Abstract
We previously performed a high-throughput screen using real-time noninvasive bioluminescence imaging of p53 transcriptional activity and identified a group of small molecules that trigger p53-like transcriptional responses in p53-deficient tumor cells. Here we further examined the anti-tumor effects of selected compounds in vitro and showed that NSC176327, a derivative of the cytotoxic plant alkaloid ellipticine, exhibited strong anti-neoplastic effects in wild-type p53, p53-mutant or p53-deficient human colon cancer cells. NSC176327 was more potent at inhibiting tumor cell growth as compared with chemotherapeutic drugs and other ellipticine derivatives and induced cell cycle arrest and apoptosis. Surprisingly, unlike what is observed with the parent compound ellipticine, a DNA damage signaling response was not observed with NSC176327 as evidenced by lack of phosphorylated histone H2AX foci in NSC176327-treated tumor cells. NSC176327 treatment caused a significant increase in p53-activated reporter signal in HCT116, SW620 and HCT116 p53−/− cells and upregulated DR5 and p21 protein expression. NSC176327 treatment also resulted in increased p73 protein expression and knockdown of transactivating isoforms of p73 in HCT116 p53−/− cells showed significant resistance to drug treatment. These results demonstrate an important role of p73 in the anti-tumor effects of NSC176327, and suggest that a close analog of ellipticine may act by a non-genotoxic mechanism targeting the p53/p73 pathway as compared with the original parent compound that targets the same pathway.
Keywords: NSC176327, ellipticine, p53, p73, colon cancer, HCT116, SW620
Introduction
p53 has been the subject of intense study over the past 30 y because of the findings that more than half of all human tumors have lost normal p53 gene function.1 The tumor suppressor role of p53 is mainly mediated by its response to stress signals and functions as a transcription factor to induce target genes.2 p53 can be activated by DNA damage, hypoxia or oncogenes3 and orchestrates a number of cellular effects by transactivating genes such as p21 which is involved in cell cycle arrest;4 p53R2 and p48 which are involved in DNA repair5 and apoptotic genes like Bax, Death Receptor 5 (DR5), Puma and Noxa.6-8 Mutant p53 or defective p53-depedent pathways are associated with tumor development and resistance to conventional radiation and chemotherapy.9 Therefore p53 and p53-dependent pathways are attractive therapeutic targets for cancer therapy.
In recent years several strategies have been developed to target p53 including: (1) delivery of wild-type p53 to cancer cells by gene therapy;10 (2) elimination of mutant p53 with adenovirus such as ONYX-015, which is undergoing clinical trials;11 (3) restoration of wild-type function of mutant p53 which led to discovery of small-molecule drugs including CP-31398,12 and PRIMA-1,13 and (4) inhibition of p53 degradation by small-molecule drugs such as nutlins which inhibit p53 binding to E3 ligase mouse double minute 2 (MDM2).14 Of note, p73, a p53 family protein that shares significant sequence homology with p53 and transactivates p53 target genes, has recently become an interesting therapeutic target.15 p73 has been shown to substitute for p53 function in p53 mutant cells and peptides and small molecules that activate p73 were reported to suppress mutant p53-bearing tumor cells in vitro and in mouse xenografts.16,17
The discovery of novel therapeutic agents will rely on not only further understanding of p53-dependent pathways but also technology advancement. For example, we previously developed a real-time noninvasive bioluminescence imaging strategy of p53 transcriptional activity in vitro and in vivo.18 Utilizing this high through-put imaging system, we performed a screen for small molecules that trigger p53-like transcriptional responses in p53-deficient tumor cells with National Cancer Institute (NCI) diversity set compounds and identified a group of small molecules that activate p53-responsive transcriptional activity in p53-deficient SW480 colon cancer cells.19 However, the anti-tumor effects and mechanisms of these compounds remain to be fully examined. Therefore in this study we evaluated the anti-tumor potential of selected compounds by assessing viability and apoptosis in tumor cell lines after compound treatment. We also determined whether DNA damage signaling was involved by monitoring protein expression of γH2AX after compound treatment. We found that NSC176327, a derivative of ellipticine, exhibited potent anti-neoplastic effects in vitro.
