Abstract
Candida albicans and Candida dubliniensis are pathogenic fungi that are highly related but differ in virulence and in some phenotypic traits. During in vitro growth on certain nutrient-poor media, C. albicans and C. dubliniensis are the only yeast species which are able to produce chlamydospores, large thick-walled cells of unknown function. Interestingly, only C. dubliniensis forms pseudohyphae with abundant chlamydospores when grown on Staib medium, while C. albicans grows exclusively as a budding yeast. In order to further our understanding of chlamydospore development and assembly, we compared the global transcriptional profile of both species during growth in liquid Staib medium by RNA sequencing. We also included a C. albicans mutant in our study which lacks the morphogenetic transcriptional repressor Nrg1. This strain, which is characterized by its constitutive pseudohyphal growth, specifically produces masses of chlamydospores in Staib medium, similar to C. dubliniensis. This comparative approach identified a set of putatively chlamydospore-related genes. Two of the homologous C. albicans and C. dubliniensis genes (CSP1 and CSP2) which were most strongly upregulated during chlamydospore development were analysed in more detail. By use of the green fluorescent protein as a reporter, the encoded putative cell wall related proteins were found to exclusively localize to C. albicans and C. dubliniensis chlamydospores. Our findings uncover the first chlamydospore specific markers in Candida species and provide novel insights in the complex morphogenetic development of these important fungal pathogens.
Introduction
The pathogenic yeast Candida albicans represents the clinically most important member of the genus Candida [1]. Although C. albicans is a harmless member of the normal microflora in healthy people, the species can cause life-threatening, disseminated infections in immunocompromised patients [2]. In medical routine diagnosis, C. albicans has been differentiated for a long time from other yeast-like fungi by a species-specific, morphogenetic characteristic, i.e. the formation of chlamydospores. These large, thick-walled, spherical cells are produced by C. albicans on specific nutrient-poor media such as rice-extract or corn meal agar at room temperature, typically from suspensor cells at the end of pseudohyphae [2], [3], [4], [5], [6]. Despite the importance of chlamydospores for species identification, even today, the biological function of these entities remains enigmatic [5], [7]. No role for chlamydospores has yet been identified in the life cycle of the microorganism or in fungal survival in the environment or in pathogenicity. Although readily inducible in vitro, chlamydospores have only rarely been observed in vivo [8], [9]. Chlamydospore formation would appear to be a complex process that undoubtedly requires specific genes and regulatory pathways that have been retained since the divergence of C. albicans and C. dubliniensis approximately 20 million years ago. The question therefore remains why have these species retained the capacity to produce these complex and unusual structures and what exactly is their purpose in the Candida life cycle?
In contrast to hyphae formation, the program of chlamydospore development has only been studied poorly at the molecular level (for review see [5]). Some of the signaling pathways which control hyphae formation in C. albicans were also found to influence chlamydospore development, e.g. those involving transcriptional regulators Efg1 and Nrg1, or the stress-activated protein kinase Hog1 [10], [11], [12]. Other genes, which are likely implicated in chlamydospore formation have also been identified, for example by screening libraries of deletion mutants or by testing individual knock-out strains for their ability to efficiently develop these morphological structures [13], [14]. However, so far no proteins have been identified which are specifically localized to chlamydospores. Such markers would be useful in the study of the morphogenetic development of these cellular entities, and would facilitate the differentiation of chlamydospores from other morphological growth forms.
Molecular analysis of chlamydospores has received increasing attention since the description of a new Candida species in 1995, C. dubliniensis. C. dubliniensis is closely related to C. albicans and displays many phenotypic characteristics that were assumed to be specific for C. albicans, including the ability to form true hyphae and chlamydospores [15], [16], [17]. Intriguingly, however, only C. dubliniensis was found to produce pseudohyphae and chlamydospores on Staib agar (syn. Guizotia abyssinica creatinine agar), where C. albicans grows as a budding yeast [18]. This species-specific characteristic was shown to be governed by a differential expression of the gene encoding the hyphal repressor Nrg1 in the two species. A C. albicans knock-out mutant in the NRG1 gene, which is known for its constitutive pseudohyphal growth [19], [20], produces chlamydospores specifically on Staib agar, similar to C. dubliniensis [12].
In the present study, we set out to identify chlamydospore specific markers in Candida. As a method, we investigated for the first time genome wide expression patterns in Candida species during chlamydospore development. In detail, global transcriptomes of C. albicans and C. dubliniensis wild-type strains as well as the C. albicans nrg1Δ mutant were monitored by RNA sequencing during growth in Staib medium. The comparison of the detected profiles allowed the identification of a set of highly expressed genes specifically related to chlamydospore development. For selected candidates, which code for putative cell wall proteins, the chlamydospore specific expression and the exclusive localization of the encoded proteins to chlamydospores was demonstrated by green fluorescent protein (GFP) fusion strains.
Materials and Methods
Strains and growth conditions
C. albicans and C. dubliniensis strains used in this work are listed in Table 1. Strains were routinely propagated on YPD agar (20 g peptone, 10 g yeast extract, 20 g glucose, 15 g agar per litre) at 30°C and stored as frozen stocks in liquid YPD medium with 15% (v/v) glycerol at –80°C. Chlamydospore formation in C. albicans and C. dubliniensis was induced by growth of the strains on rice-extract agar (Beckton, Dickinson and Company, Sparks, USA) at 25°C. Staib liquid medium was used for the specific induction of chlamydospore formation in C. dubliniensis and the C. albicans nrg1Δ mutant strain MMC3 at 25°C. Staib medium was prepared like Staib agar (syn. Guizotia abyssinica creatinine agar) as described previously [21], [22], only the agar was omitted. In brief, 50 g pulverized Guizotia abyssinica plant seeds were boiled in 1 l of distilled water for 30 min, filtered and filled up to 1 l with water. Thereafter, 1 g glucose, 1 g KH2PO4 and 1 g creatinine were added before autoclaving for 20 min at 110°C.
Table 1. C. albicans and C. dubliniensis strains used in this study.
| Candida strain | Parent | Genotypea | Reference |
| SC5314 | C. albicans wild-type strain | [42] | |
| Wü284 | C. dubliniensis wild-type strain | [43] | |
| MMC3 | CAI4 | Canrg1Δ::hisG-CaURA3-hisG/Canrg1Δ::hisG | [20] |
| Ca3512G1A/B | SC5314 | orf19.3512/orf19.3512-GFP-TACT1 | This study |
| Cd30750G1A/B | Wü284 | CD36_30750/CD36_30750-GFP-TACT1 | This study |
| Ca4170G1A/B | SC5314 | orf19.4170/orf19.4170-GFP-TACT1 | This study |
| Cd40770G1A/B | Wü284 | CD36_40770/CD36_40770-GFP-TACT1 | This study |
| Cd30750M1A/B | Wü284 | CD36_30750Δ::SAT1-FLIP/CD36_307500 | This study |
| Cd30750M2A/B | Cd30750M1A/B | CD36_30750Δ::FRT/CD36_30750 | This study |
| Cd30750M3A/B | Cd30750M2A/B | CD36_30750Δ::FRT/CD36_30750Δ::SAT1-FLIP | This study |
| Cd30750M4A/B | Cd30750M3A/B | CD36_30750Δ::FRT/CD36_30750Δ::FRT | This study |
| Cd40770M1A/B | Wü284 | CD36_40770Δ::SAT1-FLIP/CD36_40770 | This study |
| Cd40770M2A/B | Cd40770M1A/B | CD36_40770Δ::FRT/CD36_40770 | This study |
| Cd40770M3A/B | Cd40770M2A/B | CD36_40770Δ::FRT/CD36_40770Δ::SAT1-FLIP | This study |
| Cd40770M4A/B | Cd40770M3A/B | CD36_40770Δ::FRT/CD36_40770Δ::FRT | This study |
SAT1-FLIP denotes the SAT1 flipper cassette.
