Abstract
The results regarding the effects of unaccustomed eccentric exercise on muscle tissue are often conflicting and the aetiology of delayed onset muscle soreness (DOMS) induced by eccentric exercise is still unclear. This study aimed to re-evaluate the paradigm of muscular alterations with regard to muscle sarcolemma integrity and fibre swelling in human muscles after voluntary eccentric exercise leading to DOMS. Ten young males performed eccentric exercise by downstairs running. Biopsies from the soleus muscle were obtained from 6 non-exercising controls, 4 exercised subjects within 1 hour and 6 exercised subjects at 2–3 days and 7–8 days after the exercise. Muscle fibre sarcolemma integrity, infiltration of inflammatory cells and changes in fibre size and fibre phenotype composition as well as capillary supply were examined with specific antibodies using enzyme histochemistry and immunohistochemistry. Although all exercised subjects experienced DOMS which peaked between 1.5 to 2.5 days post exercise, no significant sarcolemma injury or inflammation was detected in any post exercise group. The results do not support the prevailing hypothesis that eccentric exercise causes an initial sarcolemma injury which leads to subsequent inflammation after eccentric exercise. The fibre size was 24% larger at 7–8 days than at 2–3 days post exercise (p<0.05). In contrast, the value of capillary number per fibre area tended to decrease from 2–3 days to 7–8 days post exercise (lower in 5 of the 6 subjects at 7–8 days than at 2–3 days; p<0.05). Thus, the increased fibre size at 7–8 days post exercise was interpreted to reflect fibre swelling. Because the fibre swelling did not appear at the time that DOMS peaked (between 1.5 to 2.5 days post exercise), we concluded that fibre swelling in the soleus muscle is not directly associated with the symptom of DOMS.
Introduction
The aetiology of delayed onset muscle soreness (DOMS) induced by eccentric exercise (i.e., lengthening of a contracting muscle) is still unclear [1]. The prevailing hypothesis of the mechanisms of DOMS is that the excessive strain produced during eccentric muscular contraction induces extracellular or intracellular membrane disruption that may induce hydrolysis of structural proteins such as desmin intermediate filament network, leading to myofibrillar disorganization in the form of Z-band streaming or complete disruption [2], [3]. This is followed by fibre necrosis and inflammatory cell infiltration that potentiate the nerve endings and perception of pain. Passive manipulation and active movement alter intramuscular pressure and stimulate mechanoreceptor nerve endings, contributing to the perception of soreness [4]. However, recent studies in humans provided evidence which did not support the hypothesis, e.g., voluntary eccentric exercise did not lead to desmin intermediate filament network hydrolysis [5], [6], myofibrillar disruption [5], [7] or muscle fibre inflammation and necrosis [5], [6], [8], [9], [10]. The Z-band streaming, classically proposed to be a hallmark of muscle damage after eccentric exercise [11], [12] was also proved to represent myofibril remodelling [7], [13], [14], [15], [16].
As proposed in the prevailing hypothesis, sarcolemma damage has indeed been observed in animals soon after electrical stimulation-induced eccentric muscular contractions, and the damage was often amplified later [17], [18], [19]. In contrast, studies on humans after voluntary eccentric exercise revealed no [5], [6], [20], [21], [22], [23] or only minor damage [10], [24] in muscle fibre membrane. To our knowledge, no data is available in humans with regard to the initial reactions of muscle fibre membrane to acute voluntary eccentric exercise.
Eccentric exercise has been proposed to cause intracellular fibre swelling which is associated with the subsequent muscle soreness and stiffness [3], [4], [25], [26]. Because of technique limitations in directly measuring intracellular fibre swelling, many studies measured muscle swelling using either sonography or circumference of the exercised extremity. Although many studies have reported muscle swelling in human muscles after voluntary eccentric exercise [27], [28], [29], none have shown a time course correlation between muscle swelling and DOMS. By measuring muscle fibre size and intracellular pressure, two studies observed fibre swelling after voluntary eccentric exercise [25], [30]; however, the studies only analysed muscle biopsies taken 2 days after exercise and therefore there is no information on how fibre swelling develops in relation to the time course of DOMS after eccentric exercise.
In skeletal muscle the capillary network is very dynamic and general exercise can cause adaptive changes in capillary supply [31]. In human muscles a single bout of intensive exercise has been shown to up-regulate angiogenic factors [32], whereas in animals a few days of electrical stimulation induced increased capillarization [33]. Electrical stimulation-induced eccentric exercise and downhill running have been reported to cause morphological changes in capillary luminal area and microvascular dysfunction in rat skeletal muscle [34], [35]. To date it is still unclear whether voluntary eccentric exercise in humans is also harmful to the microvascular system.
By analyzing muscle biopsies taken from human soleus muscle within 1 hour, at 2–3 days and 7–8 days after downstairs running, the present study aimed to reveal changes in 1) muscle fibre sarcolemma integrity; 2) muscle fibre swelling/fibre size; 3) microvascular system; and finally 4) to examine the time course of these changes (if any) in relation to the development of DOMS.
Materials and Methods
Ethics Statement
All participants were informed about the design of the study and were asked to refrain from unaccustomed exercise during the experimental period. Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee, Medical Faculty, Umeå University.
Subjects
Sixteen healthy males (mean age 24 years, range 21–30) participated in the study. The subjects were young and healthy sedentary medical students, and none of them were accustomed to eccentric exercise or involved in any regular exercise regime. All the subjects were asked to refrain from any strenuous physical exercise before the experiment. Ten of the subjects were randomly assigned to exercise group and the rest served as controls.
Experimental Procedures
In brief, the subjects were asked to run downstairs from the 10th floor to the ground floor and then took the elevator back to the 10th floor, and to repeat the procedure 15 times. During the downstairs running, the subjects were asked to use the ball of the foot to touch the ground to support the body mass and then to the full foot. By doing so, the soleus muscle was subjected to eccentric contraction.
Muscle Soreness Evaluation and Muscle Biopsy
Evaluation of DOMS in the soleus muscle of all subjects was described in our previous work [14]. In brief, muscle soreness was self-evaluated over the middle of the soleus muscles with the muscle contracted (loaded) and relaxed (unloaded). The muscle soreness was rated by the subjects before and after the exercise twice daily for 8.5 days on a 0–10 subject rating scale (0 = no soreness and 10 = very, very sore).