Ellipticine, 5,11-dimethyl-6H-pyrido[4,3-b] carbazole, was originally isolated from the leaves of the evergreen tree ochrosia elliptica Labill. Ellipticine was found to possess great anti-cancer activity in a variety of tumors.20 In addition, several derivatives of ellipticine have been identified or synthesized and demonstrated to be active against brain tumors.21 Although the exact mechanism of action remains unclear, ellipticine was shown to intercalate with DNA and inhibit topoisomerase II activity.22 However, recently a number of studies suggested that alternative mechanisms also play an important role in ellipticine’s anti-neoplastic effects and that derivatives of ellipticine do not necessarily act by the same mechanism as the parent compound. Ellipticine and some of its derivatives have been previously shown to rescue mutant p53 transcription function, to stimulate p53-dependent sequence specific DNA binding and to stimulate wild-type p53 (pAb1620) reactivity of mutant p53.31 We show here that unlike ellipticine, NSC176327 did not induce a DNA damage signaling response. Moreover, p73 seemed to be important in mediating NSC176327’s anti-tumor effects as knockdown of transactivating isoforms of p73 (TAp73) in HCT116 p53−/− cells showed significant resistance to compound treatment.
Results
NSC176327 inhibited tumor cell viability and induced cell cycle arrest and apoptosis
By stably expressing a human p53-reporter, PG-13-luc, which carries a firefly luciferase gene under the control of 13 p53-responsive elements, in the human colon adenocarcinoma cell line SW480 that bears a mutant p53 (R273H, P309S), we screened the NCI Developmental Therapeutics Program’s diversity set of ≈2,000 chemical agents and identified a number of small molecules that can reactivate p53 signaling in tumor cells with mutant p53.19 In the present studies we further examined the anti-cancer activity of selected compounds by evaluating tumor cell viability and cell cycle arrest and apoptosis after compound treatment. Three tumor cell lines, HCT116 with wild-type p53, SW620 with mutant p53 and HCT116 p53−/− were tested. Cell viability was determined by the MTT assay and cell cycle profile was determined by FACS analysis of PI staining. It is desirable to identify small-molecule p53 modulators that do not cause DNA damage so that synergistic effects might be achieved when compounds are used in combination with conventional radiation and chemotherapy. Therefore, we also examined the activation of DNA damage signaling in tumor cells after compound treatment by monitoring the expression level of γH2AX and evaluating cells for the presence of γH2AX foci. Based on results from three cell lines, we summarized overall anti-tumor activity and the ability to induce DNA damage signaling of these compounds (Fig. 1A). Of particular interest, NSC176327 (3-(9-methoxy-5,11-dimethylpyrido[4,3-b]carbazol-6-yl)propan-1-amine), a close derivative of ellipticine, was found to possess potent anti-cancer activity without inducing DNA damage signaling (Fig. 1B).
Figure 1. (A) Table summarizing results of the screening for the ability of compounds to induce viability loss, cell cycle arrest and apoptosis and DNA damage signaling in tumor cells. (B) Structures of ellipticine and its derivative, NSC176327.
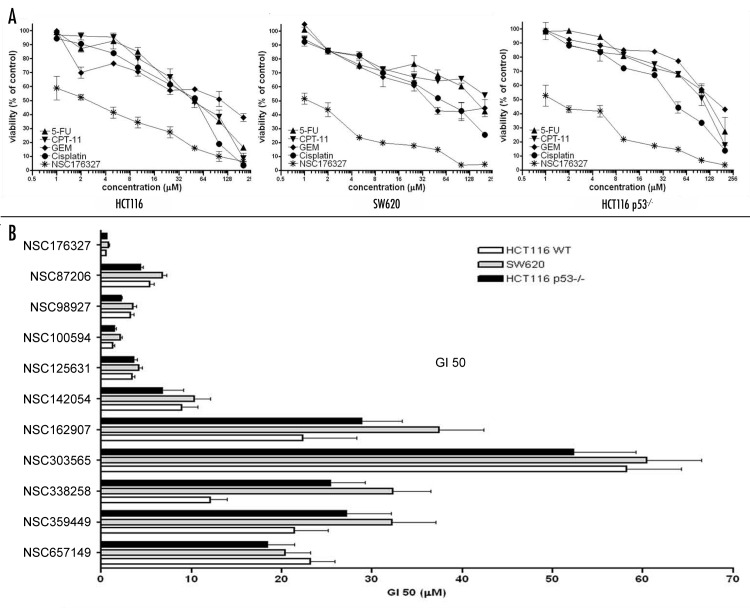
When compared with standard chemotherapeutic agents including 5-fluorouracil (5-FU), Camptothecin-11 (CPT-11), Cisplatin and Gemcitabine (GEM), NSC176327 exhibited more significant inhibition of cell viability, with ~50% inhibition observed with 1 μM NSC176327 treatment for 48 h (Fig. 2A). Moreover, the inhibition of cell viability by NSC176327 was comparable in HCT116, SW620 and HCT116 p53−/− cells, while SW620 and HCT116 p53−/− cells showed resistance to treatment of chemotherapeutic agents, suggesting that the mechanism of NSC176327’s anti-tumor effects might be independent of p53. With this finding, we extended the study to additional ellipticine derivatives from the NCI library. We tested nine compounds for viability inhibition of the same tumor cell lines. GI50 (the compound concentration that causes 50% loss of cell viability) was calculated and shown in Figure 2B. The data indicated that among ellipticine derivatives, NSC176327 was the most potent, with GI50 within 0.5–1 μM in the three tumor cell lines.