Plasmid constructions
A DNA construct for the CD36_30750-GFP reporter fusion was generated as follows: upstream sequences plus the coding region of gene CD36_30750 were amplified by PCR with primers Cd30750-1 and Cd30750-5, using genomic DNA from C. dubliniensis Wü284 as a template (all primers are listed in Table S1). Primer Cd30750-5 contains a BamHI-site which replaces the CD36_30750 stop codon. The GFP gene lacking the start codon was cloned together with the C. albicans ACT1T-terminator by use of primers GFP1 and CaACT1T-1 and the GFP-ACT1T containing plasmid pSSU1G2 (unpublished data) as a template, resulting in pJetGFPACT1T1. The ApaI-BamHI CD36_30750 fragment was cloned together with a BamHI-NcoI GFP fragment from pJetGFPACT1T1 in the ApaI/NcoI digested vector pSSU1G2, resulting in pCd30750G1. Finally, the downstream SSU1 fragment in pCd30750G1 was replaced by a PstI-SacI CD36_30750 downstream fragment obtained by PCR with primers Cd30750-6 and Cd30750-4. The resulting plasmid pCd30750G2 contains a DNA cassette which encodes CD36_30750 which is fused at its last amino acid via Gly-Ser to the GFP (Figure S1). In the same way, GFP reporter fusions were constructed for the C. dubliniensis gene CD36_40770 by use of primer pairs Cd40770-1/Cd40770-5 and Cd40770-6/Cd40770-4 and C. albicans genes orf19.3512 (primer pairs 3512-1/3512-2 and 3512-3/3512-4) and orf19.4170 (primer pairs 4170-1/4170-2 and 4170-3/4170-4), resulting in plasmids pCd40770G2, p3512G2 and p4170G2. A DNA cassette for the deletion of CD36_30750 was constructed as follows: An ApaI-XhoI fragment with CD36_30750 upstream sequences was cloned after PCR with the primers Cd30750-1 and Cd30750-2, using genomic DNA from C. dubliniensis Wü284 as a template. A SacII-SacI fragment with CD36_30750 downstream sequences was obtained by PCR with the primers Cd30750-3 and Cd30750-4. The CD36_30750 upstream and downstream fragments were successively cloned in order to flank the SAT1-flipper cassette as described before [23]. In the same way, a DNA cassette for the deletion of C. dubliniensis gene CD36_40770 was constructed, using primer pairs Cd40770-1/Cd40770-2 and Cd40770-3/Cd40770-4, respectively.
C. albicans and C. dubliniensis transformant construction
C. albicans and C. dubliniensis were transformed by an electroporation protocol [24] with gel-purified, linear DNA fragments from the generated plasmids: the ApaI-SacI fragments from pCd30750G2, pCd40770G2, p3512G2 and p4170G2 for integration of the GFP reporter fusions into one of the native alleles of the corresponding genes in the wild-type strains C. dubliniensis Wü284 and C. albicans SC5314, respectively (Figure S1). The ApaI-SacI fragments from pCd30750M2 and pCd40770M2 were used to delete genes CD36_30750 and CD36_40770, respectively, in C. dubliniensis Wü284 (Figure S2). Transformants were selected on nourseothricin (Werner Bioagents, Jena, Germany), and recycling of the selection marker by the SAT1-flipping method was carried out as described before [23]. The correct insertion of the constructs was confirmed by Southern analysis.
Southern analysis
Genomic DNA from C. albicans and C. dubliniensis was isolated as described previously [25]. A 10 µg sample of DNA was digested with appropriate restriction enzymes and separated on a 1% (w/v) agarose gel. After ethidium bromide staining, DNA was transferred by vacuum blotting onto a nylon membrane and fixed by UV cross-linking. Southern hybridization with enhanced chemiluminescence-labelled probes was performed with the Amersham ECL Direct Nucleic Acid Labelling and Detection System (GE Healthcare, Braunschweig, Germany) according to the instructions of the manufacturer.
RNA isolation and sequencing
Total RNA from C. albicans and C. dubliniensis was isolated by the hot acidic phenol method [26], purified by use of the RNeasy Mini Kit (Qiagen, Hilden, Germany) and DNase-treated on-column with the RNase-free DNase Set (Qiagen) for removing contaminations with genomic DNA. The integrity of total RNAs was analyzed on an Agilent Bioanalyzer by monitoring the RNA integrity number (RIN). Two µg of total RNA were used to extract polyadenylated RNA and to construct strand specific RNAseq libraries according to the Illumina protocol ‘Directional mRNA-Seq Sample Preparation’ (Part # 15018460 Rev. A) (Illumina, San Diego, USA). Briefly, polyadenylated RNA was enriched by two rounds of polyA selection with oligo-dT magnetic beads. The RNA was then chemically fragmented, treated with Antarctic phosphatase (NEB) and subsequently with polynucleotide kinase (NEB). V1.5 sRNA 3′Adapter was ligated to the RNA with T4 RNA ligase2 truncated (NEB), and SRA 5′ Adapter was ligated to the RNA with T4 RNA Ligase. After ligation of the SRA RT primer, the RNA was reverse transcribed with SuperScript II Reverse Transcriptase (Invitrogen). Double stranded sequencing library DNA was then produced by 12 cycles PCR with primers GX1 and GX2. DNA was purified with AMPure XP beads (Beckman Coulter International S.A.). Library quality was validated with a Bioanalyzer 2100. Each sample was sequenced for 80 cycles on one lane of the Illumina Genome Analyzer IIx platform according to the manufacturers specifications. Yields per sample were 36 to 38 Mio pass filter reads (2.9 to 3.0 Gb).
RNA-seq data-processing
In order to map raw sequence reads to the respective genomes, we applied the Bowtie algorithm (version 0.12.7) [27]. For all three datasets >70% of reads mapped. We applied MAID filtering [28] in order to identify differentially expressed genes. Instead of a constant (log) fold-change cutoff, MAID filtering applies a MA-plot-based signal intensity-dependent fold-change criterion. The advantage is that genes which are lowly expressed in both datasets are not defined to be differentially expressed. Due to the absence of biological replicates, we relied on the experience that the variance is higher for genes expressed at low level. To find genes which are differentially expressed between C. dubliniensis and C. albicans, we used the definition of orthologous pairs given by the Candida Genome Database (http://www.candidagenome.org/).
Quantitative real-time (q)RT-PCR
One hundred ng of total RNA were used to perform qRT-PCR with a one step approach using the Brilliant III SYBR Green Ultra-Fast QRT PCR master mix kit (Agilent Technologies, La Jolla, USA). RT-PCR was performed on a Stratagene Mx3005P and the threshold cycle was determined by the instrument’s MxPro software version 4.10 (Agilent Technologies, La Jolla, USA). By the ΔΔCT method [29] expression was calculated and normalized to the expression of the CaACT1/CdACT1 gene. For all samples, three biological replicates were analyzed. Data were expressed as the mean ± SD. Differences were analyzed by the two-tailed unpaired Student’s t-test, a P value of <0.05 was considered statistically significant.
Analysis of DNA/protein sequence identity and similarity
Pairwise sequence alignments were conducted by use of the free available Needleman-Wunsch global alignment tool (Needle) at The European Molecular Biology Open Software Suite (emboss, http://emboss.open-bio.org/).
