Abstract
Although gender differences in substance use disorders have been identified, few studies have examined gender differences in prescription drug dependence. The aim of this study was to examine gender differences in clinical characteristics and treatment outcomes in a large clinical trial for prescription opioid dependence. Despite no pre-treatment differences in opioid dependence severity, women reported significantly greater functional impairment, greater psychiatric severity, and higher likelihood of using opioids to cope with negative affect and pain than men. Women were also more likely than men to have first obtained opioids via a legitimate prescription and to use opioids via the intended route of administration. Men reported significantly more alcohol problems than women. There were no significant gender differences in medication dose, treatment retention, or opioid outcomes. Thus, despite the presence of pre-treatment gender differences in this population, once the study treatment was initiated, women and men exhibited similar opioid use outcomes.
Keywords: prescription opioids, opioid dependence, gender, women, treatment outcome, sex differences
1. Introduction
The number of opioid prescriptions and the abuse rates of prescription opioids have increased greatly in recent years (Joranson, Ryan, Gilson, & Dahl, 2000; Paulozzi, Budnitz, & Yi, 2006; Turk, Swanson, & Gatchel, 2008). Consistent with these trends, mortality rates (Paulozzi et al., 2006), societal costs (Birnbaum et al., 2011), and treatment-seeking (Substance Abuse and Mental Health Services Administration, 2011) related to prescription opioid use disorders have increased substantially. Accordingly, individuals seeking treatment for prescription opioid dependence now outnumber those seeking treatment for heroin dependence (Substance Abuse and Mental Health Services Administration, 2011). Most of the existing research on the nature and treatment of opioid dependence has focused on heroin-dependent individuals, but the generalizability of these findings to prescription opioid dependent populations is unclear. Early research in this area suggests that there are important differences between heroin and prescription opioid dependence, such as higher rates of clinical pain among those using prescription opioids (Brands, Blake, Sproule, Gourlay, & Busto, 2004).
One area in which prescription opioid dependence may differ from other substance use disorders (SUDs) is in rates of prevalence by gender. Despite significantly higher rates of SUDs overall among men (Substance Abuse and Mental Health Services Administration, 2011), rates of prescription opioid dependence are similar for women and men (Back, Payne, Simpson, & Brady, 2010; Green, Grimes Serrano, Licari, Budman, & Butler, 2009; Parsells Kelly et al., 2008; Tetrault et al., 2008). Although it remains unclear why these proportions reflect a higher representation of women relative to other drugs of abuse , several factors may contribute to this deviation from typical SUD patterns, such as the greater likelihood of being prescribed an opioid among women (Parsells Kelly et al., 2008) and lessened perceived risk or greater social acceptability of a substance that can be obtained via a legitimate prescription.
A large literature exists on differences between men and women in terms of substance-related problems (Greenfield et al., 2007), but it is not known whether these extend to prescription opioid users. Among the most consistent findings across substances of abuse are the presence of higher levels of functional impairment among women with SUDs relative to men (Back, Payne, et al., 2011; Hernandez-Avila, Rounsaville, & Kranzler, 2004) and greater psychiatric severity (including severity of psychiatric symptoms, rates of psychiatric disorders, and functional impairment related to psychiatric symptoms) among women (Back, Payne et al., 2011; Sordo et al., 2012). Psychiatric symptoms may be particularly problematic for women, given evidence that women are more likely than men to report using substances to manage negative affect (Monti, Rohsenow, Colby, & Abrams, 1995; Rubonis et al., 1994). In addition, women appear to be less likely to enter SUD treatment than men, but exhibit similar retention and treatment outcome once treatment is initiated (Greenfield et al., 2007).
Relatively few studies have characterized gender differences among prescription opioid abusers. Studies of mixed opioid-dependent samples (including both primary heroin and prescription opioid dependent participants) provide further support for greater impairment among women in domains such as employment and family/social functioning and higher rates of psychiatric severity (Back, Payne et al., 2011; Cicero, Lynskey, Todorov, Inciardi, & Surratt, 2008; Grella, Karno, Warda, Niv, & Moore, 2009; Wu, Ling et al., 2010). Women may also be more likely than men to first initiate opioid use via prescription opioids (Maremmani et al., 2010), initiate opioid use at a later age, exhibit a shorter time between initiation of use and regular use (i.e., a telescoping course), and less likely to seek treatment for an opioid use disorder (Back, Payne, Simpson, & Brady, 2010).