Figure 2. (A) HCT116, SW620 and HCT116 p53−/− cells were treated with different concentrations of NSC176327, 5-FU, CPT-11, Cisplatin or GEM and cell viability was measured by MTT assay after 48 h. (B) GI50 (the compound concentration that causes 50% loss of cell viability measured by MTT assay) after 48 h treatment of NSC176327 and other ellipticine derivatives from NCI library in HCT116, SW620 and HCT116 p53−/− cells.
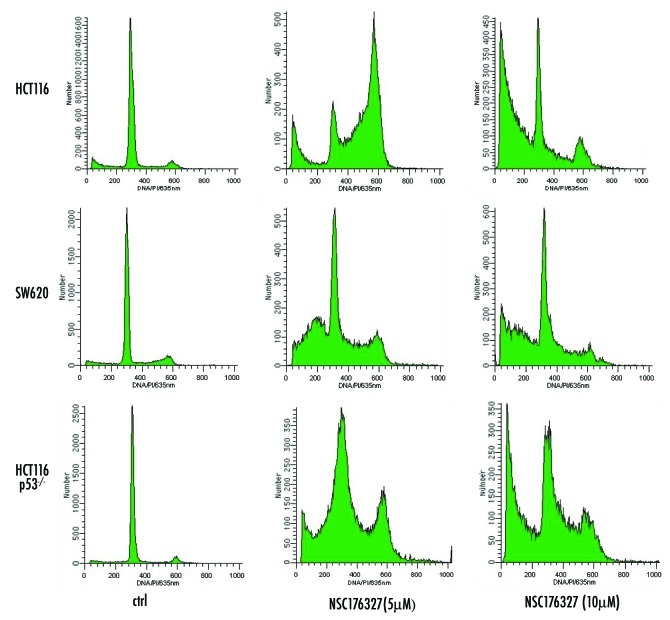
We further evaluated the ability of NSC176327 to induce cell cycle arrest and apoptosis in tumor cells. We found that at 48 h, treatment with 5 μM NSC176327 induced cell cycle arrest in HCT116, SW620 and HCT116 p53−/− cells while 10 μM of NSC176327 induced a subG1 peak characteristic of apoptosis in these cell lines (Fig. 3). Interestingly, induction of cell cycle arrest was less evident in SW620 cells. These data suggest that whether tumor cells undergo cell cycle arrest or apoptosis after drug treatment depends on both the concentration of the drug and the cell type.
Figure 3. HCT116, SW620 and HCT116 p53−/− cells were treated with 5 μM or 10 μM NSC176327 for 48 h and cell cycle profiles were determined by flow cytometry analysis of PI staining.
Lack of histone H2AX phosphorylation in NSC176327-treated tumor cells
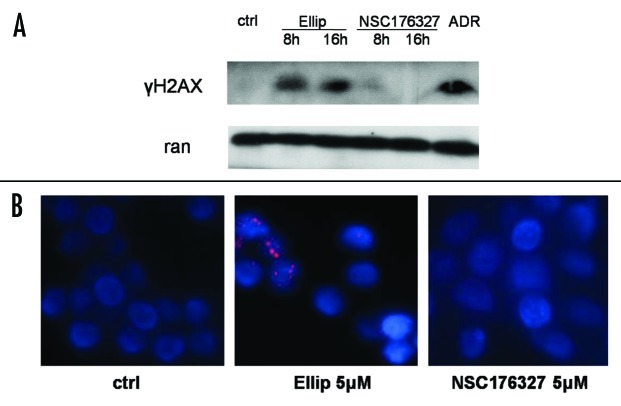
To confirm whether DNA damage signaling is involved in the mechanism of action of NSC176327, we tested by -immunobloting the expression level of γH2AX as well as by detecting γH2AX foci formation with immunofluorescence. While 10 μM of ellipticine treatment induced strong γH2AX expression in SW620 cells, we did not observe γH2AX expression after treatment of NSC176327 at the same concentration for 8 h or 16 h (Fig. 4A). Adriamycin was included as positive control. Immunofluorescence also showed that γH2AX foci were formed in SW620 cells after ellipticine treatment but not NSC176327 treatment (Fig. 4B). These data are consistent with previous reports showing that ellipticine causes DNA damage signaling by intercalating with DNA and inhibiting topoisomerase II activity and suggest that a close derivative of ellipticine may act by a different mechanism from the parent compound.
