Abstract
Background
Chronic rhinosinusitis (CRS) is one of the most common chronic conditions in the United States. There is a significant subpopulation of CRS patients who remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy. Antimicrobial photodynamic therapy (aPDT) is a non-invasive non-antibiotic broad spectrum antimicrobial treatment. Our previous in vitro studies demonstrated that aPDT reduced CRS polymicrobial planktonic bacteria and fungi by >99.9% after a single treatment. However, prior to human treatment, the effectiveness of aPDT to eradicate polymicrobial biofilms in a maxillary sinus cavity must be demonstrated.
Objective
The objective of this study was to demonstrate the effectiveness of a non-invasive aPDT treatment of antibiotic resistant biofilms known to cause CRS in a novel anatomically correct maxillary sinus in vitro model using an enhanced photosensitizer solution.
Methods
Antibiotic resistant polymicrobial biofilms of Pseudomonas aeruginosa and MRSA were grown in an anatomically correct novel maxillary sinus model and treated with a methylene blue/EDTA photosensitizer and 670nm non-thermal activating light. Cultures of the biofilms were obtained before and after light treatment to determine efficacy of biofilm reduction.
Results
The in vitro maxillary sinus CRS biofilm study demonstrated that aPDT reduced the CRS polymicrobial biofilm by >99.99% after a single treatment.
Conclusions
aPDT can effectively treat CRS polymicrobial antibiotic resistant Pseudomonas aeruginosa and MRSA biofilms in a maxillary sinus cavity model.
Keywords: chronic sinusitis, biofilms, photodynamic therapy, maxillary sinus model
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the facial sinuses and nasal passages that is defined as lasting longer than 12 weeks or occurring more than 4 times per year with symptoms usually lasting more than 20 days.1 The National Institute for Health Statistics estimates that CRS is one of the most common chronic conditions in the United States affecting an estimated 37 million Americans.2 The potential etiologies of CRS include bacteria, viruses, allergies, fungi, superantigens, exotoxins and microbial biofilms. Importantly, CRS is also considered to be a significant factor that can exacerbate asthma, chronic lung diseases, eczema, otitis media and chronic fatigue.3-6
In clinical practice there is a significant subpopulation of patients with CRS who remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy.1,7-12 One theory for the reason for treatment failure is thought to be related to the destruction of the sinus mucociliary defense by the chronic sinus infection resulting in the development of secondary antibiotic resistant microbial colonization of the sinuses and biofilm formation.1,7-12 The biofilm is then thought to contribute to a local inflammatory response resulting in many of the CRS clinical symptoms.7-12 It is increasingly reported that methicillin resistant Staphylococcus aureus (MRSA) and multidrug resistant Pseudomonas aeruginosa are found in the clinical isolates of CRS patients and are a cause of antibiotic treatment failures.1,7-12 Antibiotic resistant strains of these bacteria also significantly contribute to poor clinical results with the presence of antibiotic resistant bacteria in clinical isolates as high as 30%.13 CRS with its chronic indolent course, resistance to antibiotics and acute exacerbations has a clinical course that parallels that of other persistent biofilm related inflammatory diseases.13 Due to the failure of standard therapies to control and cure CRS, other novel non-antibiotic therapies that are able to destroy biofilms and antibiotic resistant bacteria are needed.
Our previous studies have demonstrated the in vitro effectiveness of methylene blue (MB) based antimicrobial photodynamic therapy (aPDT) as a broad spectrum non-antibiotic polymicrobial treatment that is able to completely eradicate antibiotic resistant planktonic bacteria and fungi as well as significantly reduce CRS polymicrobial antibiotic resistant biofilm by >99.9% after a single treatment.14 As well the mechanism of action of MB aPDT and its human clinical safety has been previously discussed.14
The present studies evaluate the effectiveness of aPDT using an enhanced MB/EDTA photosensitizer on polymicrobial biofilms in a unique in vitro anatomically correct maxillary sinus model. EDTA was chosen as an additive to MB as it strongly reduces thiazine dyes under red light.15 During the electron transfer from EDTA to the MB, a large number of free radicals with significant quantum yields are generated, which are capable of destroying bacteria.16,17 This unique maxillary sinus model approximates the anatomy and enclosed conditions as is found in the human maxillary sinus thereby allowing for the accurate evaluation of the antimicrobial efficacy of the entire human aPDT treatment method.