Muscle biopsies were taken from the lateral border of the middle of the soleus muscle (accessible through contraction of the triceps surae). Biopsies from the soleus muscle were obtained under local anaesthesia from 6 controls, 4 exercised subjects within 1 hour (1 h group) and 6 exercised subjects at 2–3 days (2–3 day group) and 7–8 days (7–8 day group; the contralateral leg) after the exercise. The muscle biopsy was mounted in embedding medium (Tissue Tek®, O.C.T. Compound, Miles laboratories, Naperville, IL, USA), frozen in propane chilled with liquid N2 (−160 °C) and stored at −80 °C until used.
Histochemistry and Immunohistochemistry (IHC)
Serial muscle cross-sections were cut at −25 °C by using a Reichert Jung cryostat (Leica, Nussloch, Germany). Eight µm thick sections were stained with haematoxylin-eosin and a modified Gomori trichrome staining for basic histopathology including detection of degenerative processes and inflammation [36].
Five µm thick sections were processed for IHC with different previously characterized antibodies. For fibre phenotype type classification, serial cross sections were stained with monoclonal antibodies (mAbs) against different adult and developmental myosin heavy chain (MyHC) isoforms [37]: A4.840 (strong affinity for MyHCI), A4.74 (strong affinity for MyHCIIa), N2.261 (strong affinity to MyHCIIa, weak affinity to MyHCI, no affinity to MyHCIIx), BF-35 (strong affinity for all MyHC isoforms except IIx), F1.652 (strong affinity to embryonic MyHC) and NCL-MHCn (strong affinity to fetal MyHC). All antibodies, except NCL-MHCn, were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Biological Sciences, Iowa City, Iowa, USA. NCL-MHCn was obtained from Novocastra Lab, Newcastle, UK.
A panel of antibodies was used for visualization of muscle fibre membrane integrity. Monoclonal Abs Dys1, Dys2, Dys3 (Novocastra laboratories, Newcastle upon Tyne, UK) and polyclonal antibody 5EM96 (gift from Dr. S. C. Watkins, CBI, Pittsburgh, PA, USA) stains muscle fibre plasma membrane protein dystrophin, and mAb 5H2 against laminin α2-chain (Novocastra Lab, Newcastle, UK) stains the basement membranes of muscle fibres. Muscle fibre membrane integrity was also evaluated through identification of cellular distribution of plasma proteins fibronectin and fibrinogen. Fibronectin has been previously used as an indicator for membrane injury in muscle fibre [18] and fibrinogen as a sensitive marker for membrane damage in cardiomyocytes after myocardial infarction [38]. Myocardial tissue samples obtained from experimental myocardial infarction in pigs were used as control for specificity of the staining reaction. MAb 52BF12 was used for plasma protein fibronectin [39]. Three different antibodies against plasma protein fibrinogen were used: mAb F 9902 (Sigma, Copenhagen, Denmark), polyclonal Ab A0080 (Dako, Carpinteria, USA) and polyclonal Ab Pc 056 (The Binding site Inc., San Diego, CA, USA).
Identification of capillaries was performed with mAb 4C7 against laminin α5-chain in the capillary basement membrane (Chemicon, Temecula, Calif., USA) [40] and Ulex europaeus agglutin 1 lectin (UEA-1, Dako, Glostrup, Denmark), an endothelial cell marker that previously has been shown to specifically recognize capillaries in skeletal muscle. The mAb 4C7 labels the basement membrane of capillaries strongly and the basement membrane of muscle fibre weakly [39]. To identify monocytes/macrophages, mAb F7135 (Dako, Glostrup, Denmark) against CD68 was used [41].
The IHC staining follows the same procedures as described previously [14]. Visualization of bound primary antibodies was performed by indirect fluorescence, using Alexa 488 (green) or Alexa 568 (red) conjugated secondary antibodies (Molecular Probes Inc., Eugene, USA) [14], or by indirect peroxidase-antiperoxidase (PAP) staining [37]. Control sections were treated as above, except that the primary antibody was exchanged with non-immune serum.
Sarcolemma Integrity
Analysis of the muscle sarcolemma integrity was performed on sections stained with antibodies against dystrophin, laminin α2-chain, fibronectin and fibrinogen. From each muscle sample randomly chosen areas covering a minimum of 300 fibres were scanned and photographed using a Nikon microscope (Eclipse, E800, Tokyo, Japan) equipped with a Spot RT camera (Diagnostic Instrument, Sterling Heights, USA). The sections stained with the antibodies against dystrophin and laminin α2-chain were examined for disruption or absence of staining from membrane, whereas the sections stained with antibodies against plasma proteins were examined for intracellular staining outlined by double labelling of dystrophin or laminin. The number of fibres with visible lack of staining on membrane or intracellular staining was counted separately and expressed as percentage of total fibres in the photographed area.
Morphometric Analysis
For estimation of muscle fibre size, fibre type classification and capillary network, four randomly chosen areas in each of the sections stained with antibodies against laminin α2-chain, UEA-I lectin and sections double stained for α5-chains and different MyHC isoforms were scanned and photographed with a MTI CCD 72 video camera (DAGE-MTI, Michigan City, USA) through a Zeiss microscope (Axiophot, Carl Zeiss, Oberkochen, Germany) equipped with an image analysis system (IBAS, Kontron Elektronik GMBH, Eching, Germany).
To estimate fibre size and capillary supply, the circumference of each fibre and capillary was traced on digital image along the periphery of the basement membrane revealed by staining for laminin α5-chain and UEA-I lectin. The IBAS program calculated the area of each individual muscle fibre in the muscle fibre cross-section. Fibre size variability as coefficient of variation (CV) was estimated according to the formula CV = (SD/mean fibre diameter) x 100%. Capillary density (CD) was calculated as the total number of capillary per mm2 muscle cross-section and capillary per fibre (C/F) was expressed as the ratio CD/number of fibre per mm2 cross-sectional tissue. For the analysis of the number of capillaries around each individual fibre (CAF), all capillaries within a distance of 5 µm from each muscle fibre were included. The variable of CAF related to the cross-sectional area of each individual fibre (CAFA) was calculated according to the formula: CAF/fibre cross-sectional area x 103. On average, 349 fibres per sample (totally 6821 fibres) were individually analysed for capillary supply, which is beyond the reported requirement (50 fibres/sample) for a reliable analysis of the capillary network in normal limb muscles [42].