Figure 4. (A) SW620 cells were treated with 10 μM ellipticine or NSC176327 for 8 or 16 h and protein expression level of γH2AX was determined by western blot. Adriamycin (1 μg/ml for 12 h) was included as positive control and ran was included as loading control. (B) SW620 cells were treated with 5 μM ellipticine or NSC176327 for 12 h and γH2AX foci formation was detected by immunofluorescence.
p53 transcriptional activity and target protein expression after NSC176327 treatment
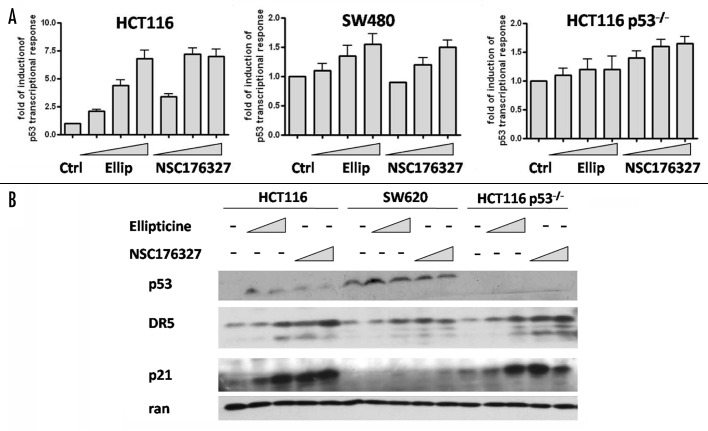
The ability of NSC176327 to induce a p53-like transcriptional response in wild-type, p53-mutant and p53-null tumor cells was examined. Using the same strategy, PG-13-luc was introduced and stably expressed in HCT116 and HCT116 p53−/− cells. Both ellipticine and NSC176327 treatment for 12 h induced a concentration-dependent increase of p53 transcriptional activity in HCT116 cells, with the fold induction as high as 7.5-fold. Increased p53 transcriptional response was also observed in SW480 cells after NSC176327 and ellipticine treatment but the magnitude was much less (~1.5 fold). By contrast, in HCT116 p53−/− cells, only NSC176327 treatment induced a significant increase of p53 transcriptional response (Fig. 5A).
Figure 5. (A) HCT116, SW480 and HCT116 p53−/− cells were treated with different concentrations of ellipticine or NSC176327 for 12 h and fold induction of p53 transcriptional response was measured by bioluminescence imaging of human p53 reporter, PG-13-luc. (B) HCT116, SW620 and HCT116 p53−/− cells were treated with different concentrations of ellipticine or NSC176327 for 16 h and protein expression level of p53, p21 and DR5 was determined by western blot. Ran was included as loading control.
We next determined whether protein expression of p53 target genes correlated with p53 transcriptional activity that we observed after ellipticine or NSC176327 treatment. p53 target proteins p21 and DR5 were chosen for western blot analysis because of their important roles in cell cycle arrest and apoptosis. We found that there was a concentration-dependant increase of DR5 protein expression after both ellipticine and NSC176327 treatment in HCT116, SW620 and HCT116 p53−/− cells and that NSC176327 seemed to exhibit more potent effects (Fig. 5B). This is surprising since ellipticine treatment did not induce p53 transcriptional response in HCT116 p53−/− cells. Similarly, p21 protein expression was upregulated in HCT116 and HCT116 p53−/− cells after compound treatment. However, in SW620 cells p21 protein expression was detectable only after treatment of 10 μM NSC176327, which in part explains why induction of cell cycle arrest was relatively less evident in SW620 cells after NSC176327 treatment.