Fluorescence microscopy
Fluorescence microscopy was performed with a Zeiss Axio-Observer Z1 microscope equipped with a Zeiss HXP120C illuminator. Images were acquired by use of the corresponding filter settings for green fluorescent protein (GFP) and parallel/overlay transmission images. The cells were inspected with a x40 objective. Surface plot analysis to localize the fluorescence signal of CdCsp1/2-GFP was performed with ImageJ 1.46r.
Results and Discussion
C. dubliniensis wild type and the C. albicans nrg1Δ mutant form chlamydospores during growth in Staib liquid medium
As previously reported, the C. dubliniensis wild type and the C. albicans nrg1Δ mutant produce chlamydospores during growth on Staib agar, in contrast to the C. albicans wild type [12]. First, we proved whether a similar, expected growth phenotype of the three analyzed strains is also displayed in Staib liquid medium [30], since liquid culture conditions facilitated the planned transcriptome analysis. We found an incubation for 28 h at 25°C optimal for chlamydospore analysis in Staib liquid medium. At this time point, both the C. dubliniensis wild type as well as the C. albicans nrg1Δ mutant exclusively grew in form of pseudohyphae, almost all of which produced chlamydospores at their terminal ends (Figure 1). It has to be noted that the C. albicans nrg1Δ mutant constitutively forms pseudohyphae, but not chlamydospores. Instead, the formation of chlamydospores by C. albicans nrg1Δ pseudohyphae is specifically induced in Staib medium, hence allowing the identification of putative chlamydospore related genes by comparative gene expression analysis.
Figure 1. Differential chlamydospore development by the analyzed Candida strains in Staib liquid medium.

C. dubliniensis wild type Wü284 and the C. albicans nrg1Δ mutant MMC3 form chlamydospores, in contrast to the C. albicans wild type SC5314. The fungal strains were grown for 28 h in Staib medium at 25°C and inspected by microscopy (scale bar: 10 µm).
Comparative RNA sequencing identifies putative chlamydospore specific genes
Total RNA from the three tested Candida strains, i.e. C. albicans wild type SC5314, C. albicans MMC3 (nrg1Δ) and C. dubliniensis wild type Wü284, was isolated after 28 h of growth in Staib liquid medium and used for global RNA sequence analysis (Materials and Methods). The complete results of pairwise relative gene expression comparisons of the three strains is depicted in Table S2. In order to identify differentially regulated genes, we applied the stringent MAID filtering approach (MA-plot-based signal intensity-dependent fold-change) [28], permitting the removal of genes which are expressed at a low level in both compared conditions/strains. A set of putative chlamydospore formation related genes was obtained by comparing datasets from the C. albicans nrg1Δ mutant strain and the C. dubliniensis wild type with the C. albicans wild type. By this approach, we identified 25 strongly up- and 8 downregulated genes, respectively (Figure 2, Table 2), most of which were uncharacterized. Since chlamydospore related gene expression has not been monitored before on a global scale, the identification of many unknown function/uncharacterized genes points to the assumed specificity of the chlamydospore developmental program. Interestingly, many of the highly upregulated genes encode putative cell wall/plasma membrane associated proteins, including PGA13 and PGA55 [31], [32]. This finding suggests that chlamydospore cell walls contain a characteristic composition of proteins. Based on our findings specified further on in this work we designated the two highly upregulated genes orf19.3512/CD36_30750 and orf19.4170/CD36_40770 as C. albicans and C. dubliniensis ‘Chlamydospore Specific Protein 1 and 2′, i.e. ca/cdCSP1 and ca/cdCSP2, respectively (Table 2).
Figure 2. Identification of chlamydospore specific genes in Candida.
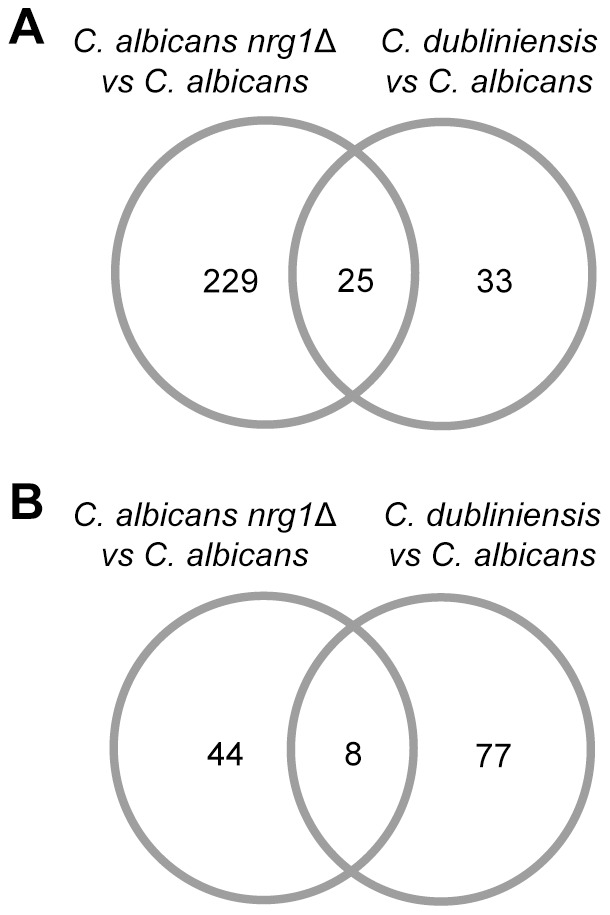
Venn diagram of genes which were ≥ two fold up- (A) and downregulated (B) in both the C. albicans nrg1Δ mutant and the C. dubliniensis wild type during growth in Staib medium.
Table 2. Strongly differentially expressed genes in C. albicans nrg1Δ and C. dubliniensis vs. C. albicans in Staib medium.
| orf19 ID | Namea | Dub ID | Relative Expression (log2fold)b | |
| C. albicans nrg1 Δ vs. C. albicans | C. dubliniensis vs. C. albicans | |||
| orf19.3512 | CSP1 | CD36_30750 | 9.60 | 17.20 |
| orf19.654 | CD36_30570 | 9.50 | 17.90 | |
| orf19.4170 | CSP2 | CD36_40770 | 8.47 | 12.31 |
| orf19.4463 | CD36_03620 | 8.38 | 13.47 | |
| orf19.2317 | CD36_10300 | 7.13 | 9.93 | |
| orf19.6315 | CD36_30140 | 6.49 | 8.29 | |
| orf19.6420 | PGA13 | CD36_34100 | 6.47 | 6.93 |
| orf19.2638 | CD36_53200 | 6.44 | 9.25 | |
| orf19.4011 | CD36_54780 | 6.20 | 10.82 | |
| orf19.2583 | CD36_26850 | 5.61 | 5.83 | |
| orf19.6741 | CD36_87380 | 5.35 | 8.82 | |
| orf19.207 | PGA55 | CD36_23160 | 4.84 | 6.93 |
| orf19.3330.3 | POX18 | CD36_01320 | 4.34 | 7.04 |
| orf19.3885 | CD36_31810 | 3.93 | 7.48 | |
| orf19.4688 | DAG7 | CD36_41020 | 3.70 | 4.58 |
| orf19.2457 | CD36_05580 | 3,44 | 4.83 | |
| orf19.4783 | CD36_08720 | 2.96 | 5.74 | |
| orf19.6569 | CD36_71400 | 2.84 | 5.21 | |
| orf19.4264 | CD36_52290 | 2.84 | 5.03 | |
| orf19.4459 | CD36_03600 | 2.74 | 5.16 | |
| orf19.6920 | CD36_71220 | 2.64 | 4.82 | |
| orf19.2506 | CD36_80940 | 2.48 | 4.55 | |
| orf19.4953 | CD36_12310 | 2.36 | 4.94 | |
| orf19.6788 | CD36_87050 | 2.07 | 4.49 | |
| orf19.5645 | MET15 | CD36_40270 | 1.92 | 5.28 |
| orf19.508 | QDR1 | CD36_29520 | –6.43 | –6.32 |
| orf19.4773 | AOX2 | CD36_08630 | –6.03 | –6.19 |
| orf19.7150 | NRG1 | CD36_73890 | –4.37 | –4.98 |
| orf19.7554 | CD36_34960 | –3.21 | –5.31 | |
| orf19.1189 | CD36_60240 | –2.49 | –5.49 | |
| orf19.2251 | AAH1 | CD36_21260 | –1.78 | –4.55 |
| orf19.1193 | GNP1 | CD36_60280 | –1.75 | –4.99 |
| orf19.4555 | ALS4 | CD36_64610 | –1.63 | –10.47 |
Denominations CSP1 and CSP2 were proposed in the present study.