Materials and Methods
Anatomically Accurate in vitro Human Maxillary Sinus Model
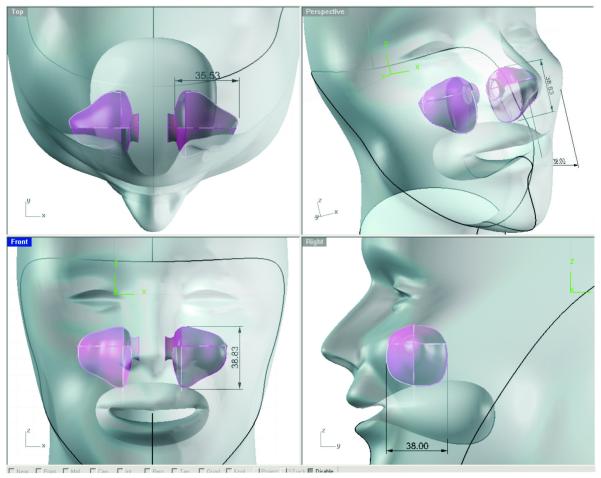
The anatomically accurate human maxillary sinus model was designed off of 3-dimensional human CT scans of the maxillary sinus and the model’s dimensions were derived from the average dimensions of 10 random male and female human maxillary sinuses (Figure 1). The sinus model was constructed of a solid food grade platinum cure silicone material with an anatomically correct sinus cavity inside. The silicone was 40 shore A, solid but not stiff so as to replicate a bio-material like soft tissue. The opening of the sinus (maxillary sinus osteum) in the model was anatomically correct so that the administration of the photosensitizer and the light device would be tested under anatomically correct conditions. The outer housing of the sinus model was designed to be placed in the rocker/tumbler device for biofilm formation.
Figure 1.
Anatomically accurate maxillary sinus model
Bacterial Culture and Inocula
Stock cultures of methicillin resistant Staphylococcus aureus (MRSA) ATCC # 33592 and antibiotic resistant Pseudomonas aeruginosa ATCC # 9027 were grown aerobically overnight on tryptic soy agar, harvested individually, and suspended in phosphate buffered saline (PBS) solution at a concentration of ~2×108 CFU/ml and ~3×106 CFU/ml, respectively. Cell densities were measured as a function of absorbance at 420 nm using a Genesys 10 spectrophotometer (Thermo Scientific, Pittsburgh, PA). P. aeruginosa inocula was then serial diluted to a final concentration of ~3×104 CFU/ml. The two suspended inocula were mixed 1:1 and diluted in tryptic soy broth to achieve the final biofilm inoculum.
Biofilm Growth
Biofilms were grown inside the in vitro maxillary sinus model using a validated gyrorotory shaker method.18,19 Mixed species biofilm inoculum, prepared as described above, was pipetted into sterile silicone models of adult human maxillary sinus cavities. The models were then allowed to shake for 24 hours using an Innova 200 platform gyrorotary shaker (New Brunswick Scientific Co, Edison NJ) at 35°C.18,19 Prior to the study, the consistent presence of mixed species biofilm growth covering the inside of the in vitro maxillary sinus model was verified using the crystal violet staining method20,21 and microbiological testing in 20 consecutive inoculated in vitro maxillary sinus models.