p73 is involved in the anti-tumor effects of NSC176327
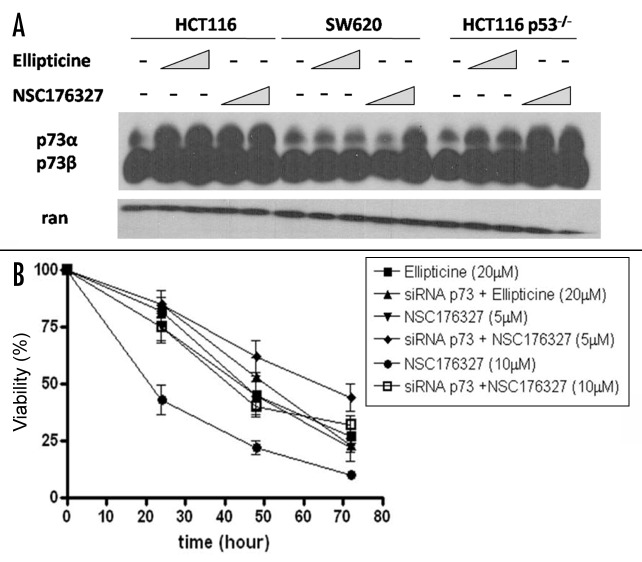
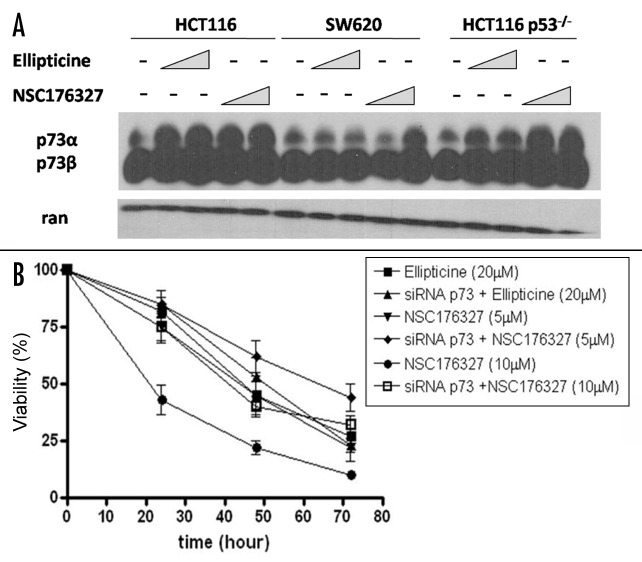
The findings that NSC176327 treatment caused similar levels of viability loss and apoptosis in wild-type p53 cells, p53-mutant cells and p53-null cells and that NSC176327 induced a p53-like transcriptional response in HCT116 p53−/− cells suggest that alternative pathways are involved in its anti-tumor mechanism. p73 is a p53 family protein recently found to substitute p53 function in p53-mutant or -null cells. We tested whether NSC176327 could upregulate p73. As shown in Figure 6A, treatment with ellipticine or NSC176327 both increased p73 protein expression in HCT116 cells but only NSC176327 treatment significantly upregulated p73 expression in SW620 and HCT116 p53−/− cells. More importantly, knockdown of TAp73 in HCT116 p53−/− cells by transducing cells with retroviral vector containing p73 RNAi significantly inhibited NSC176327’s anti-tumor activity (Fig. 6B). In contrast, viability loss induced by ellipticine treatment was not affected by p73 knockdown. These data indicate an important and specific role of p73 in NSC176327’s anti-tumor effects and potentially important differences between the mechanism of action of ellipticine vs. its derivative NSC176327.
Figure 6. (A) HCT116, SW620 and HCT116 p53−/− cells were treated with different concentrations of ellipticine or NSC176327 for 12 h and protein expression level of p73 was determined by western blot. Ran was included as loading control. (B) HCT116 p53−/− cells or HCT116 p53−/− cells transduced with retroviral vector containing TAp73 RNAi were treated with 5 μM or 10 μM NSC176327 or 20 μM ellipticine for 24, 48 or 72 h and cell viability was measured by MTT assay.
Discussion
In this study we examined the anti-tumor activity of a group of small-molecule compounds previously identified through screening using bioluminescence imaging of p53 transcriptional activity. We showed that NSC176327, a derivative of the plant alkaloid ellipticine, exhibited potent tumor-suppressing effects. NSC176327 induced viability loss, cell cycle arrest and apoptosis and upregulation of p53 target protein expression in wild-type p53, p53-mutant or p53-deficient tumor cells. Unlike the parent compound ellipticine, the mechanism of action of NSC176327 did not seem to involve DNA damage signaling. In addition, p73 was found to play a critical role in NSC176327’s anti-tumor effects. Our study suggests a strategy to screen, identify, evaluate and characterize potential p53 family modulators and provides important insights to p53-targeting cancer therapy. In particular, avoidance of genotoxic damage can be part of the screening process.