Fibre type classification was based on the staining pattern of the fibres for the mAbs directed against different MyHC isoforms. According to a staining scheme of these antibodies (Fig. 1), the fibres were classified into fibre phenotypes containing solely slow MyHCI (type I), fast MyHCIIa (type IIA) or fast MyHCIIx (type IIX) or hybrid fibres co-expressing MyHCI+IIa (type I+IIA) or MyHCIIa+IIx (type IIAX).
Figure 1. Scheme of fibre phenotype classification.
The staining intensity of the four different antibodies in all fibres were briefly classified into “unstained”, “weak”, “Moderate” and “Strong”. An individual fibre was classified into a specific fibre type according to its staining intensities with the four different antibodies against different MyHC isoforms.
The sections stained with antibodies F1.652 and NCL-MHCn were examined for detection of fibres containing developmental MyHCs. All positively stained fibres were counted and expressed as percentage of total fibres. For analysis of infiltration of inflammatory cells, the sections stained with mAb against CD68 were scanned thoroughly to identify positively stained monocytes/macrophages. The number of fibres with infiltration of monocytes/macrophages was counted and expressed as number of cells per mm2 muscle cross-section. Necrotic muscle fibres containing monocytes/macrophages were not included in the counting.
Statistical Analysis
Statistical analyses were performed using the StatView program (SAS Company, Berkley, CA, USA). Data are presented as mean ± SD. Since no indication of non-normality was observed within each group, a t-test for unpaired data was used. Because the biopsies taken at 2–3 days and at 7–8 days were from the same group of subjects, data of the two groups was further analysed by using a paired sample t-test. Statistical significance was set at p<0.05.
Results
DOMS and Sarcolemma Injury
All exercised subjects experienced severe DOMS that reached peak values (7.8±1.4 for loaded and 7.7±1.7 for unloaded) between 1.5 to 2.5 days and returned to pre-exercise level by 6 days post-exercise. There was no significant difference in the mean values of DOMS between the loaded and unloaded soleus muscles at any time [14]. The dynamic changes of DOMS following the time course after the downstairs running have been published previously [14].
No visible lesions were observed in muscle fibre sarcolemma proteins revealed by staining for dystrophin and laminin (Fig. 2). Staining for plasma fibronectin was negative in all muscle samples, except for one biopsy from the 1 h group, where a few fibres contained some small and weakly stained subsarcolemmal dots (not shown). In the same fibres, where intracellular staining for fibronectin was observed, similar subsarcolemmal dots revealed by double staining for fibrinogen were present as well. However, intracellular staining for fibrinogen was more often observed in fibres where staining for fibronectin was negative. The intracellular staining for fibrinogen was usually seen as a rim adjacent to the plasmalemma close to capillaries and exhibited a diminishing gradient towards the centre of the fibres (Fig. 2). The intracellular staining for fibrinogen was observed in a low proportion of fibres (<5%) in both the controls and exercised groups. Statistical analysis of the number of fibres with intracellular staining for fibrinogen did not reveal any significant difference between control and any post exercise groups.
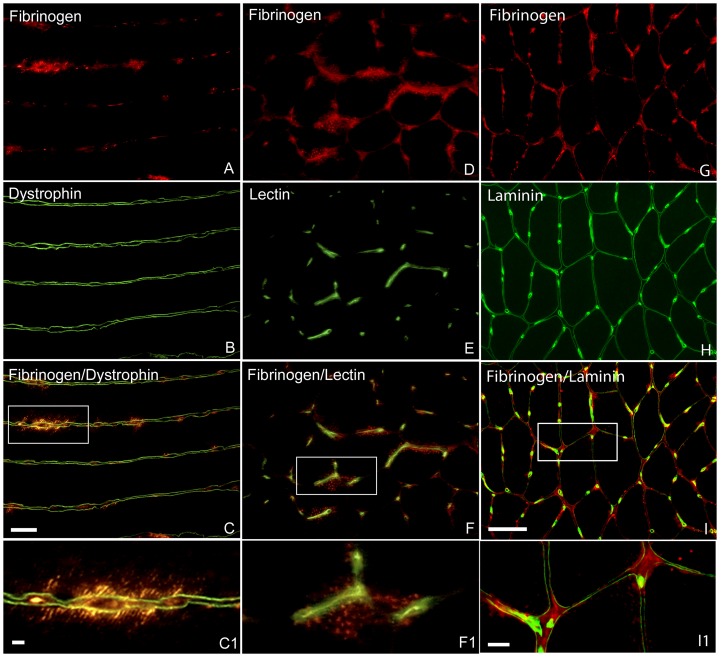
Figure 2. Immunofluorescence staining for visualization of muscle fibre sarcolemma integrity.
A–C, one longitudinal section from a biopsy of 1 h group double stained for fibrinogen (A) and dystrophin (B). C is merged images of A and B, and C1 is enlargement of boxed areas in C. Several sites of intracellular staining for fibrinogen outlined by dystrophin were seen along the length of a fibre, whereas staining for dystrophin did not reveal distinct disruption of plasma membrane. D–F, one transverse section from a biopsy of 1 h group double stained for fibrinogen (D) and lectin (E). F is merged images of D and E, and F1 is enlargement of boxed areas in F. Intracellular staining for fibrinogen was seen close to capillaries revealed by lectin staining. G–I, one transverse section from a biopsy of control group double stained for fibrinogen (G) and laminin (H). I is merged images of G and H, and I1 is enlargement of boxed areas in I. Several sites of distinct extracellular staining for fibrinogen outlined by laminin were seen. Bars 50 µm
In the samples of experimental myocardial infarction, all the three antibodies against fibrinogen showed distinct intracellular staining in necrotic cardiomyocytes as previously shown [38]. However, in the soleus muscle samples, positive staining for fibrinogen as small dots was seen in some areas in both muscle fibres and extracellular matrix (Fig. 2).