aPDT Biofilm Efficacy Studies
After the 24 hour biofilm growth period, the tryptic soy broth was emptied by inversion of the sinus model. The residual planktonic organisms were then removed by forcefully flushing the sinus model with 20 ml of sterile 0.9% saline delivered using a stainless steel cannula. Residual saline was emptied out by inversion of the sinus model. After rinsing, an aqueous solution of varying concentrations of ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, St. Louis, MO), ethanol (Pharmco-Aaper, Brookfield, CT), and methylene blue (American Regent, Shirley, NY) was pipetted into the sinus models. The 11 treatment conditions studied were as follows: untreated controls, including PBS, 0.03% MB, 0.03% MB + 5% EtOH, 1.25mM EDTA + 5% EtOH + 0.05% MB and light alone; saline rinse only; and aPDT treatment groups including 0.03% MB, 0.75mM EDTA + 5% EtOH + 0.01%MB, 0.75mM EDTA + 10% EtOH + 0.03%MB, 0.75mM EDTA + 5% EtOH + 0.05%MB or 1.25mM EDTA + 5% EtOH + 0.03%MB. The EDTA/ETOH/MB solution was allowed to incubate in the sinus model for 3.5 minutes before being removed by inversion of the sinus model. Using the Sinuwave® balloon catheter (Figure 2), sinus models were uniformly illuminated with 670 nm non-thermal laser light at a fluence rate of 150 mW/cm2 for a period of 8 minutes in the aPDT treatment group only. (Figure 3) In the control groups, the Sinuwave balloon catheter was placed but no illumination provided. Immediately following illumination, the Sinuwave® balloon catheter was removed and the sinus model was swabbed using a calcium alginate swab from right lateral posterior to left lateral anterior. Swabs were placed in recovery media (0.5% Tween-80® in PBS) and sonicated for 15 minutes using a model 250HT sonicator (VWR, West Chester, PA). Serial dilutions were performed in PBS and samples were plated on TSA media plates for 48 hours growth before assessing viable colony counts to determine aPDT efficacy. All experimental and control conditions were repeated nine times, and overall average viable colony counts were used to calculate bacterial kill rate.
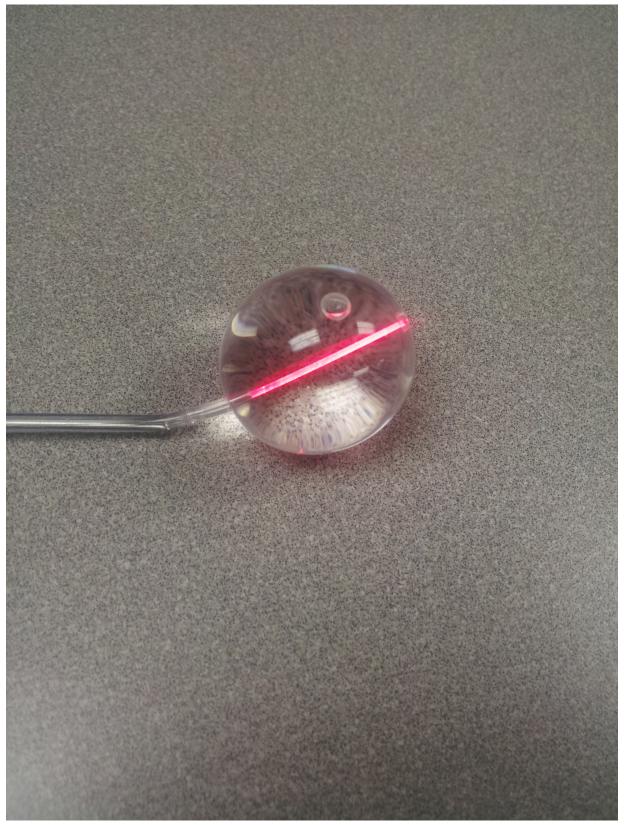
Figure 2.