Downregulation of genes is indicated by a minus (–), followed by the logarithmised log fold change value.
The hyphal repressor Nrg1 was previously found to govern the differential chlamydospore phenotype of C. albicans and C. dubliniensis in Staib medium [12]. Therefore, it appears reasonable that some of the detected genes were previously related to filamentation and/or annotated as putative targets of Nrg1. Examples include PGA13, PGA55 and IHD1, which encode putative glycosylphosphatidylinositol (GPI)-anchored proteins [31], the latter being named as ‘induced during hyphae development’ [20], [33]. Other candidate target genes are the strongly upregulated unknown function gene orf19.6741 as well as UME6, which is described as a filament specific regulator in C. albicans (Table 2; Table S2) [34], [35]. Notably, it has been reported that a differential expression of the UME6 gene contributes to the varied ability of C. albicans and C. dubliniensis to form filaments [36]. Confirming prior results on NRG1 expression during chlamydospore development [12], we found the gene to be strongly downregulated in C. dubliniensis versus the C. albicans wild-type strain during growth in Staib medium (Table 2, Table S2).
Analysis of RNAseq data did not detect a particular differential induction of genes that have previously been identified by mutant screening to be important for efficient chlamydospore formation in C. albicans, such as ISW2, MDS3, RIM13, RIM101, SCH9 and SUV3 [14]. Supporting former findings on the role of the stress-activated protein kinase Hog1 for chlamydospore production in C. albicans, HOG1 transcription was detected in our experiments to be elevated in C. dubliniensis and the C. albicans nrg1Δ mutant, suggesting that the cells grown under these conditions may be experiencing osmotic or nutritional stress.
Other genes of interest which were strongly upregulated during chlamydospore development in our assay were those which by differential expression patterns were previously detected during switching or mating in C. albicans. For example, expression of orf19.2317 and DAG7 was shown to be inducible by alpha-pheromone [37], and orf19.2506 was reported to be opaque cell specific [38]. In addition, one of the most strongly downregulated genes associated with chlamydospore development was QDR1/CD36_29520. This gene, which encodes a putative transporter, was previously detected in C. albicans to also experience strong differential expression in white versus opaque cells [39]. The discovery of mating in C. albicans has revealed that the life cycle of this microorganism is more complex than originally assumed. In this context, the interest in chlamydospore development should also be restimulated.
Sequence specificity of the four genes which were most strongly upregulated during chlamydospore formation
Intriguingly, the four most strongly upregulated C. albicans genes and C. dubliniensis homologues, repectively, encode putative cell wall proteins which display considerable similarity (Table 3) (http://old.genedb.org/genedb/cdubliniensis/; http://www.candidagenome.org/). Given their chlamydospore related expression and the fact that only these two Candida species form chlamydospores, we asked whether these genes are specific for C. dubliniensis and C. albicans in the genus Candida. The Candida Gene Order Browser (GCOB) is an online tool for visualising the syntenic context of genes from multiple Candida genomes (http://cgob.ucd.ie; [40]). Among 14 species included in GCOB, caCSP1 (orf19.3512) and orf19.654 related genes were only found in C. albicans and C. dubliniensis. In case of C. albicans caCSP2 (orf19.4170), a homologue was in addition to C. dubliniensis also detected in C. tropicalis (CTRG_01767). A comparison of C. albicans caCSP2 and C. tropicalis CTRG_01767 on the level of the deduced proteins revealed identity/similarity of 38.9/47.7%, whereas the proteins encoded by caCSP2 and cdCSP2 showed identity/similarity of 83.8/86.7% (Table 3). Whether the identified C. tropicalis gene encodes a functional homologue of CSP2 is questionable, especially since C. tropicalis is not known to produce chlamydospores. In the case of orf19.4463, which was absent from the other inspected Candida species, the homologous gene CD36_03620 in C. dubliniensis is annotated as a pseudogene which contains several stop codons. The deduced proteins encoded by orf19.4463 and CD36_03620 displayed identity/similarity of 30.6/38.3% (Table 3), but the genes showed 59.6% identity on the level of DNA. Overall, the application of GCOB uncovered that the identified, putative chlamydospore related C. albicans and C. dubliniensis genes are not widely distributed in the genus Candida. This observation further underlines a putative specific role of these factors during chlamydospore development in C. albicans and C. dubliniensis.
Table 3. Protein sequence identity/similarity among gene products encoded by the four genes most strongly upregulated during chlamydospore formation.
| C. albicans | C. dubliniensis | % identity/similarity | % identity (DNA)a |
| caCSP1 | cdCSP1 | 80.1/83.2 | 78.3 |
| orf19.654 | CD36_30570 | 80.2/89.2 | 84.1 |
| caCSP2 | cdCSP2 | 83.8/86.7 | 82.0 |
| orf19.4463 | CD36_03620 | 30.6/38.3 | 59.6 |
Per cent identity of the C. albicans and C. dubliniensis homologues on the level of DNA.
Per cent similarity (lower left) and identity (upper right) among the gene products is given.
Expression of ca/cdCSP1 and ca/cdCSP2 is specifically correlated with chlamydospore development
In search of chlamydospore specific markers two pairs of C. albicans and C. dubliniensis homologues were selected from our identified set of chlamydospore development related genes for detailed analysis, i.e. cdCSP1/caCSP1 and cdCSP2/caCSP2. According to the C. dubliniensis genome database, these genes putatively encode cell wall associated proteins (http://old.genedb.org/genedb/cdubliniensis/). A comparison of the deduced amino acid sequences revealed that homologues cdCSP1 and caCSP1 display 80.1/83.2% identity/similarity. Homologues cdCSP2 and caCSP2 are identical/similar to 83.8/86.7% (Table 3). The expression of these highly upregulated, putative chlamydospore related C. dubliniensis and C. albicans genes was confirmed by qRT-PCR analysis (Figure 3). The results show that cdCSP1 and cdCSP2 were upregulated >1000-fold in C. dubliniensis during growth in Staib medium in comparison to growth in YPD medium. In accordance, expression levels of the C. albicans homologues caCSP1 and caCSP2 were higher in the C. albicans nrg1Δ mutant than in the C. albicans wild type during growth in Staib versus YPD medium. These observations made the two selected C. albicans and C. dubliniensis genes promising candidates for chlamydospore specific markers.
Figure 3. Induced expression levels of genes CSP1 and CSP2 during chlamydospore formation.