Studies examining gender differences in prescription opioid abuse and dependence have yielded mixed results. Back and colleagues (Back, Lawson, Singleton, & Brady, 2011) conducted clinical interviews with a sample of non-treatment-seeking men and women with prescription opioid dependence. In this group, women reported a later age of first use of prescription opioids, but shorter time to regular use, consistent with the telescoping pattern of use that has been reported commonly (Hernandez-Avila et al., 2004; Randall et al., 1999), although not universally (Keyes, Martin, Blanco, & Hasin, 2010), among women with a variety of SUDs. Women were also significantly less likely to report intranasal use and more likely than men to report using opioids to cope with negative affect (Back, Lawson et al., 2011). In a secondary analysis of a large sample of over 29,000 patients seeking treatment for SUDs, women were more likely to report both use and abuse of prescription opioids in the previous month (Green et al., 2009). Women reporting prescription opioid abuse reported more co-occurring psychiatric problems, but were less likely to report a problem with pain (Green et al., 2009). Route of administration of opioids did not differ by gender.
Overall, previous studies have yielded somewhat inconsistent results with respect to gender differences in opioid use disorders, highlighting the need for additional research to clarify the nature of gender differences to inform assessment, prevention, and treatment efforts in this population. In particular, relatively little is known about gender differences in factors that are unique to prescription drug dependence (e.g., whether the source of the drug was from a legitimate prescription). Moreover, understanding differences in treatment outcome for women and men is important to inform the continued development, testing, and implementation of treatments. The notable difference in prevalence by gender for prescription opioid dependence relative to other SUDs highlights the importance of examining gender differences in this population.
The aim of the current study was to examine gender differences in a large clinical trial of treatment for prescription opioid dependence. We addressed two research questions evaluating whether there were gender differences in: (1) baseline clinical characteristics including substance use, pain, and affective variables; and, (2) treatment variables, including medication dosing, retention, and outcome. Data were drawn from the Prescription Opioid Addiction Treatment Study (POATS) (Weiss et al., 2011; Weiss et al., 2010), a large, multi-site clinical trial conducted in the National Drug Abuse Treatment Clinical Trials Network. Consistent with previous research, we hypothesized that women would report: (1) more problems with medical and social functioning relative to men, (2) greater psychiatric severity relative to men, and (3) more frequent use of opioids to cope with negative affect. In addition, exploratory analyses were conducted to examine gender differences in other relevant baseline clinical characteristics. Because few studies to date have examined treatment retention and outcome in prescription opioid dependence, exploratory analyses were conducted to examine whether retention, outcomes, and medication dosing differed by gender.
2. Methods
A full description of POATS study methods and main outcomes is available elsewhere (Weiss et al., 2011; Weiss et al., 2010). The methods relevant to the current analysis are presented below.
2.1. Participants
Participants age 18 years or older were recruited from a geographically diverse (urban, rural, and suburban) selection of 10 substance abuse treatment facilities in the United States, selected based on location in an area with a high prevalence of prescription opioid dependence as well as logistical factors (e.g., staffing resources). Eligible participants met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (American Psychiatric Association, 1994) criteria for a diagnosis of opioid dependence with physiological dependence on prescription opioids. Participants were excluded for the following reasons: substantial heroin use (defined as more than 4 days of use in the past month, a history of injection heroin use, or a history of having met opioid dependence diagnostic criteria as a result of heroin use alone); a medical or psychiatric condition contraindicated with the study medication or that would interfere with participation; dependence on alcohol, sedatives, or stimulants requiring immediate medical attention; participation in methadone or buprenorphine maintenance treatment in the past 30 days; a major pain event in the 6 months prior to enrollment or chronic pain requiring ongoing management; current participation in substance abuse treatment (not including self-help); or other logistical barriers to participation (e.g., plans to move from the local area, refusal to provide locator information). See Weiss et al. (2010) for a detailed description of study inclusion/exclusion criteria.
2.2. Procedures
Following provision of informed consent, participants completed a baseline assessment consisting of interviewer-administered, biological (urine drug screen), and self-report measures of substance use and comorbid problems.