Sinuwave® sinus illumination catheter
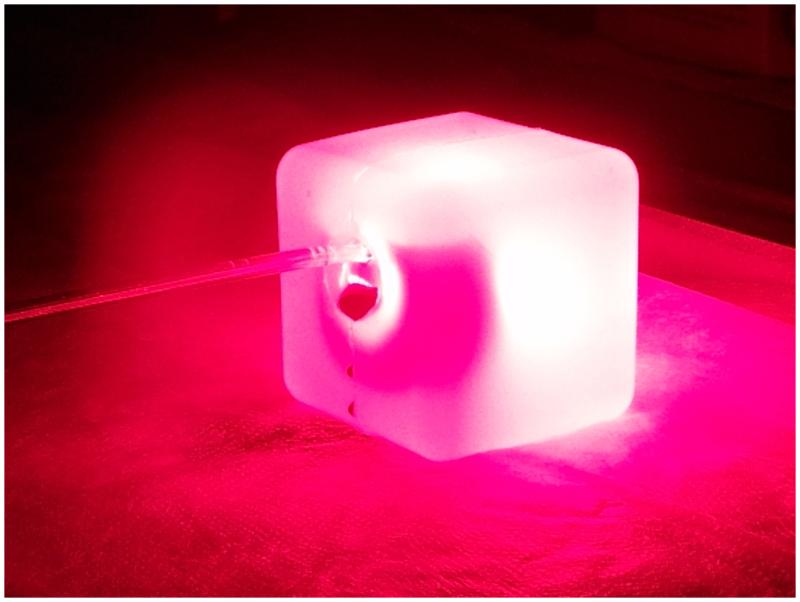
Figure 3.
Maxillary sinus model illuminated with the Sinuwave® sinus illumination catheter and 670nm light
Data Analysis
Counts for all experimental and control conditions were averaged and expressed as CFU/ml. Kill rate was calculated as surviving CFU/ml in experimental conditions vs. control (no light and no photosensitizer) and expressed as a log10 reduction from control for each individual organism. Results from the untreated and treatment groups were logarithmically transformed and compared using a two-tailed unequal variance Students T-test at a significance level of p<0.05.
Results
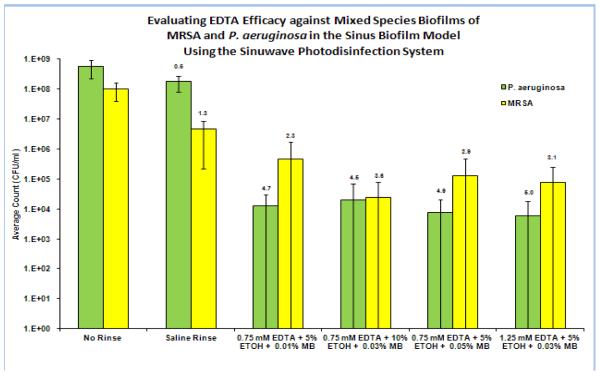
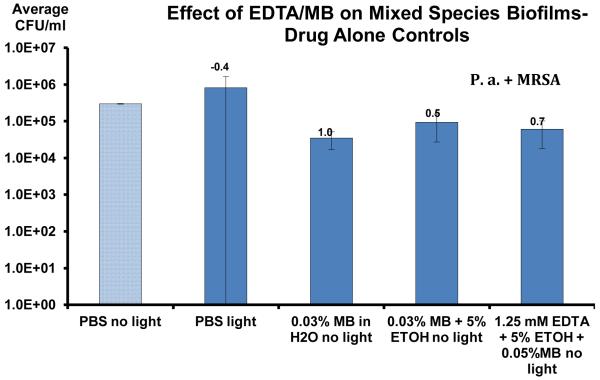
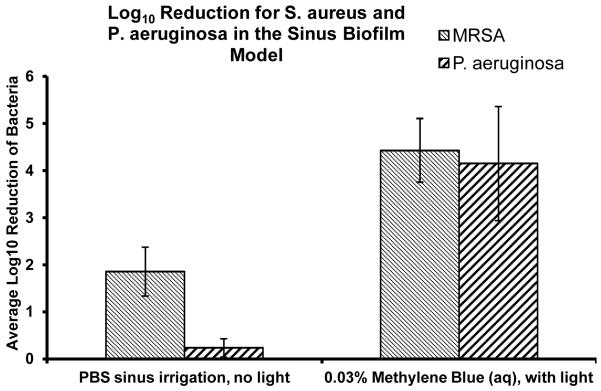
All of the aPDT treatment groups demonstrated statistically significant reductions (p<0.005) (Table 1) in both MRSA and antibiotic resistant Pseudomonas aeruginosa biofilms. Saline irrigation alone reduced the MRSA biofilm CFU by 1.3 log10 and Pseudomonas by 0.6 log10. The best treatment results in biofilm reduction were achieved with aPDT using the photosensitizer 1.25mM EDTA + 5% EtOH + 0.03%MB in the presence of 670nm light at a fluence rate of 150 mW/cm2 resulting in a 5 log10 (99.99%) reduction in Pseudomonas aeruginosa biofilm and a 3.1 log10 (99.9%) reduction in MRSA biofilm after a single treatment. The control groups of light alone and MB or MB/EDTA photosensitizer alone demonstrated no statistical decrease in CFU compared to the untreated control (Table 2). 0.03% MB aPDT resulted in a 4.1 log10 reduction of MRSA and a 3.9 log10 reduction of Pseudomonas aeruginosa (Table 3). The addition of low concentrations of EDTA (1.25mM) to MB aPDT resulted in a statistically significant (p<0.05) additional 1.1 log10 CFU reduction of Pseudomonas aeruginosa biofilm (5 log10 CFU reduction), as compared to MB aPDT alone (3.9 log10 CFU reduction), in the in vitro maxillary sinus biofilm model (Table 1).