C. dubliniensis Wü284, C. albicans SC5314 and the C. albicans nrg1Δ mutant were grown for 28 h in Staib medium and YPD medium, respectively, before total RNA was isolated. (A) qRT-PCR measurements detected a strong upregulation of cdCSP1 and cdCSP2 gene expression levels in C. dubliniensis during growth in Staib versus YPD medium. (B) Similarly, the C. albicans homologues caCSP1 and caCSP2 were found to be upregulated in the chlamydospore producing C. albicans nrg1Δ mutant stronger than in C. albicans wild-type yeast cells. The results are the means ±SD from three biological replicates, ‘*’ indicates that the detected differences were significant (P<0.05).
Localization of chlamydospore specific markers in C. dubliniensis and C. albicans
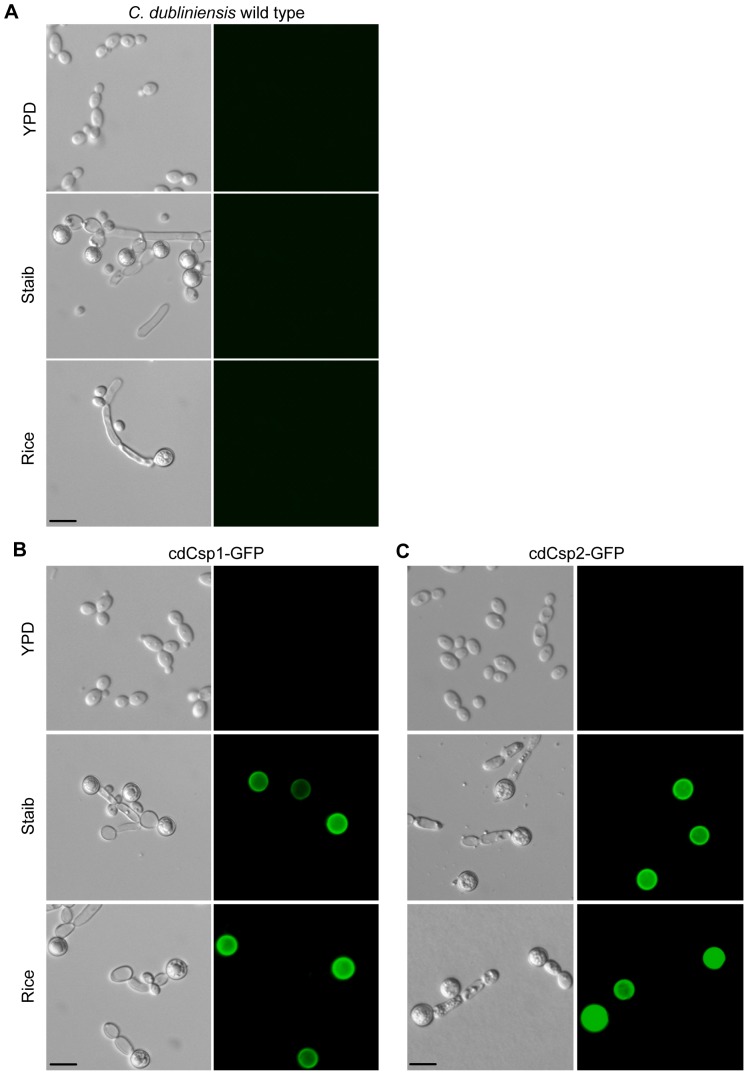
The putative chlamydospore related expression of the C. dubliniensis genes cdCSP1 and cdCSP2 was next analysed in the context of morphologic development. In order to define the expression on a cellular level and the morphotype specific localization of the encoded proteins, DNA cassettes for translational fusions with the green fluorescent protein (GFP) were constructed and integrated into one of the corresponding alleles of the C. dubliniensis wild type Wü284 (Figure S1 and data not shown). Cells of the wild type and resulting reporter strains Cd30750G1A/B (for cdCSP1) and Cd40770G1A/B (for cdCSP2) were grown in Staib medium for 28 h and inspected by fluorescence microscopy. Growth in YPD medium was used as a control. As demonstrated in Figure 4, the analysed proteins were not only specifically and highly abundant in C. dubliniensis cells grown in Staib medium, but were exclusively expressed and located in chlamydospores. In these entities, fluorescence was most intensive at the cell surface, thus supporting the putative function of cdCsp1 and cdCsp2 as cell wall proteins. Localization of cdCsp1/2 to chlamydospore cell walls was further supported by a surface plot analysis, shown as example for cdCsp1 (Figure S3). Expression of these proteins was not detected in yeast cells or pseudohyphae. Most notably, fluorescence was even not detected in suspensor cells, which carry chlamydospores at their terminal ends and presumably share a continous outer layer with them [41]. In order to find out whether the identified proteins cdCsp1 and cdCsp2 are also specifically expressed in C. dubliniensis chlamydospores induced by conditions different from Staib medium, the reporter strains were also monitored during growth on rice-extract agar. Like in Staib medium, the investigated gene products were found to be specifically localized to chlamydospores (Figure 4). C. dubliniensis wild-type cells grown in YPD as well as under the tested chlamydospore inducing conditions were used as negative controls in order to exclude unspecific autofluorescence.
Figure 4. Proteins encoded by cdCSP1 and cdCSP2 are specifically expressed and located in chlamydospores.
C. dubliniensis wild type Wü284 (A) and the C. dubliniensis GFP reporter strains Cd30750G1A/B (cdCsp1-GFP) (B) and Cd40770G1A/B (cdCsp2-GFP) (C) were grown in YPD and Staib liquid medium for 28 h at 25°C, and on rice-extract agar for 3 d at 25°C, respectively, and inspected by phase contrast and fluorescence microscopy. Fluorescence microscopy demonstrated that the genes of interest are specifically induced during growth in Staib medium and that the encoded proteins exclusively localize to chlamydospores. The two independently constructed A/B-GFP reporter strains behaved identically and only one of them is shown (scale bar: 10 µm).
Next, we investigated whether the identified chlamydospore related factors are also specifically expressed in C. albicans chlamydospores. For this purpose, GFP reporter fusions with the homologous C. albicans genes caCSP1 and caCSP2, respectively, were constructed and integrated into the genome of the wild type SC5314 at the corresponding loci (Figure S1 and data not shown). The wild type and the resulting reporter strains Ca3512G1A/B (for caCSP1) and Ca4170G1A/B (for caCSP2) were grown on rice-extract agar and inspected by fluorescence microscopy. Like in C. dubliniensis, the monitored C. albicans proteins were also specifically expressed and localized in chlamydospores (Figure 5). This finding supported the notion that the proteins encoded by genes ca/cdCSP1 and ca/cdCSP2 are the first identified, strictly chlamydospore related factors in Candida. Moreover, these proteins appear to be useful as markers for these morphological entities, and the constructed GFP reporter may be useful tools for future research.
Figure 5. Proteins encoded by C. albicans caCSP1 and caCSP2 are specifically expressed and located in chlamydospores.