Resistance of p53-mutant or -null tumors to conventional radiation and chemotherapeutic agents is a major obstacle in cancer therapy. Here we also showed that SW620 and HCT116 p53−/− cells displayed resistance to standard chemotherapeutic agents (Fig. 2A). Therefore identifying compounds that reactivate or bypass the p53-dependent pathway is important to enhance conventional cancer therapy. Moreover, in order to achieve synergistic effects, the candidate compound should possess a different mechanism of action from conventional therapy, which is mainly through DNA damage signaling. In this respect, NSC176327 seems to be a good candidate since it induced p53 transcriptional response and endogenous p53 target gene expression in p53-mutant and p53-null cells without activating DNA damage signaling (Figs. Four and 5). This is interesting since it is well established that ellipticine causes DNA damage signaling by intercalating with DNA and inhibiting topoisomerase II. However, since its isolation a large number of ellipticine derivatives have been synthesized and some of them have been shown to act independent of DNA damage signaling. For example, Celiptium (NSC264137) and Detalliptinium (NSC311152), two clinically administrated ellipticine derivatives in the treatment of breast cancer, were compared with a putative topoisomerase II inhibitor in vivo and demonstrated to be 50 times less potent for DNA strand breakage despite that their IC50 are in close range.23 Another ellipticine derivative NSC338258 was shown to exhibit cytotoxicity in myeloma cells independent of topoisomerase II inhibition.24 These studies are consistent with our findings and suggest that a close derivative to ellipticine might act by different mechanisms to suppress tumors.
The observations that the anti-tumor effects (Figs. Two and 3) and the ability to induce p53 transcriptional activity of NSC176327 are comparable in wild-type p53 cells, p53-mutant cells and p53-null cells suggested the existence of an alternative target. Recently the important role of p73 in cancer therapy has emerged. p73 shares significant sequence homology with p53 and can bind to the p53-specific DNA-binding sequence and activates transcription of p53 target genes. Its function in tumorigenesis has been demonstrated by heterozygous deletion of p73, which resulted in various tumor predispositions in mice.25 Unlike p53, p73 is rarely mutated in tumors. However, it has been shown that mutant p53 bound and inactivated p73. Importantly, p73 was induced by a variety of chemotherapeutic agents and the restoration of wild-type p53 function in p53 mutant cells to enhance chemosensitivity was mediated by p73.15 Therefore we tested whether p73 was involved in the anti-tumor mechanism of NSC176327. We found that NSC176327, but not ellipticine, induced p73 protein expression in p53-mutant and -null cells and knockdown of TAp73 reduced the cytotoxicity of NSC176327 in p53-null cells (Fig. 6). This indicates that, like several other drug candidates,16,17 p73 is an important target of NSC176327. It is not clear how NSC176327 activates p73 but possible mechanisms include (1) binding to iASPP, the inhibitory member of the ASPP family, and displacing iASPP from p73;26 and (2) interfering with mutant p53-p73 complex to release p73.
Of note, ellipticine and its derivatives seem to activate a panel of cellular responses to mediate cytotoxicity in tumors. For example, ellipticine has been reported to generate free radicals,27 inhibit cytochrome P4501A1,28 induce endoplasmic reticulum stress,29 and activate the mitochondrial apoptotic pathway.24 Interestingly, studies have shown that ellipticine has the ability to restore mutant p53 function. An analysis has indicated that ellipticine and its analogs were more potent against cell lines that harbor mutant p53 in growth inhibition assays.30 It was also shown that ellipticine could restore the wild-type conformation and transactivation function of transfected or endogenous p53 mutants.31 Recently ellipticine was reported to increase the nuclear localization of p53 in cancer cells with a resultant increase in the transactivation of the p21 promoter.32 In agreement, we found that ellipticine increased p53 transcriptional response in SW480 cells and that although ellipticine treatment did not induce p53 transcriptional response in HCT116 p53−/− cells, p21 and DR5 protein expression were upregulated (Fig. 5).
In conclusion, we identified NSC176327 as a potent anti-neoplastic agent against human colon cancer cells. The mechanism of action is independent of DNA damage but involves activation of p73. Therefore NSC176327 represents an excellent drug candidate to be tested in various tumor models in combination with radiation and chemotherapy. However, other molecular targets of NSC176327 remain to be found since knockdown of TAp73 in HCT116 p53−/− cells only partially rescued cell death after compound -treatment (Fig. 6B). Future directions include structural and pharmaceutical refinement of the compound and preclinical testing of general toxicity and pharmacokinetic and pharmacodynamic profile.
Materials and Methods
Materials and cell culture
All small compounds including ellipticine analogs were obtained from the Developmental Therapeutics Program at the National Cancer Institute. Chemotherapeutic agents including 5-Fluorouracil (5-FU), Camptothecin-11 (CPT-11), Gemcitabine (GEM), Cisplatin were obtained from pharmacy of the Hospital of the University of Pennsylvania. HCT116, HCT116 p53−/− and SW620 cells were maintained at 37°C in 5% CO2 in DMEM supplemented with FCS (10%), L-glutamine, penicillin and streptomycin.