Table 1.
MB/EDTA aPDT of Sinuwave Photodisinfection System against MRSA and Pseudomonas aeruginosa biofilms in an in vitro maxillary sinus model. The small numbers at the top of each bar indicate the average log10 reduction compared to the untreated control.
|
Table 2.
Photosensitizer only and light only controls against MRSA and Pseudomonas aeruginosa biofilms in an in vitro maxillary sinus model. The small numbers at the top of each bar indicate the average log10 reduction compared to the phosphate buffered saline control.
|
Table 3.
MB aPDT of Sinuwave Photodisinfection System against MRSA and Pseudomonas aeruginosa biofilms in an in vitro maxillary sinus model.
|
Discussion
CRS is one of the most common chronic conditions in the United States affecting an estimated 37 million Americans2-6 and a significant number of patients with CRS remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy.3-12 One of the hypothesized causes of recalcitrant CRS is the polymicrobial biofilm colonization of the sinuses resulting in a local inflammatory response, edema and ultimate infection. Due to the failure of standard therapies to control and cure CRS, other novel non-antibiotic therapies that are able to destroy biofilms and antibiotic resistant bacteria and fungi which may contribute to the chronic recurrent nature of CRS, are needed.
Our previous studies demonstrated that MB PDT is highly effective in the photoeradication of pathogenic Candida and various antibiotic resistant gram positive and gram negative bacteria that are commonly associated with chronic recurrent sinusitis.14 The present studies evaluate the effectiveness of aPDT using an enhanced MB photosensitizer formulation with EDTA on polymicrobial biofilms in a unique in vitro maxillary sinus model. This unique maxillary sinus model approximates the anatomy of the human maxillary sinus thereby allowing for the accurate evaluation of the antimicrobial efficacy of the entire human aPDT treatment method. However, a limitation of the in vitro maxillary sinus model is that it is not mucosalized and therefore does not reflect the physiology of the human maxillary sinus.
The present studies demonstrate that low concentrations of EDTA added to MB results in improved aPDT efficacy of multispecies biofilm bacterial kill, particularly antibiotic resistant Pseudomonas aeruginosa, and therefore indicate that MB/EDTA PDT may be an effective treatment method for the control or eradication of antibiotic resistant bacterial biofilms that are one of the major contributing causes of CRS. MB/EDTA PDT may be particularly beneficial to patients with CRS who have a predominant colonization with Pseudomonas aeruginosa, such as cystic fibrosis patients. Further studies to evaluate the safety and effectiveness of this non-invasive treatment of CRS are ongoing including ciliary beat frequency studies and a planned human clinical trial.
Acknowledgements
Supported by NIH Grants: R43 AI094706
Footnotes
Financial Disclosure:
MAB: Chief Scientific Officer PhotoBiologix, consultant Sinuwave, Inc.
Stockholder PhotoBiologix and Sinuwave Inc
NL: stockholder Sinuwave, Inc.