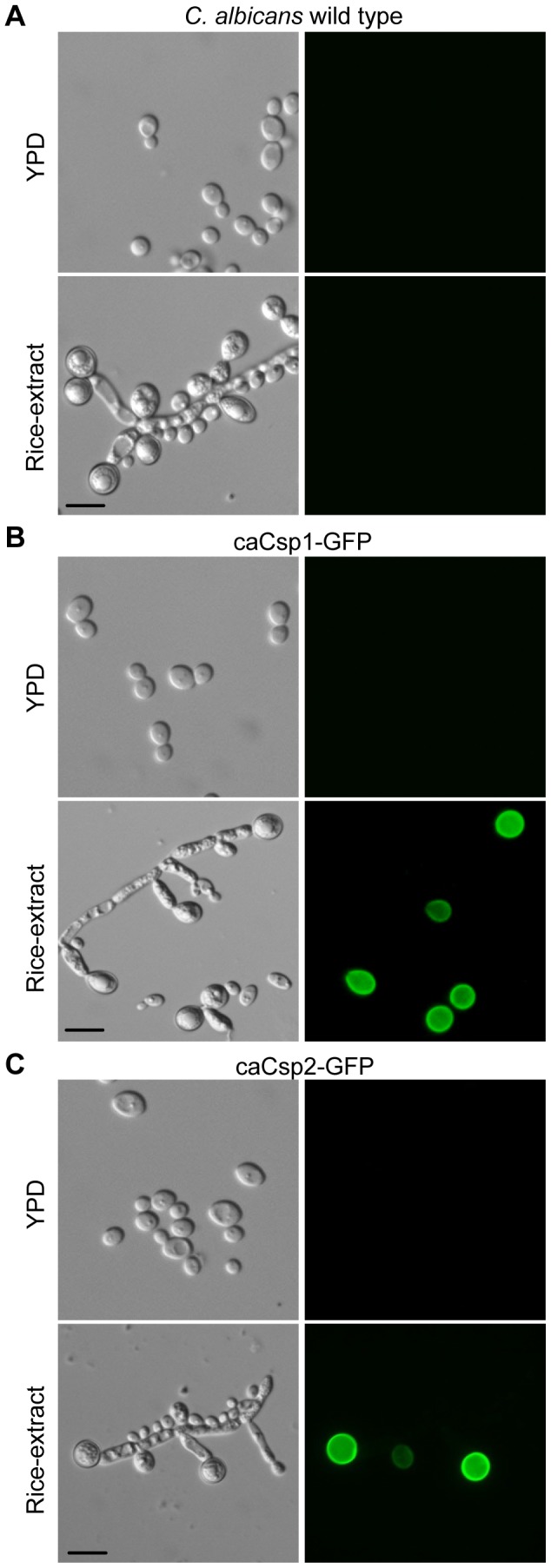
C. albicans wild type SC5314 (A) and the C. albicans GFP reporter strains Ca3512G1A/B (caCsp1-GFP) (B) and Ca4170G1A/B (caCsp2-GFP) (C) were grown in YPD medium for 28 h at 25°C and on rice-extract agar for 3 d at 25°C, respectively, before the fungal cells were inspected by phase contrast and fluorescence microscopy. Note that the monitored proteins exclusively localize to chlamydospores. The two independently constructed A/B-GFP reporter strains behaved identically and only one of them is shown (scale bar: 10 µm).
Analysis of C. dubliniensis mutants in cdCSP1 and cdCSP2
Since the function of chlamydospores is unknown, we were not able to investigate the role of the identified chlamydospore specific proteins. Nevertheless, in case of C. dubliniensis, we tested whether the two analysed genes cdCSP1 and cdCSP2 are required for efficient chlamydospore development. Deletion mutants Cd30750M4A/B (cdcsp1Δ) and Cd40770M4A/B (cdcsp2Δ) were constructed in the wild-type strain Wü284 by use of the SAT1-flipper technology (Figure S2). An altered ability of these mutants to produce chlamydospores in Staib medium was not observed (Figure S4). No difference to the wild type was also detected when these mutants were assayed for germ tube formation in cell culture medium or when growth sensitivity was tested in the presence of calcofluor white, congo red, menadion or hydrogen peroxide (data not shown). However, as indicated above (Table 3), the chlamydospore specific genes cdCSP1, CD36_30570, cdCSP2 and CD36_03620 display a high degree of similarity. Therefore, redundant functions of the encoded proteins during chlamydospore assembly can not be excluded, thereby masking a potential phenotype of the single knock-out mutants in either cdCSP1 or cdCSP2. Future construction and phenotypic analyses of multiple knock-out strains in all these related genes would allow further insights in their potential structural functions. Moreover, mutants in the identified chlamydospore specific genes may further be investigated once a functional role of chlamydospores is known.
Conclusion
To date, it remains obscure whether the ability to produce chlamydospores has any impact on the basic life cycle or the adaptation of C. albicans and C. dubliniensis to their human host. Interestingly, however, especially these two pathogenic Candida species, which are usually not found in the environment [2], [16], can form these mysterious morphological structures. In order to get novel insights into the biological role of chlamydospores, the molecular analysis of their development and structural assembly appears therefore to be of particular interest. In the present study, we addressed this issue by the identification of genes which encode chlamydospore specific factors. We took advantage of the observation that C. albicans and C. dubliniensis display a species specific difference in the regulation of chlamydospore formation in response to environmental growth conditions, i.e. by incubation in Staib medium [18]. The knowledge that species specific chlamydospore production under these conditions is controled by the differential expression of the transcriptional repressor Nrg1 further supported the identification of chlamydospore specific genes. In general, the identification of chlamydospore associated factors may be difficult, given the observation that chlamydospore production is usually correlated with pseudohyphae formation – although it is not clear whether these two phenotypes depend on each other or represent independent, co-regulated pathways. In this context, the discovered chlamydospore specific proteins, together with the provided GFP reporter constructs will further help elucidating the genetic control of chlamydospore related gene expression in Candida.
In general, knowledge of the identified ‘chlamydospore-specific’ markers may have particular practical value for chlamydospore identification as well as for further detailed studies on chlamydospore formation, maintenance and germination. Future studies on supposed ‘chlamydospore specific’ markers will elucidate whether such factors participate in additional processes as well, for example during host adaptation or mating. If chlamydospores played no role in the life cycle of C. albicans and C. dubliniensis one would have expected either or both species to have lost the capability to synthesise them. However, since these related pathogenic species are the only yeasts to have been observed to produce chlamydospores it remains to be seen how these fungi benefit from this phenotype. Pursuing research on chlamydospores may not only identify a role for these intriguing cells, but may also help clarify the complete life cycle of C. albicans and solve the riddle, why C. albicans has not lost the ability to form these striking cellular structures during evolution.
Supporting Information
Construction of GFP reporter strains. (A) The structure of the insert of plasmid pCd30750G2 containing the CD36_30750(cdCSP1)-GFP reporter fusion is shown on top. At the bottom, the genomic structure of the CD36_30750 locus in strain Wü284 is shown. The CD36_30750 coding region is represented by the white arrow, the upstream and downstream regions by solid lines. The GFP gene, which is fused to the last codon (before the stop codon) of CD36_30750, is symbolized by the hatched arrow. The caSAT1 selection marker is marked by a grey arrow. Probes for Southern analysis of transformants are indicated by black bars. Restriction sites used to obtain the linear fragment and for Southern analysis are: A, ApaI; SI, SalI; ScI, SacI. (B) Southern hybridization of SalI-digested genomic DNA of parental strain C. dubliniensis Wü284 (lane 1) and GFP reporter strains Cd30750G1A (lane 1) and Cd30750G1B (lane 2) with the CD36_30750-specific probe 1. The sizes of the hybridizing fragments (in kilobases) are given on the left side of the blot, their identities on the right. (C) Southern hybridization of XbaI-digested genomic DNA of parental strain C. albicans SC5314 (lane1) and the GFP reporter strains Ca3512G1A (lane 2) and Ca3512G1B (lane 3) with the orf19.3512-specific probe 1. A restriction site polymorphism allows the differentiation of the two homologous wild-type alleles. Reporter strains Ca3512G1A/B containing the orf19.3512(caCSP1)-GFP fusion were constructed in the same way as the C. dubliniensis GFP-reporter strains.