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay of cell viability
MTT reduction assay kit from Sigma Aldrich was used to determine cell viability. Cells were seeded in 96-well plates at 1 x 105 per well for 24 h. After treatment with small molecule compounds or chemotherapeutic agents at different concentrations for 48 h, MTT (15 μg/well) was added into culture which was further incubated for 2 h. The reaction was stopped by adding 100 μl of solubilization solution to dissolve the formazan crystals. Plates were read at wavelength of 570 nm and subtracted background absorbance at 690 nm. Growth Inhibition 50% (GI50) is the concentration of test drug where 100 x (T - T0)/(C - T0) = 50.
Flow cytometry assay
Adherent cells in a six-well plate were trypsinized and collected in 15-ml centrifuge tubes, to which were added the originally floating cells. The collected cells were ethanol-fixed and stained with propidium iodide (PI) (Sigma). The DNA content of the stained cells was then measured by using an Epics Elite flow cytometer (Beckman Coulter).
Real-time noninvasive bioluminescence imaging
SW480 human colon cancer cells, stably expressing a p53 reporter, PG13-luc, were seeded in 96-well black plates with clear bottom (Corning) at a density of 5 x 104 cells per well. Compounds were added to the well at different concentrations. p53 transcriptional activity was imaged by using an IVIS imaging system (Xenogen, Alameda, CA) during a time period ranging from 24 to 72 h.
Western blotting
Cells were collected, and protein concentration was quantified by the Bio-Rad protein assay before SDS/PAGE. Proteins were transferred to a PVDF membrane (Immobilon-P, Millipore) by a semidry transfer apparatus (Bio-Rad). The membranes with transferred proteins were blotted with 10% W/V nonfat dry milk and then incubated with the primary antibody and subsequently secondary antibodies, which were labeled by horseradish peroxidase, or near IR dyes. Signals were either visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) and exposed to an X-ray film or scanned by the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). Anti-p53 DO-1 was obtained from Santa Cruz Biotechnology, and anti-p73 (AB-1) and anti-p21 (AB-1) were obtained from Calbiochem. Anti-DR5 antibody was obtained from Cayman Chemical (Ann Arbor, MI) and anti-γ-H2AX antibody was purchased from Upstate.
Immunofluoresence
Cells were seeded in the 4-chamber slides (Fisher Scientific) at 5 x 104 per well. After treatment, cells were washed with PBS, fixed with paraformaldehyde and permeabilized with 0.01% Triton X-100. Blocking solution (1% BSA) was added for 30 min at 37°C followed by primary antibody incubation for 2 h. Samples were washed and incubated with secondary antibody goat anti-mouse PE for h at 37°C. After washing, samples were stained in for DAPI for 30 sec. Mounting solution (90% glycerol) was added and slides were examined by fluorescence microscope.
Si-TAp73 retrovirus construction
The pBS/U6 vector containing TAp73 RNAi was kindly provided by Leif W. Ellisen (Harvard Medical School, Boston), from which the expression cassette was removed and recombined to pSIREN-RetroQ retroviral vector (Clontech), which was reconstructed to express a blasticidin-resistant marker.
Acknowledgments
We thank David T. Dicker for excellent assistance with flow cytometry and Leif W. Ellisen at Harvard Medical School (Boston, MA) for providing pBS/U6-si-TAp73 vector. This work was supported by NIH grants CA105008, CA123258, CA75138 and the Littlefield AACR grant in metastatic colon cancer research. This work was presented in part at the 14th International p53 workshop in Shanghai, China in October 2008.