LP and AG: no financial disclosures
Conflict of Interest:
MAB, NL: Financial disclosure
LP, AG: none
References
- 1.Marple BF, Stankiewicz JA, Baroody FM, et al. Diagnosis and Management of Chronic Rhinosinusitis in Adults. Postgraduate Medicine. 2009;121(6):121–39. doi: 10.3810/pgm.2009.11.2081. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics, NCHS . Summary Health Statistics for US Adults, 2002. Centers for Disease Control, US Department of Health and Human Services; Hyattsville, MD: 2002. Chronic sinusitis. [Google Scholar]
- 3.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–9. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 4.Hamilos DL. Chronic sinusitis. J Allergy Clin Immunol. 2000;106:213–27. doi: 10.1067/mai.2000.109269. [DOI] [PubMed] [Google Scholar]
- 5.Somerville LL. Hidden factors in asthma. Allergy Asthma Proc. 2001;22:341–5. [PubMed] [Google Scholar]
- 6.Chester AC. Health impact of chronic sinusitis. Otolaryngol Head Neck Surg. 1999;114:842–9. doi: 10.1016/S0194-59989670122-5. [DOI] [PubMed] [Google Scholar]
- 7.Desrosiers MY, Kilty SJ. Treatment alternatives for chronic rhinosinusitis persisting after ESS: What to do when antibiotics, steroids and surgery fail. Rhinology. 2008;46:3–14. [PubMed] [Google Scholar]
- 8.Cohen M, Kofonow J, Nayak JV, et al. Biofilms in chronic rhinosinusitis: A review. Am J Rhinol Allergy. 2009 May-Jun;23(3):255–60. doi: 10.2500/ajra.2009.23.3319. [DOI] [PubMed] [Google Scholar]
- 9.Foreman A, Jervis-Bardy J, Wormald P. Do Biofilms Contribute to the Initiation and Recalcitrance of Chronic Rhinosinusitis? Laryngoscope. 2011;121:1085–1091. doi: 10.1002/lary.21438. [DOI] [PubMed] [Google Scholar]
- 10.Singhal D, Foreman A, Jervis-Bardy J, Wormald P. Staphylococcus aureus biofilms: Nemesis of Endoscopic Sinus Surgery. Laryngoscope. 2011;121:1578–1583. doi: 10.1002/lary.21805. [DOI] [PubMed] [Google Scholar]
- 11.Wood AJ, Fraser, Swif S, Amirapu S, Douglas RG. Are biofilms associated with an inflammatory response in chronic rhinosinusitis? Int Forum Allery Rhinol. 2011;1:335–339. doi: 10.1002/alr.20060. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Wang D, Sun X, Hu L, Yu H, Wang J. Relationship between bacterial biofilm and clinical features of patients with chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2012;269:155–163. doi: 10.1007/s00405-011-1683-y. [DOI] [PubMed] [Google Scholar]
- 13.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001 Jul 14;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 14.Biel MA, Sievert C, Usacheva M, Teichert M, Balcom J. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. Int Forum Allergy Rhinology. 2011 Oct;1(5):329–334. doi: 10.1002/alr.20089. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal MK, Manna PCH. Effect of DNA and other polyanions on the EDTA induced photoreduction of thionine. Makromol Chem. 1982;183:2811–21. [Google Scholar]
- 16.Bonneau R, Joussot-Dubien J, Faure J. Mechanism of photoreduction of thiazine dyes by EDTA studied by flash photolysis. Photochem Photobiol. 1973;17:313–19. [Google Scholar]
- 17.Pal MK, Mazumdar KK. Photoreduction of dyes catalysed by organic and bio-molecules. Histochemistry. 1974;40:267–74. doi: 10.1007/BF00501962. [DOI] [PubMed] [Google Scholar]
- 18.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Canadian Journal of Veterinary Research. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlin KL, Coll OP, Ceri H. Interspecies biofilms of Pseudomonas aeruginosa and Burkholderia capacia. Canadian Journal of Microbiology. 2001;47:949–954. [PubMed] [Google Scholar]
- 20.Merritt JH, Kadouri DE, O’Toole AG. Curr Protoc Microbiol. Vol. 22. John Wiley & Sons, Inc; Aug, 2011. Growing and Analyzing Static Biofilms; pp. 1B.1.1–1B.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]