Necrotic Fibre and Infiltration of Inflammatory Cells
Routine histological staining showed that the fibres in both the controls and exercised samples had generally a polygonal shape and were tightly packed in well-ordered fascicles. A few monocytes/macrophages revealed by antibody against CD68 were seen in between fibres in both control and post exercise groups (Fig. 3). No significant difference was observed in the number of extracellular monocytes/macrophages per unit muscle cross-sectional area (CSA) between control (5.3±3.3 per µm2) and post exercise groups (1 h, 6.0±1.7 per µm2; 2–3 day, 11.9±3.3 per µm2; 7–8 day, 5.3±3.5 per µm2). Intracellular staining for CD68 was only observed in a few fibres that appeared to be necrotic (Fig. 3). Cells with intracellular staining for CD68 were not included in the data counting.
Figure 3. Immunofluorescence staining for visualization of developmental and inflammatory cells.
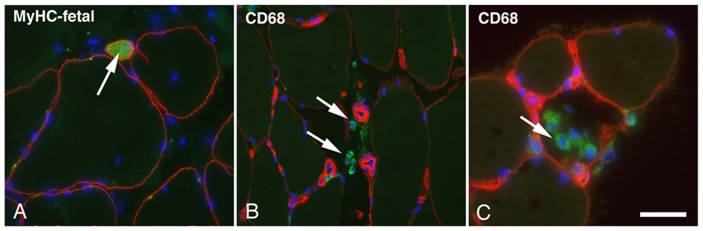
A, one transverse sections from biopsy of 7–8 day group double stained with antibodies against MyHC-fetal (green) and dystrophin (red). One small neonatal fibre was seen between normal sized fibres (arrow). B and C, transvers sections from biopsies of 2–3 day group double stained with antibodies against CD68 (green) and laminin (red). Several extracellular monocytes/macrophages (arrows) were seen in B whereas in C monocytes/macrophages were seen inside a necrotic fiber. Nuclei (blue) were visualized in all sections by using mounting medium containing DAPI. Bar 100 µm
Developmental MyHCs
A few fibres expressing developmental MyHCs were observed in the 2–3 day and 7–8 day groups, but not in the control or 1 h group (Fig. 3). These fibres were generally extremely small in size in the 2–3 day group (<100 µm2) and somewhat larger (<400 µm2) in the 7–8 day group.
Muscle Fibre Phenotype Composition
The staining pattern for the different MyHC mAbs distinguished fibres containing MyHCI and MyHCIIa in all subjects and nearly all samples contained a small proportion of hybrid fibres co-expressing MyHCI+IIa or MyHCIIa+IIx. The control soleus muscle was characterized by a very high proportion of fibres containing slow MyHCI (68%±9) and a population of fibres containing fast MyHCIIa (26%±8). No significant difference in proportion of the different fibre phenotypes was observed between any group, except that one subject contained a low proportion of fibres expressing MyHCIIx at 2–3 days (1%±4) and 7–8 days (2%±4).
Muscle Fibre Size
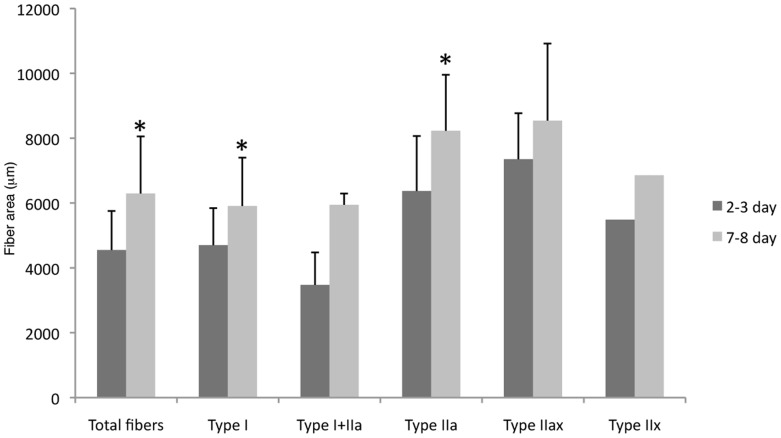
The mean fibre size was 5894 µm2±845 for the control group and 4930 µm2±1418 for the 1 h group, 4987 µm2±1367 for the 2–3 day group and 6588 µm2±1551 for the 7–8 day group. The fibre size at 7–8 days was significantly larger than that at 2–3 days (p<0.05; Fig. 4). Analysis of fibre size with respect to fibre phenotype revealed that type I and type IIa fibres in the 7–8 day group were significantly larger than in the 2–3 day group (Fig. 5). Variability in fibre size, expressed as CV, was approximately in the same range at 2–3 days and at 7–8 days (34% vs. 30%).
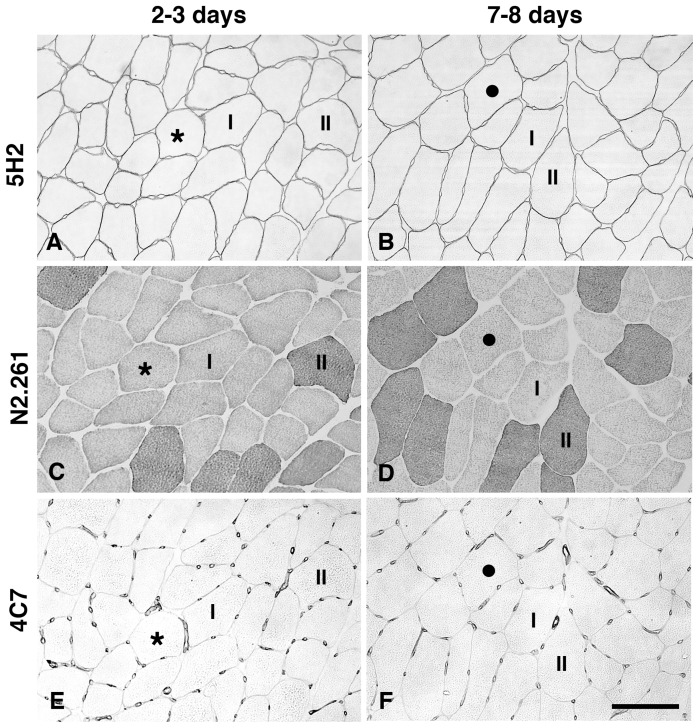
Figure 4. Immunohistochemical staining for visualization of muscle fibre size, fibre phenotype and capillary supply.