Glossary
Abbreviations:
- DR5
death receptor 5
- NCI
national cancer institute
- 5-FU
5-fluorouracil
- CPT-11
camptothecin-11
- GEM
gemcitabine
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- TAp73
transactivating form of p73
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/7461
References
- 1.Crawford LV, Lane DP. An immune complex assay for SV40 T antigen. Biochem Biophys Res Commun. 1977;74:323–9. doi: 10.1016/0006-291X(77)91411-5. [DOI] [PubMed] [Google Scholar]
- 2.el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–57. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 3.Vousden KH. Activation of the p53 tumor suppressor protein. Biochim Biophys Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 4.el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–74. [PubMed] [Google Scholar]
- 5.Sax JK, El-Deiry WS. p53 downstream targets and chemosensitivity. Cell Death Differ. 2003;10:413–7. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666–72. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 9.El-Deiry WS. The role of p53 in chemosensitivity and radiosensitivity. Oncogene. 2003;22:7486–95. doi: 10.1038/sj.onc.1206949. [DOI] [PubMed] [Google Scholar]
- 10.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–27. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 12.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–10. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 13.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–8. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 14.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 15.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr. Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–10. doi: 10.1016/S1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 16.Bell HS, Dufes C, O’Prey J, Crighton D, Bergamaschi D, Lu X, et al. A p53-derived apoptotic peptide derepresses p73 to cause tumor regression in vivo. J Clin Invest. 2007;117:1008–18. doi: 10.1172/JCI28920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci U S A. 2008;105:6302–7. doi: 10.1073/pnas.0802091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Kim SH, El-Deiry WS. Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci U S A. 2006;103:11003–8. doi: 10.1073/pnas.0604507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo YC, Kuo PL, Hsu YL, Cho CY, Lin CC. Ellipticine induces apoptosis through p53-dependent pathway in human hepatocellular carcinoma HepG2 cells. Life Sci. 2006;78:2550–7. doi: 10.1016/j.lfs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Jurayj J, Haugwitz RD, Varma RK, Paull KD, Barrett JF, Cushman M. Design and synthesis of ellipticinium salts and 1,2-dihydroellipticines with high selectivities against human CNS cancers in vitro. J Med Chem. 1994;37:2190–7. doi: 10.1021/jm00040a011. [DOI] [PubMed] [Google Scholar]
- 22.Garbett NC, Graves DE. Extending nature’s leads: the anticancer agent ellipticine. Curr Med Chem Anticancer Agents. 2004;4:149–72. doi: 10.2174/1568011043482070. [DOI] [PubMed] [Google Scholar]
- 23.Multon E, Riou JF, LeFevre D, Ahomadegbe JC, Riou G. Topoisomerase II-mediated DNA cleavage activity induced by ellipticines on the human tumor cell line N417. Biochem Pharmacol. 1989;38:2077–86. doi: 10.1016/0006-2952(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 24.Tian E, Landowski TH, Stephens OW, Yaccoby S, Barlogie B, Shaughnessy JD., Jr. Ellipticine derivative NSC 338258 represents a potential new antineoplastic agent for the treatment of multiple myeloma. Mol Cancer Ther. 2008;7:500–9. doi: 10.1158/1535-7163.MCT-07-0524. [DOI] [PubMed] [Google Scholar]
- 25.Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–73. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Bell HS, Ryan KM. Targeting the p53 family for cancer therapy: ‘big brother’ joins the fight. Cell Cycle. 2007;6:1995–2000. doi: 10.4161/cc.6.16.4614. [DOI] [PubMed] [Google Scholar]
- 27.Kovacic P, Ames JR, Lumme P, Elo H, Cox O, Jackson H, et al. Charge transfer-oxy radical mechanism for anti-cancer agents. Anticancer Drug Des. 1986;1:197–214. [PubMed] [Google Scholar]
- 28.Lesca P, Rafidinarivo E, Lecointe P, Mansuy D. A class of strong inhibitors of microsomal monooxygenases: the ellipticines. Chem Biol Interact. 1979;24:189–97. doi: 10.1016/0009-2797(79)90007-3. [DOI] [PubMed] [Google Scholar]
- 29.Hägg M, Berndtsson M, Mandic A, Zhou R, Shoshan MC, Linder S. Induction of endoplasmic reticulum stress by ellipticine plant alkaloids. Mol Cancer Ther. 2004;3:489–97. [PubMed] [Google Scholar]
- 30.Shi LM, Myers TG, Fan Y, O’Connor PM, Paull KD, Friend SH, et al. Mining the National Cancer Institute Anticancer Drug Discovery Database: cluster analysis of ellipticine analogs with p53-inverse and central nervous system-selective patterns of activity. Mol Pharmacol. 1998;53:241–51. doi: 10.1124/mol.53.2.241. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y, Li C, Chen L, Sebti S, Chen J. Rescue of mutant p53 transcription function by ellipticine. Oncogene. 2003;22:4478–87. doi: 10.1038/sj.onc.1206777. [DOI] [PubMed] [Google Scholar]
- 32.Xu GW, Mawji IA, Macrae CJ, Koch CA, Datti A, Wrana JL, et al. A high-content chemical screen identifies ellipticine as a modulator of p53 nuclear localization. Apoptosis. 2008;13:413–22. doi: 10.1007/s10495-007-0175-4. [DOI] [PubMed] [Google Scholar]