The treatment portion of the study consisted of two phases implemented in an adaptive treatment study design (i.e., progression through procedures was determined based on outcomes at specified decision points). In Phase 1, all participants received brief treatment with buprenorphine-naloxone (BUP) and were randomly assigned (stratified by lifetime heroin use and chronic pain) to either standard medical management alone (SMM) or SMM plus individual opioid drug counseling (ODC). Phase 1 consisted of a 4-week buprenorphine (BUP) taper and eight weeks of follow-up. Upon conclusion of Phase 1, participants who met criteria for “successful outcome” (defined in detail below) completed the study. Those with unsuccessful outcomes were re-randomized to SMM or SMM+ODC for Phase 2, which consisted of 12 weeks of buprenorphine treatment, four weeks of taper, and eight weeks of follow-up.
Participants received the study medication at each SMM weekly visit and doses were adjusted by study physicians in accordance with best practice guidelines, with doses ranging from 8-32 mg daily during active treatment (i.e., not including induction or taper). Key elements of SMM treatment included medication adherence and response monitoring, assessment of opioid craving and self-help participation, pain assessment, identification of other medical problems, and referrals for medical services if indicated. ODC sessions consisted of 13 educational/skills training modules related to opioid relapse prevention, addiction and recovery.
2.3. Measures
The Composite International Diagnostic Interview (CIDI) (World Health Organization, 1997) was administered to determine DSM-IV diagnoses of substance use disorders, major depressive disorder, and posttraumatic stress disorder. The CIDI has demonstrated strong inter-rater and retest reliability and good validity (Andrews & Peters, 1998). The Addiction Severity Index (ASI) ( McLellan et al., 1992), a widely used interviewer-administered measure of functional domains relevant to substance abuse, was used here as an index of functional impairment and substance abuse severity in baseline analyses. The ASI has well-established reliability and validity in substance abusing samples (McLellan, Cacciola, Alterman, Rikoon, & Carise, 2006). Composite scores reflect severity of problems in the following domains: alcohol use, drug use, legal, medical, employment, psychiatric, and family/social.
The Beck Depression Inventory II (BDI) (Beck, Steer, Ball, & Ranieri, 1996), a 21-item self-report measure of depressive symptoms over the 2 weeks prior to administration, was used as an index of depressive symptom severity. The BDI has demonstrated strong psychometric properties (Beck et al., 1996) and has been validated in samples of patients with substance use disorders (Lykke, Hesse, Austin, & Oestrich, 2008). The Brief Pain Inventory Short Form (BPI) (Keller et al., 2004) is a 9-item self-report measure of pain outcomes. A composite score reflecting severity of pain in the past 24 hours was used as an estimate of pain severity in this analysis. The BPI has been extensively validated in different clinical populations (Gjeilo, Stenseth, Wahba, Lydersen, & Klepstad, 2007; Tan, Jensen, Thornby, & Shanti, 2004). The Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a 6 item self-report measure that provides an index of the severity of nicotine dependence. Self-report on the FTND is associated with nicotine intake (Heatherton et al., 1991; Courvoisier & Etter, 2010).
Measures of opioid withdrawal (Clinical Opiate Withdrawal Scale) (Tompkins et al., 2009) and craving were also included to examine gender differences in withdrawal and craving at baseline. Craving was assessed using a 3-item visual analogue scale originally designed to measure cocaine craving (Weiss, Griffin, & Hufford, 1995) adapted for use with opioids, in which participants were asked to rate current craving, cue-induced craving, and ability to resist craving. Finally the Pain and Opioid Analgesic Use History, a self-report measure developed for the purpose of this study (Weiss et al., 2011), assesses a range of variables related to pain and opioid use, such as first source of opioids (e.g., legitimate prescription, drug dealer) and motives for use (e.g., to get high, to cope with pain).
Treatment outcomes were based on the primary outcomes from the POATS study (Weiss et al., 2011). Outcome was defined dichotomously, as a “successful” or “unsuccessful” outcome. Specifically, in Phase 1 a successful outcome was defined as ≤ 4 days of opioid use in the previous month, < 2 consecutive urine drug screens positive for opioids (and no more than 1 missing urine), and no additional substance abuse treatment (not including self-help). In Phase 2, successful outcome was defined as no opioid use in the final treatment week and abstinence on ≥ 2 of the previous 3 weeks (as assessed by urine drug screen and self-report).