Serial transverse sections from one biopsy in the 2–3 day group (A, C, E) and one from the 7–8 day group (B, D, F) stained with antibodies 5H2 for fibre profile (A, B), N2.261 for fibre phenotypes (C, D) and 4C7 for capillaries (E, F). Most fibres are larger in size in the 7–8 days group (B) compared to the 2–3 days group (A). Type I and type II fibres are marked (C, D). There was no distinct difference in number of capillaries around each fibre between the two groups (E, F) despite the fibre size was larger in the 7–8 day group (F). Stars and black dots mark same fibre in series sections. Bar 100 µm
Figure 5. Comparison in fibre size between 2–3 day and 7–8 day groups.
Fibre size in 7–8 day group was significantly larger than in 2–3 day group. Among the five different fibre phenotypes, only type I and type IIa fibres presented larger fibre size in 7–8 day group than in 2–3 day group (*p<0.05).
Capillary Supply
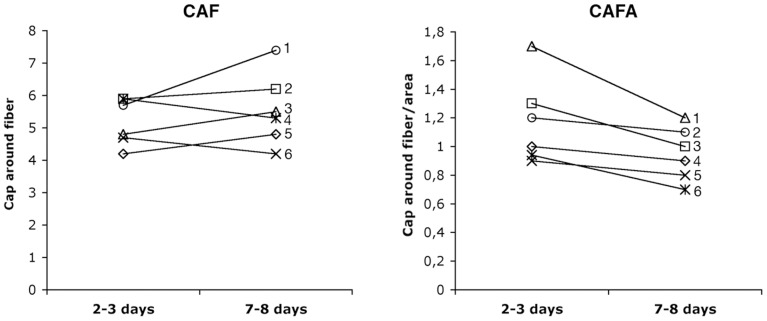
At the group level no significant difference in capillary parameters was observed between the control and the post-exercise groups, although a decreasing trend in capillary density (CD) was observed from 2–3 days to 7–8 days (330±6.1 vs. 289±71 cap/mm2; p = 0.07). Paired analysis of the subjects in the 2–3 day and 7–8 day groups revealed a significant difference in CAF in 5 of the 6 subjects, where 3 subjects had higher values and 2 subjects had lower values at 7–8 days than at 2–3 days (p<0.05; Fig. 6). When CAF was further processed by fibre size (CAFA), paired comparison showed lower CAFA value in 5 of the 6 subjects at 7–8 days compared to 2–3 days (p<0.05; Fig. 6).
Figure 6. Comparison of individual values in CAF and CAFA from the same subjects in 2–3 day and 7–8 day groups.
Compared to 2–3 day group, CAF was higher in three subjects (1, 3, 5) and lower in two subjects (4, 6), whereas CAFA was lower in five subjects (1, 3, 4, 5, 6) in 7–8 day group (p<0.05).
Discussion
The present study shows that the downstairs running leading to DOMS did not result in significant sarcolemma injury or serious inflammatory reactions in the soleus muscle. Thus, the hypothesis that eccentric exercise induces initial sarcolemma injury which triggers subsequent muscle fiber inflammation and necrosis seems not applicable for the human soleus muscle. The enlarged fiber size at 7–8 days post exercise was interpreted to represent fibre swelling due to edema. Since fiber swelling did not appear at the time of maximal DOMS (between 1.5 to 2.5 days post exercise), it seems not to be directly associated with the aetiology of DOMS.
Sarcolemma Injury
Previous studies have mainly used two different methods to evaluate sarcolemma integrity after eccentric exercise: direct visualization through examination of muscle biopsy and indirect evaluation by measuring serum levels of muscle enzymes such as creatine kinase (CK), lactate dehydrogenase (LDH) and glutamic oxaloacetic transaminase (GOT), and of muscle proteins like slow myosin heavy chain (MyHC) fragments and myoglobin [1].
Many studies using animal models of eccentric muscular contraction have reported large proportions (around 10–30%) of muscle fibres with discontinuous or complete disruption of sarcolemma as evidenced by lack of staining for dystrophin, dystroglycans and sarcoglycans [43], [44] and by intracellular staining for serum albumin, fibronectin and Evans blue [19], [43], [44], [45]. In comparison, voluntary eccentric exercise of human muscles usually does not induce similar serious injury in sarcolemma integrity. In an early study on humans [10], ultra-marathon running (mainly downhill) induced very few fibres (in gastrocnemius muscle) with sarcolemma disruption. Using isolated high intensity eccentric exercise, two studies reported sarcolemma injury (in vastus lateralis muscle [46] and in biceps brachii muscle [47]), but the injury was only limited in a few of the subjects/biopsies (1 out of 8 subjects or 2 out of 24 biopsies [46], and 9 out of 61 biopsies [47]). Similarly, in a recent study, sarcolemma injury was only observed in 1 out of 18 biopsies (or 1 out of 9 subjects) from vastus lateralis muscle taken during (at 3 days) and after 6 days maximum voluntary eccentric exercise of knee joint [23]. The present study did not observe any significant lesion revealed by either sarcolemma proteins or by plasma proteins in any of the post exercise groups. The results of the study indicate that human soleus muscle sarcolemma was not as susceptible to the downstairs running as animal muscles to isolated eccentric exercise [18], [19], [43], [44], [45]. Importantly, the study did not observe sarcolemma damage at 1 hour after the downstairs running, indicating the previous hypothesis that early sarcolemma injury triggers subsequent deleterious reactions in muscles after eccentric exercise is not applicable to the human soleus muscles. The results of all these studies collectively indicate that sarcolemma disruption occurs in a minority of exercised subjects irrespective of how the voluntary eccentric exercise is performed.