(TIF)
Construction of C. dubliniensis knock-out mutants in cdCSP1 ( CD36_30750 ) and cdCSP2 ( CD36_40770 ), respectively. (A) Structure of the deletion cassette from plasmid pCd30750M2 (top), which was used for deletion of both CD36_30750 alleles, and genomic structure of the CD36_30750 locus in strain Wü284 (bottom). The CD36_30750 coding region is represented by the white arrow, the upstream and downstream regions by the solid lines. The SAT1 flipper cassette is represented by a grey rectangle bordered by FRT sites (black arrows). The 34-bp FRT sites are not drawn to scale. The probes which were used for Southern analysis of the transformants are indicated by the black bars. Restriction sites used to cut out the linear fragment from the plasmid and for Southern analysis are given: A, ApaI; SI, SalI; ScI, SacI. (B) Southern hybridization of SalI-digested genomic DNA of parental strain Wü284 (lane 1), heterozygous CD36_30750Δ mutants Cd30750M2A (lane 2) and Cd30750M2B (lane 3), homozygous CD36_30750Δ mutants Cd30750M4A (lane 4) and Cd30750M4B (lane 5) with the CD36_30750-specific probe 1. The sizes of the hybridizing fragments (in kilobases) are given on the left side of the blot, and their identities on the right. (C) Southern hybridization of EcoRV-digested genomic DNA of parental strain Wü284 (lane 1), heterozygous CD36_40770Δ mutants Cd40770M2A (lane 2) and Cd40770M2B (lane 3), homozygous CD36_40770Δ mutants Cd40770M4A (lane 4) and Cd40770M4B (lane 5) with the CD36_40770-specific probe 1. The sizes of the hybridizing fragments are given on the left side of the blot, and their identities on the right.
(TIF)
Protein localization of cdCsp1-GFP in chlamydospores. Fluorescence microscopy pictures of C. dubliniensis chlamydospores of strain Cd30750G1A after growth for 3 d at 25°C on rice-extract agar were analysed by surface plot analysis to localize the fluorescence signal of cdCsp1-GFP. (A) The yellow rectangle marks the area for the surface plot analysis. The intensity of fluorescence signal of cdCsp1-GFP within the defined region was determined by plot analysis (not shown) and surface plot analysis (B). The highest brightness was detected within the outer layer of the chlamydospore, suggesting that cdCsp1 is particularly located within the chlamydospore cell wall.
(TIF)
Phenotypic analysis of C. dubliniensis knock-out mutants in genes cdCSP1 and cdCSP2 , respectively. C. dubliniensis wild type Wü284 and mutant strains Cd30750M4A/B (cdcsp1Δ) and Cd40770M4A/B (cdcsp2Δ) were grown for 28 h in Staib medium, before cellular morphology was inspected by microscopy. Like the wild type control, both mutants efficiently produced chlamydospores under the tested conditions. For each mutant, the two independently constructed transformants (A/B) behaved identically and only one of them is shown.
(TIF)
Primers used in this study.
(DOC)
Identification of genes that were differentially regulated during chlamydospore development. The table contains the relative expression values (log2fold) for all C. albicans and C. dubliniensis genes detected in the comparative RNAseq analyses, i.e. the C. albicans nrg1Δ mutant versus C. albicans wild type, C. dubliniensis wild type versus C. albicans wild type as well as C. dubliniensis wild type versus the C. albicans nrg1Δ mutant.
(XLS)
Acknowledgments
We thank Corinne Peter from the Genomic Technologies Facility of the University of Lausanne for her assistance with RNAseq library preparation.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (DFG grant STA 1147/1-1) and the Hans Knoell Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, et al. (2001) International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol 39: 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odds FC (1988) Candida and Candidosis: A Review and Bibliography. London: Baillière Tindall.
- 3. Bernhardt E (1946) Time saving in the preparation of corn meal agar and in the identification of yeast-like fungi. Mycologia 38: 228–229. [Google Scholar]
- 4.Kreger-van Rij NJW (1984) The yeasts, a taxonomic study. Amsterdam: Elsevier Science Publishers B. V.
- 5. Staib P, Morschhäuser J (2007) Chlamydospore formation in Candida albicans and Candida dubliniensis--an enigmatic developmental programme. Mycoses 50: 1–12. [DOI] [PubMed] [Google Scholar]
- 6. Taschdjian CL (1953) A simple prepared identification medium for Candida albicans . Mycologia 45: 474–475. [Google Scholar]
- 7. Citiulo F, Moran GP, Coleman DC, Sullivan DJ (2009) Purification and germination of Candida albicans and Candida dubliniensis chlamydospores cultured in liquid media. FEMS Yeast Res 9: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 8. Chabasse D, Bouchara JP, de Gentile L, Chennebault JM (1988) Candida albicans chlamydospores observed in vivo in a patient with AIDS. Ann Biol Clin 46: 817–818. [PubMed] [Google Scholar]
- 9. Cole GT, Seshan KR, Phaneuf M, Lynn KT (1991) Chlamydospore-like cells of Candida albicans in the gastrointestinal tract of infected, immunocompromised mice. Can J Microbiol 37: 637–646. [DOI] [PubMed] [Google Scholar]
- 10. Alonso-Monge R, Navarro-Garcia F, Roman E, Negredo AI, Eisman B, et al. (2003) The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans . Eukaryot Cell 2: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonneborn A, Bockmuhl DP, Ernst JF (1999) Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun 67: 5514–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staib P, Morschhäuser J (2005) Differential expression of the NRG1 repressor controls species-specific regulation of chlamydospore development in Candida albicans and Candida dubliniensis . Mol Microbiol 55: 637–652. [DOI] [PubMed] [Google Scholar]
- 13. Melo NR, Moran GP, Warrilow AG, Dudley E, Smith SN, et al. (2008) CYP56 (Dit2p) in Candida albicans: characterization and investigation of its role in growth and antifungal drug susceptibility. Antimicrob Agents Chemother 52: 3718–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nobile CJ, Bruno VM, Richard ML, Davis DA, Mitchell AP (2003) Genetic control of chlamydospore formation in Candida albicans . Microbiology 149: 3629–3637. [DOI] [PubMed] [Google Scholar]
- 15. Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, et al. (2009) Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans . Genome Res 19: 2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan DJ, Moran GP, Pinjon E, Al-Mosaid A, Stokes C, et al. (2004) Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans . FEMS Yeast Res 4: 369–376. [DOI] [PubMed] [Google Scholar]
- 17. Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC (1995) Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141: 1507–1521. [DOI] [PubMed] [Google Scholar]
- 18. Staib P, Morschhäuser J (1999) Chlamydospore formation on Staib agar as a species-specific characteristic of Candida dubliniensis . Mycoses 42: 521–524. [DOI] [PubMed] [Google Scholar]
- 19. Braun BR, Kadosh D, Johnson AD (2001) NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. EMBO J 20: 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, et al. (2001) NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans . EMBO J 20: 4742–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staib F, Arasteh K (2001) Chlamydospore formation on Staib agar. Observations made before Candida dubliniensis was described. Mycoses 44: 23–27. [DOI] [PubMed] [Google Scholar]
- 22. Staib F, Seibold M, Antweiler E, Frohlich B, Weber S, et al. (1987) The brown colour effect (BCE) of Cryptococcus neoformans in the diagnosis, control and epidemiology of C. neoformans infections in AIDS patients. Zentralbl Bakteriol Mikrobiol Hyg [A] 266: 167–177. [DOI] [PubMed] [Google Scholar]
- 23. Reuss O, Vik A, Kolter R, Morschhäuser J (2004) The SAT1 flipper, an optimized tool for gene disruption in Candida albicans . Gene 341: 119–127. [DOI] [PubMed] [Google Scholar]
- 24. Köhler GA, White TC, Agabian N (1997) Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol 179: 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, et al. (1994) Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J Clin Microbiol 32: 1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, et al.. (1989) Current protocols in molecular biology. New York: John Wiley & Sons, Inc.