2.4. Data Analysis
First, hypothesized baseline gender differences were tested based on previous literature and theoretical considerations. Second, given that little research has been conducted in this area to date, exploratory analyses examining additional baseline variables of interest (source of opioids, route of opioid administration, history of heroin use, history of injection opioid use, craving, pain coping motives, nicotine dependence severity, pain severity, and withdrawal) were also conducted. For these analyses, alpha was adjusted to control for multiple comparisons using Sidak correction (Sidak, 1967). For analyses of motives for use, linear regression models were used to control for the effect of severity of negative affect and pain as potential confounders of this association. Third, differences between men and women in treatment variables including buprenorphine dosing, treatment retention (assessed as percentage of sessions attended), and treatment outcome (defined as a successful opioid outcome, consistent with the main outcome trial) were examined.
3. Results
The sample consisted of 653 prescription opioid dependent patients, including 261 women (40% of the sample) and 392 men. The mean age of the sample was 33.2 years (SD = 10.2; range = 18-77) and the average completed education was 13 years (SD = 2.2, range = 7-22). The sample predominantly self-reported race as White (91%), followed by Black/African American (3%). Half of the sample had never been married, 28% were currently married, and 16% were divorced. The majority of participants were employed either full-time (63%) or parttime (18%), followed by unemployed (13%), and a small portion of students (4%), retired or on disability (2%), and in the military (<1%). The most commonly used opioids were long-acting oxycodone (total sample = 35.2%, men = 43.4%, women = 23.0%), hydrocodone (total sample = 32.3%, men = 26.3%, women = 41.4%), and oxycodone (total sample = 18.5%, men = 17.3%, women = 20.3%). These gender differences were significant for long-acting oxycodone (F[1,651] = 29.73, p <.001) and hydrocodone (F[1,651] = 16.7, p <.001).
As shown in Table 1, women (n = 261) and men (n = 392) did not differ significantly with respect to age, marital status, or race (all ps > .13). Men reported significantly more years of education; however, the magnitude of this difference was minimal (women = 12.7 years, men = 13.3 years).
Table 1.
Gender Differences in Baseline Clinical Characteristics
| Characteristics | Women (n = 261) |
Men (n = 392) |
Test Statistic (t, χ2) |
P |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age | 33.4 (9.8) | 33.1 (10.4) | 0.35 | .73 |
| Race (% White) | 90.8% | 91.8% | 0.20 | .67 |
| Employment (% full time) | 49.0% | 72.2% | 36.00 | <.001* |
| Marital status (% married) | 55.9% | 46.2% | 5.98 | .02* |
| Education (years) | 12.67 (2.16) | 13.25 (2.15) | −3.36 | .001* |
| Baseline Clinical Variables | ||||
| First obtained opioids via legitimate prescription |
62.8% | 47.4% | 14.91 | <.001* |
| Opioid use by non-accepted route of administration |
70.9% | 81.4% | 9.8 | .002* |
| History of heroin use | 18.0% | 24.0% | 3.3 | .07 |
| History of injection opioid use | 4.2% | 5.1% | 0.27 | .60 |
| Craving Scale | 8.2 (2.0) | 7.5 (2.2) | 4.06 | <.001* |
| Coping motives (pain) | 23.2 (9.7) | 20.0 (9.2) | 4.31 | <.001* |
| Coping motives (negative affect) |
32.6 (18.7) | 25.4 (17.0) | 5.11 | <.001* |
| FTND | 3.6 (2.8) | 3.0 (2.9) | 2.53 | .01* |
| BPI | 3.0 (2.6) | 2.7 (2.4) | 1.78 | .08 |
| COWS | 12.5 (4.4) | 12.1 (3.8) | 1.33 | .18 |
| BDI | 25.5 (12.4) | 20.0 (10.9) | 5.99 | <.001* |
| Lifetime MDD diagnosis | 47.5% | 20.2% | 54.43 | <.001* |
| Lifetime PTSD diagnosis | 11.9% | 5.4% | 10.10 | .001* |
Note. statistically significant (for baseline clinical variables this controls for multiple comparisons); Craving Scale = visual analogue craving scale, FTND = Fagerstrom Test for Nicotine Dependence, BPI = Brief Pain Inventory, pain severity scale, COWS = Clinical Opioid Withdrawal Scale, BDI = Beck Depression Inventory, MDD = major depressive disorder, PTSD = posttraumatic stress disorder. For all scales higher scores reflect greater severity. Dichotomous variables are reported as percent of male or female subgroups. Remaining variables are reported as means (standard deviations).