Previous study has shown that disruptions of cell membranes are common in vivo and that the disrupted membranes can reseal within a minute even when a hole is up to 1 µm in diameter [48]. It is commonly believed that structural changes in muscles induced by eccentric exercise can be regarded as a continuum from mild changes to pathological-like changes [1]. We propose that the sarcolemma injuries in humans induced by voluntary eccentric exercise are mostly from mild to moderate in extent and therefore most injuries are temporary and reversible. The temporary injury in sarcolemma may allow the efflux of muscle enzymes such as CK, LDH and GOT, and even large molecular proteins like MyHC fragments and myoglobin into the plasma. Since such sarcolemma injuries are usually resealed very fast, no visible lesion in sarcolemma is available even if the level of muscle enzymes and muscle proteins are high in the serum. This might explain the poor correlation between serum CK level and changes in muscle histology [49].
In contrast, the electrical stimulation-induced eccentric muscular contractions might result in sarcolemma injuries too extensive in size to be resealed in animals (rabbit digitorum longus muscle [18] and rabbit and rat tibialis anterior muscle [18], [19]) and in humans (vastus lateralis muscle [5]). This might lead to calcium influx and subsequent proteinase activation and hydrolysis of structural proteins like desmin and sarcolemma proteins, followed by complete sarcolemma disruption and fibre necrosis [5], [18], [19]. Of course, sarcolemma damage to such an extent will allow efflux of muscle enzymes and muscle proteins, and even influx of serum proteins such as fibronectin, albumin and tetranectin [1], [5], [18], [19].
Sarcolemma injury in humans after voluntary eccentric exercise seems to be limited mainly in a few biopsies/subjects [10], [23], [46], [47], which indicates individual variation in sensitivity of muscle fibre sarcolemma to the same mode of eccentric exercise. This is consistent with the observation that post-exercise plasma CK activity demonstrates large variation across individuals with high responders, median responders and low responders [49]. Interestingly, some studies (including the present study) have shown that sarcolemma injury [10], [23], [46], [47] occurred often in the same biopsies/subjects where fibre necrosis were observed [5], [10], [46], [50], [51]. Currently, not much data is available about the relationship between sarcolemma injury and fibre necrosis after eccentric exercise; however, it seems plausible to speculate a close correlation between the two events, especially when sarcolemma injury is extensive.
The present study applied downstairs running to expose the soleus muscle to eccentric exercise. Compared with other studies using isolated maximal eccentric contraction on humans [5], [46], [47], [50], [51] and animals [17], [18], [19], the exercise mode used in the study might explain the lack of significant sarcolemma injury and inflammatory reaction.
Fibrinogen has been previously proved to be a good marker for cardiomyocyte damage after myocardial infarction [38]. However, in the present study, some small intracellular staining for fibrinogen existed not only in post exercise muscles (<5%) but also in controls (around 3.7%). In addition, in some areas, positive staining for fibrinogen was also present in extracellular matrix. As a control for the antibodies, the samples of the experimental myocardial infarction were also tested in the study, and all the three different antibodies against fibrinogen revealed only distinct intracellular staining in the necrotic cardiomyocytes. On basis of the results, we concluded that plasma fibrinogen might be a good marker for necrotic fibres, but not for detection of mild membrane damage.
Necrotic Fibres and Infiltration of Inflammatory Cells
In the present study, very few necrotic fibres and a nearly negligible infiltration of monocytes/macrophages were observed in the exercised muscles. The findings were consistent with previous observations on humans after voluntary eccentric exercise [10], [20], [22], [30], [50], [51]. Studies on lower body muscles (vastus lateralis) failed to present fibre necrosis after high intensity of knee eccentric activity (30 min eccentric cycling [20], [22] and repetitive knee eccentric extension [30]). Even ultra-marathon footrace (160 km, mainly downhill) only resulted in very few necrotic fibres (1%) in gastrocnemius muscles [10]. Studies on upper body muscles (biceps brachii), however, indeed revealed fibre necrosis after maximal voluntary eccentric contractions of the elbow flexors, but it occurred only in a few of the exercise subjects/biopsies (3 out of 15 biopsies [50], and 8 out of 23 subjects [51]). These results seem in support of the observation that the upper body muscles are more prone to exercise-induced damage than lower body muscles [52].
In animal models of electrical stimulation-induced eccentric muscle contraction, massive muscular inflammation and necrotic fibres (over 30% necrotic fibres) were observed (in rabbit digitorum longus muscle [18] and in both rabbit and rat tibialis anterior muscle [18], [19]). In this context, the study of Crameri et al (5) is of special interest and relevance. Massive fibre necrosis was observed in vastus laterlis muscles subjected to electrical stimulation-induced eccentric muscle contractions whereas in the same subjects, no fibre necrosis was observed in the muscle of the contralateral leg after maximal voluntary eccentric contractions. The authors suggested that the difference in the extent of muscle injury between the voluntary eccentric exercise and the electrical stimulation-induced eccentric muscle contraction may be due to the difference in motor unit recruitment pattern. This may also explain the difference in the extent of fibre necrosis and inflammation between animals and humans. Alternatively, the difference in exercise protocol may play an important role in the extent of muscle injury.
Developmental MyHCs
In the 2–3 day group, a few extremely small fibres expressed developmental MyHC. Such fibres were also observed in a very low number in the 7–8 day group, but these were relatively larger in size. In adult limb muscles, small fibres expressing developmental MyHC have been associated with newly formed/regenerated muscle fibres [53]. Satellite cells are considered to be involved in the process of repair and formation of new fibres after muscle damage [54]. Although we found no signs of serious muscle damage in the post exercise muscles, the findings of small fibres expressing developmental MyHCs in the post-exercise muscle biopsies may imply that the downstairs running induced a very restricted regenerative process in the soleus muscle.