- 27. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hecker M, Goertsches RH, Engelmann R, Thiesen HJ, Guthke R (2009) Integrative modeling of transcriptional regulation in response to antirheumatic therapy. BMC Bioinformatics 10: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Staib P, Morschhäuser J (2005) Liquid growth conditions for abundant chlamydospore formation in Candida dubliniensis . Mycoses 48: 50–54. [DOI] [PubMed] [Google Scholar]
- 31. De Groot PW, Hellingwerf KJ, Klis FM (2003) Genome-wide identification of fungal GPI proteins. Yeast 20: 781–796. [DOI] [PubMed] [Google Scholar]
- 32. Gelis S, de Groot PW, Castillo L, Moragues MD, Sentandreu R, et al. (2012) Pga13 in Candida albicans is localized in the cell wall and influences cell surface properties, morphogenesis and virulence. Fungal Genet Biol 49: 322–331. [DOI] [PubMed] [Google Scholar]
- 33. Nantel A, Dignard D, Bachewich C, Harcus D, Marcil A, et al. (2002) Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol Biol Cell 13: 3452–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, et al. (2008) UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell 19: 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeidler U, Lettner T, Lassnig C, Muller M, Lajko R, et al. (2009) UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans . FEMS Yeast Res 9: 126–142. [DOI] [PubMed] [Google Scholar]
- 36. O'Connor L, Caplice N, Coleman DC, Sullivan DJ, Moran GP (2010) Differential filamentation of Candida albicans and Candida dubliniensis Is governed by nutrient regulation of UME6 expression. Eukaryot Cell 9: 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dignard D, Whiteway M (2006) SST2, a regulator of G-protein signaling for the Candida albicans mating response pathway. Eukaryot Cell 5: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsong AE, Miller MG, Raisner RM, Johnson AD (2003) Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115: 389–399. [DOI] [PubMed] [Google Scholar]
- 39. Lan CY, Newport G, Murillo LA, Jones T, Scherer S, et al. (2002) Metabolic specialization associated with phenotypic switching in Candida albicans . Proc Natl Acad Sci U S A 99: 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fitzpatrick DA, O'Gaora P, Byrne KP, Butler G (2010) Analysis of gene evolution and metabolic pathways using the Candida Gene Order Browser. BMC Genomics 11: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jansons VK, Nickerson WJ (1970) Induction, morphogenesis, and germination of the chlamydospore of Candida albicans . J Bacteriol 104: 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gillum AM, Tsay EY, Kirsch DR (1984) Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198: 179–182. [DOI] [PubMed] [Google Scholar]
- 43. Morschhäuser J, Ruhnke M, Michel S, Hacker J (1999) Identification of CARE-2-negative Candida albicans isolates as Candida dubliniensis . Mycoses 42: 29–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Construction of GFP reporter strains. (A) The structure of the insert of plasmid pCd30750G2 containing the CD36_30750(cdCSP1)-GFP reporter fusion is shown on top. At the bottom, the genomic structure of the CD36_30750 locus in strain Wü284 is shown. The CD36_30750 coding region is represented by the white arrow, the upstream and downstream regions by solid lines. The GFP gene, which is fused to the last codon (before the stop codon) of CD36_30750, is symbolized by the hatched arrow. The caSAT1 selection marker is marked by a grey arrow. Probes for Southern analysis of transformants are indicated by black bars. Restriction sites used to obtain the linear fragment and for Southern analysis are: A, ApaI; SI, SalI; ScI, SacI. (B) Southern hybridization of SalI-digested genomic DNA of parental strain C. dubliniensis Wü284 (lane 1) and GFP reporter strains Cd30750G1A (lane 1) and Cd30750G1B (lane 2) with the CD36_30750-specific probe 1. The sizes of the hybridizing fragments (in kilobases) are given on the left side of the blot, their identities on the right. (C) Southern hybridization of XbaI-digested genomic DNA of parental strain C. albicans SC5314 (lane1) and the GFP reporter strains Ca3512G1A (lane 2) and Ca3512G1B (lane 3) with the orf19.3512-specific probe 1. A restriction site polymorphism allows the differentiation of the two homologous wild-type alleles. Reporter strains Ca3512G1A/B containing the orf19.3512(caCSP1)-GFP fusion were constructed in the same way as the C. dubliniensis GFP-reporter strains.
(TIF)
Construction of C. dubliniensis knock-out mutants in cdCSP1 ( CD36_30750 ) and cdCSP2 ( CD36_40770 ), respectively. (A) Structure of the deletion cassette from plasmid pCd30750M2 (top), which was used for deletion of both CD36_30750 alleles, and genomic structure of the CD36_30750 locus in strain Wü284 (bottom). The CD36_30750 coding region is represented by the white arrow, the upstream and downstream regions by the solid lines. The SAT1 flipper cassette is represented by a grey rectangle bordered by FRT sites (black arrows). The 34-bp FRT sites are not drawn to scale. The probes which were used for Southern analysis of the transformants are indicated by the black bars. Restriction sites used to cut out the linear fragment from the plasmid and for Southern analysis are given: A, ApaI; SI, SalI; ScI, SacI. (B) Southern hybridization of SalI-digested genomic DNA of parental strain Wü284 (lane 1), heterozygous CD36_30750Δ mutants Cd30750M2A (lane 2) and Cd30750M2B (lane 3), homozygous CD36_30750Δ mutants Cd30750M4A (lane 4) and Cd30750M4B (lane 5) with the CD36_30750-specific probe 1. The sizes of the hybridizing fragments (in kilobases) are given on the left side of the blot, and their identities on the right. (C) Southern hybridization of EcoRV-digested genomic DNA of parental strain Wü284 (lane 1), heterozygous CD36_40770Δ mutants Cd40770M2A (lane 2) and Cd40770M2B (lane 3), homozygous CD36_40770Δ mutants Cd40770M4A (lane 4) and Cd40770M4B (lane 5) with the CD36_40770-specific probe 1. The sizes of the hybridizing fragments are given on the left side of the blot, and their identities on the right.
(TIF)
Protein localization of cdCsp1-GFP in chlamydospores. Fluorescence microscopy pictures of C. dubliniensis chlamydospores of strain Cd30750G1A after growth for 3 d at 25°C on rice-extract agar were analysed by surface plot analysis to localize the fluorescence signal of cdCsp1-GFP. (A) The yellow rectangle marks the area for the surface plot analysis. The intensity of fluorescence signal of cdCsp1-GFP within the defined region was determined by plot analysis (not shown) and surface plot analysis (B). The highest brightness was detected within the outer layer of the chlamydospore, suggesting that cdCsp1 is particularly located within the chlamydospore cell wall.
(TIF)
Phenotypic analysis of C. dubliniensis knock-out mutants in genes cdCSP1 and cdCSP2 , respectively. C. dubliniensis wild type Wü284 and mutant strains Cd30750M4A/B (cdcsp1Δ) and Cd40770M4A/B (cdcsp2Δ) were grown for 28 h in Staib medium, before cellular morphology was inspected by microscopy. Like the wild type control, both mutants efficiently produced chlamydospores under the tested conditions. For each mutant, the two independently constructed transformants (A/B) behaved identically and only one of them is shown.
(TIF)
Primers used in this study.
(DOC)
Identification of genes that were differentially regulated during chlamydospore development. The table contains the relative expression values (log2fold) for all C. albicans and C. dubliniensis genes detected in the comparative RNAseq analyses, i.e. the C. albicans nrg1Δ mutant versus C. albicans wild type, C. dubliniensis wild type versus C. albicans wild type as well as C. dubliniensis wild type versus the C. albicans nrg1Δ mutant.
(XLS)