, et al. (2008) Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol 180: 5413–5422. [DOI] [PubMed] [Google Scholar]
- 45. Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, et al. (2008) PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe 4: 147–158. [DOI] [PubMed] [Google Scholar]
- 46. Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J (2008) The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe 3: 293–303. [DOI] [PubMed] [Google Scholar]
- 47. Zaidman-Remy A, Herve M, Poidevin M, Pili-Floury S, Kim MS, et al. (2006) The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24: 463–473. [DOI] [PubMed] [Google Scholar]
- 48. De Gregorio E, Spellman PT, Rubin GM, Lemaitre B (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A 98: 12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B (2002) The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J 21: 2568–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39: 715–720. [DOI] [PubMed] [Google Scholar]
- 51. Paredes JC, Welchman DP, Poidevin M, Lemaitre B (2011) Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 35: 770–779. [DOI] [PubMed] [Google Scholar]
- 52. Tsuda M, Langmann C, Harden N, Aigaki T (2005) The RING-finger scaffold protein Plenty of SH3s targets TAK1 to control immunity signalling in Drosophila. EMBO Rep 6: 1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Danen-van Oorschot AA, Voskamp P, Seelen MC, van Miltenburg MH, Bolk MW, et al. (2004) Human death effector domain-associated factor interacts with the viral apoptosis agonist Apoptin and exerts tumor-preferential cell killing. Cell Death Differ 11: 564–573. [DOI] [PubMed] [Google Scholar]
- 54. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G (2007) Genome regulation by polycomb and trithorax proteins. Cell 128: 735–745. [DOI] [PubMed] [Google Scholar]
- 55. Schwartz YB, Pirrotta V (2007) Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8: 9–22. [DOI] [PubMed] [Google Scholar]
- 56. Bocca SN, Muzzopappa M, Silberstein S, Wappner P (2001) Occurrence of a putative SCF ubiquitin ligase complex in Drosophila. Biochem Biophys Res Commun 286: 357–364. [DOI] [PubMed] [Google Scholar]
- 57. Hattori K, Hatakeyama S, Shirane M, Matsumoto M, Nakayama K (1999) Molecular dissection of the interactions among IkappaBalpha, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IkappaBalpha. J Biol Chem 274: 29641–29647. [DOI] [PubMed] [Google Scholar]
- 58. Hatakeyama S, Kitagawa M, Nakayama K, Shirane M, Matsumoto M, et al. (1999) Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci U S A 96: 3859–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Willems AR, Goh T, Taylor L, Chernushevich I, Shevchenko A, et al. (1999) SCF ubiquitin protein ligases and phosphorylation-dependent proteolysis. Philos Trans R Soc Lond B Biol Sci 354: 1533–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beinke S, Belich MP, Ley SC (2002) The death domain of NF-kappa B1 p105 is essential for signal-induced p105 proteolysis. J Biol Chem 277: 24162–24168. [DOI] [PubMed] [Google Scholar]
- 61. Kwon D, Yoon JH, Shin SY, Jang TH, Kim HG, et al. (2012) A comprehensive manually curated protein-protein interaction database for the Death Domain superfamily. Nucleic Acids Res 40 (D1): D331–D336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16: 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chinchore Y, Gerber GF, Dolph PJ (2012) Alternative pathway of cell death in Drosophila mediated by NF-kappaB transcription factor Relish. Proc Natl Acad Sci U S A 109: E605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The dRYBP loss of function phenotype is modulated by mutant alleles of the Imd pathway. (A) Crossing scheme. The engrailed-Gal4 (en-Gal4) driver was used to express UAS-dRYBPRNAi and UAS-VDRCRNAi in the wing. Female virgins en-Gal4/CyO;UAS-dRYBPRNAi /MKRS were crossed with males UAS-VDRCRNAi and maintained at 29°C. From these crosses, the en-Gal4;UAS-VDRCRNAi progeny were analyzed for wing phenotypes associated with the particular VDRCRNAi line under study and the en-Gal4;UAS-dRYBPRNAi,/UAS-VDRCRNAi progeny were analyzed for the penetrance of the wing blister phenotype. (B) Wild-type wing. (C) en-Gal4>dRYBPRNAi wing showing a blister (arrow) in the posterior compartment. (D) Quantification of flies with wing blisters of the indicated genotypes. en-Gal4>dRYBPRNAi/UAS-GFP was used as a control. Importantly, this screen merely shows genetic interaction between dRYBP and Imd pathway components. It is not yet clear why the penetrance of blister phenotype in our screen can be modulated by mutations in genes involved in the innate immune response or why the penetrance is modulated by mutations in both activators and repressors of the Imd pathway. The wing phenotypes are probably due to these factors involved in other biological processes [38], [63].
(TIF)
Mass spectrometry data and localization of a Death Domain in the Relish protein. (A) Mass spectrometry results showing the scores for the indicated dRYBP interacting proteins. (B) Alignment of Human p105 and Drosophila Relish protein sequence. Indicated in green is the predicted Death Domain (DD) sequence (www.deathdomain.org). (C) Magnification of the predicted DD domain. (D) Alignment of other DD containing proteins with the predicted DD domain in the Relish proteins.
(TIF)