Muscle Fibre Swelling
Muscle fibre swelling due to oedema has been associated with the mechanisms of DOMS induced by eccentric exercise [55]. Muscle fibre swelling evaluated by increased muscle fiber size and intramuscular pressure has been observed 2 days post exercise in human tibialis anterior muscle after 400 submaximal eccentric contractions [25] and vastus lateralis muscle after repetitive maximal eccentric exercise [30]. However, since the two studies did not examine serial biopsies following the time course of DOMS after the eccentric exercise, the association between muscle fibre swelling and DOMS was still inconclusive. In the present study, we observed a 24% greater increase in muscle fibre size at 7–8 days than at 2–3 days in the same group subjects. The increased fibre size was interpreted to reflect muscle fibre swelling. However, a single bout of eccentric exercise has been shown to stimulate net protein synthesis within 4–8 hours after exercise [56]. Thus, we could not exclude the possibility that increased protein synthesis is partly responsible for the dramatic enlargement in fibre size at 7–8 days after the exercise. Yet, the results of the relatively similar fibre size in different fibre phenotypes and the significant lower CAFA values in 5 out of the 6 subjects at 7–8 days compared to 2–3 days suggest that the larger fibre size was most likely due to fibre swelling. Since fibre swelling appeared at 7–8 days, not at 2–3 days when DOMS peaked, we conclude that fibre swelling is not associated with the aetiology of DOMS in human soleus muscle after the downstairs running.
Muscle Vascularization
In previous studies on rats, exhaustive eccentric exercise of spinotrapezius muscle [34] and 300 electrical stimulation-induced eccentric contractions of gastrocnemius muscles resulted in damage to microcirculation and thereby the delivery and exchange of O2 and substrates [35]. The damage was exhibited more often in the capillary lumen rather than capillary collapse or capillary degeneration. In this study, we did not observe significant alterations in capillary network at the group level. However, a significant increase in the capillary network was observed 7–8 days post exercise in some individuals, whereas a regression of the capillary network was found in others. The different responses to the downstairs running might reflect individual variation in physical fitness or inherent genetic differences. Although it is not possible to make any reliable conclusions about the effect of eccentric exercise on the capillary network, the present results indicate that the same exercise could lead to completely different reactions in the microvascular system in different subjects.
Conclusions
The present study showed that the downstairs running did not induce early injury in sarcolemma or severe muscle fibre inflammation and necrosis. The result disproves the previous hypothesis that eccentric exercise leads to initial sarcolemma injury which triggers subsequent deleterious reactions such as muscle fibre inflammation and necrosis. The study revealed that the downstairs running indeed induced fibre swelling, but it appeared at 7–8 days, not at 2–3 days post exercise when DOMS peaked. The result also disproved previous hypothesis that muscle fibre swelling is directly associated with the symptom of DOMS. However, the study examined only the soleus muscles after downstairs running; thus, a definite conclusion cannot be made before evaluations of other muscles are performed in the same way. Furthermore, individual variation may confound the interpretation of the data since in the study only the 2–3 day group and the 7–8 day group are from the same subjects.
Acknowledgments
We thank M Enerstedt and A-K Olofsson for excellent technical assistance.
Funding Statement
This work was supported by grants from the Swedish National Centre for Research in Sports (139/07, 89/10, 105/11), the Swedish Research Council (12X-03934) and the Medical Faculty of Umeå University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There is no actual or potential conflict of interest including financial, personal or other relationships with other people or organizations.
References
- 1. Paulsen G, Mikkelsen UR, Raastad T, Peake JM (2012) Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18: 42–97. [PubMed] [Google Scholar]
- 2. Lieber RL, Friden J (2002) Morphologic and mechanical basis of delayed-onset muscle soreness. J Am Acad Orthop Surg 10: 67–73. [PubMed] [Google Scholar]
- 3. Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil/Association of Academic Physiatrists 81: S52–69. [DOI] [PubMed] [Google Scholar]
- 4. Lewis PB, Ruby D, Bush-Joseph CA (2012) Muscle soreness and delayed-onset muscle soreness. Clin Sports Med 31: 255–262. [DOI] [PubMed] [Google Scholar]
- 5. Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, et al. (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583: 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu JG, Malm C, Thornell LE (2002) Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol 118: 29–34. [DOI] [PubMed] [Google Scholar]
- 7. Yu JG, Carlsson L, Thornell LE (2004) Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: an ultrastructural and immunoelectron microscopic study. Histochem Cell Biol 121: 219–227. [DOI] [PubMed] [Google Scholar]
- 8. Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, et al. (2004) Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol 556: 983–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malm C, Yu JG (2012) Exercise-induced muscle damage and inflammation: re-evaluation by proteomics. Histochem Cell Biol 138: 89–99. [DOI] [PubMed] [Google Scholar]
- 10. Crenshaw AG, Friden J, Hargens AR, Lang GH, Thornell LE (1993) Increased technetium uptake is not equivalent to muscle necrosis: scintigraphic, morphological and intramuscular pressure analyses of sore muscles after exercise. Acta Physiol Scand 148: 187–198. [DOI] [PubMed] [Google Scholar]
- 11. Friden J, Sjostrom M, Ekblom B (1981) A morphological study of delayed muscle soreness. Experientia 37: 506–507. [DOI] [PubMed] [Google Scholar]
- 12. Friden J, Lieber RL (1992) Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc 24: 521–530. [PubMed] [Google Scholar]
- 13. Yu JG, Furst DO, Thornell LE (2003) The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochem Cell Biol 119: 383–393. [DOI] [PubMed] [Google Scholar]
- 14. Yu JG, Thornell LE (2002) Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: a high resolution immunocytochemical study. Histochem Cell Biol 118: 171–179. [DOI] [PubMed] [Google Scholar]
- 15. Carlsson L, Yu JG, Moza M, Carpen O, Thornell LE (2007) Myotilin: a prominent marker of myofibrillar remodelling. Neuromuscul Disord 17: 61–68. [DOI] [PubMed] [Google Scholar]
- 16. Carlsson L, Yu JG, Thornell LE (2008) New aspects of obscurin in human striated muscles. Histochem Cell Biol 130: 91–103. [DOI] [PubMed] [Google Scholar]
- 17. Friden J, Lieber RL (2001) Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171: 321–326. [DOI] [PubMed] [Google Scholar]
- 18. Lieber RL, Thornell LE, Friden J (1996) Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80: 278–284. [DOI] [PubMed] [Google Scholar]
- 19. Komulainen J, Takala TE, Kuipers H, Hesselink MK (1998) The disruption of myofibre structures in rat skeletal muscle after forced lengthening contractions. Pflugers Arch 436: 735–741. [DOI] [PubMed] [Google Scholar]
- 20. Malm C, Nyberg P, Engstrom M, Sjodin B. Lenkei R, et al. (2000) Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529 Pt 1: 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newham DJ, McPhail G, Mills KR, Edwards RH (1983) Ultrastructural changes after concentric and eccentric contractions of human muscle. Neurol Sci 61: 109–122. [DOI] [PubMed] [Google Scholar]
- 22. Friden J, Sjostrom M, Ekblom B (1983) Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 4: 170–176. [DOI] [PubMed] [Google Scholar]
- 23. Costa A, Orosz Z, Apor P, Csaba N, Siamilis S, et al. (2009) Impact of repeated bouts of eccentric exercise on sarcolemma disruption in human skeletal muscle. Acta Physiol Hung 96: 189–202. [DOI] [PubMed] [Google Scholar]
- 24. Stauber WT, Clarkson PM, Fritz VK, Evans WJ (1990) Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol 69: 868–874. [DOI] [PubMed] [Google Scholar]
- 25. Friden J, Sfakianos PN, Hargens AR, Akeson WH (1988) Residual muscular swelling after repetitive eccentric contractions. J Orthop Res 6: 493–498. [DOI] [PubMed] [Google Scholar]
- 26. Proske U, Allen TJ (2005) Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 33: 98–104. [DOI] [PubMed] [Google Scholar]
- 27. Chleboun GS, Howell JN, Conatser RR, Giesey JJ (1998) Relationship between muscle swelling and stiffness after eccentric exercise. Med Sci Sports Exerc 30: 529–535. [DOI] [PubMed] [Google Scholar]
- 28. Zainuddin Z, Newton M, Sacco P, Nosaka K (2005) Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J Athl Train 40: 174–180. [PMC free article] [PubMed] [Google Scholar]
- 29. Jayaraman RC, Reid RW, Foley JM, Prior BM, Dudley GA, et al. (2004) MRI evaluation of topical heat and static stretching as therapeutic modalities for the treatment of eccentric exercise-induced muscle damage. Eur J Appl Physiol 93: 30–38. [DOI] [PubMed] [Google Scholar]
- 30. Crenshaw AG, Thornell LE, Friden J (1994) Intramuscular pressure, torque and swelling for the exercise-induced sore vastus lateralis muscle. Acta Physiol Scand 152: 265–277. [DOI] [PubMed] [Google Scholar]
- 31. Laughlin MH, Roseguini B (2008) Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59 Suppl 771–88. [PMC free article] [PubMed] [Google Scholar]
- 32. Bloor CM (2005) Angiogenesis during exercise and training. Angiogenesis 8: 263–271. [DOI] [PubMed] [Google Scholar]
- 33. Brown MD, Hudlicka O (2003) Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14. [DOI] [PubMed] [Google Scholar]
- 34. Kano Y, Padilla DJ, Behnke BJ, Hageman KS, Musch TI, et al. (2005) Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J Appl Physiol 99: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 35. Kano Y, Sampei K, Matsudo H (2004) Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand 180: 291–299. [DOI] [PubMed] [Google Scholar]
- 36.Dubowitz V, Sewry CA (2007) Muscle biopsy. A practical approach. Bailliere Tindall, City.
- 37. Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellof F (2002) Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem 50: 171–183. [DOI] [PubMed] [Google Scholar]
- 38. Holmbom B, Lindstrom M, Naslund U, Thornell LE (1991) A method for enzyme- and immunohistochemical staining of large frozen specimens. Histochem 95: 441–447. [DOI] [PubMed] [Google Scholar]
- 39. Vartio T, Laitinen L, Narvanen O, Cutolo M, Thornell LE, et al. (1987) Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci 88: 419–430. [DOI] [PubMed] [Google Scholar]
- 40. Stal PS, Lindman R (2000) Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat 197 (Pt 2): 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Micklem K, Rigney E, Cordell J, Simmons D, Stross P, et al. (1989) A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol 73: 6–11. [DOI] [PubMed] [Google Scholar]
- 42. Porter MM, Koolage CW, Lexell J (2002) Biopsy sampling requirements for the estimation of muscle capillarization. Muscle Nerve 26: 546–548. [DOI] [PubMed] [Google Scholar]
- 43. Lehti TM, Kalliokoski R, Komulainen J (2007) Repeated bout effect on the cytoskeletal proteins titin, desmin, and dystrophin in rat skeletal muscle. J Muscle Res Cell Motil 28: 39–47. [DOI] [PubMed] [Google Scholar]
- 44. Lovering RM, De Deyne PG (2004) Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol 286: C230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNeil PL, Khakee R (1992) Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140: 1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 46. Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, et al. (2004) Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol-London 558: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulsen G, Egner I, Raastad T, Reinholt F, Owe S, et al. (2012) Inflammatory markers CD11b, CD16, CD66b, CD68, myeloperoxidase and neutrophil elastase in eccentric exercised human skeletal muscles. Histochem Cell Biol Dec 7.. [DOI] [PubMed] [Google Scholar]
- 48. McNeil PL, Steinhardt RA (1997) Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol 137: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clarkson PM, Nosaka K, Braun B (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24: 512–20. [PubMed] [Google Scholar]
- 50. Lauritzen F, Paulsen G, Raastad T, Bergersen LH, Owe SG (2009) Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J Appl Physiol 107: 1923–1934. [DOI] [PubMed] [Google Scholar]
- 51. Paulsen G, Egner IM, Drange M, Langberg H, Benestad HB, et al. (2010) A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand J Med Sci Sports 20: e195–e207. [DOI] [PubMed] [Google Scholar]
- 52. Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, et al. (2005) Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol 95: 179–85. [DOI] [PubMed] [Google Scholar]
- 53. Schiaffino S, Gorza L, Dones I, Cornelio F, Sartore S (1986) Fetal myosin immunoreactivity in human dystrophic muscle. Muscle nerve 9: 51–58. [DOI] [PubMed] [Google Scholar]
- 54. Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE (1999) Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol 111: 189–195. [DOI] [PubMed] [Google Scholar]
- 55. Lieber RL, Friden J (1999) Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport 2: 253–265. [DOI] [PubMed] [Google Scholar]
- 56. Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ (2005) Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288: E1153–E1159. [DOI] [PubMed] [Google Scholar]