3.1. Baseline Clinical Characteristics
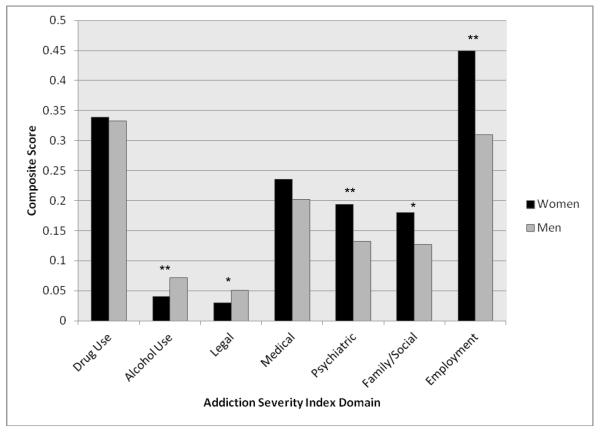
Results of analyses of clinical characteristics are presented in Table 1.There were no statistically significant differences between men and women in severity of opioid dependence as assessed by the drug use severity composite score of the ASI (see Figure 1), age of opioid dependence onset (women = 29.4 years, men = 29.1 years; t[651] = 0.41, p = .68), endorsement of opioid abstinence as a goal (37.5% of women, 36.7% of men; χ2 = 0.83, p = .87), or previous receipt of treatment for an opioid use disorder (31.0% of women, 30.9% of men; χ2 = 0.86, p = .87). Women reported significantly more opioid craving (t[651] = 4.06, p < .001), whereas men reported greater alcohol use severity (t[620] = −3.65, p < .001). Men were also more likely to have previously used heroin; however, this difference did not reach significance. Additionally, women were significantly more likely to have their first source of opioids be a legitimate prescriber (e.g., obtained by a physician rather than illegal means; p < .001).
Figure 1.
Addiction Severity Index Composite Scores at Baseline by Gender
Women reported significantly more family/social functional impairment relative to men (t[584] = 3.02, p < .01), but similar levels of medical functional impairment (t[644] = 1.29, p = .20). Consistent with findings suggesting greater impairment among women, there was also evidence for significantly worse employment functioning among women (t[642] = 6.17, p < .001). Results from the ASI composite scores by gender are presented in Figure 1.
Women exhibited greater psychiatric severity as indicated by both the psychiatric severity composite score of the ASI (t[636] = 3.99, p < .001) and depressive symptoms on the BDI (t[651] = 5.99, p < .001). Consistent with these findings, women were more likely to meet criteria for lifetime diagnoses of major depressive disorder (χ2 = 54.43, p < .001) and posttraumatic stress disorder (χ2 = 10.10, p < .01).
Women also reported more coping motives for use of opioids (e.g., use when feeling anxious or tense; t[651] = 5.11, p < .001). To determine whether this effect remained significant when controlling for psychiatric severity, a linear regression was conducted with coping motives as the dependent variable and gender (dummy-coded) and psychiatric severity (assessed by the ASI) as independent variables. Both gender (beta = −.15, t = −4.10, p < .001) and psychiatric severity (beta = .30, t = 7.94, p < .001) were uniquely associated with coping motives, predicting 13% of the variance in scores. Despite no significant differences in reported pain severity, women also reported a significantly more frequent use related to motives for coping with physical symptoms (t[651] = 4.31, p < .001).
3.2. Dosing, Treatment Retention, and Outcome
There were no significant gender differences in the maximum prescribed daily dose of buprenorphine in either Phase 1 (t[632] = −0.76, p = .45) or Phase 2 (t[354] = 0.60, p = .55). In Phase 1, there were no significant gender differences in session attendance (SMM: t[651] = 1.22, p = .22; ODC: t[327] = 0.81, p = .42), with women attending 86% of SMM sessions and 71% of ODC sessions and men attending 83% of SMM and 69% of ODC sessions. Similarly, there were no significant differences in session attendance in Phase 2 for either SMM (83% for women, 82% for men) or ODC (64% for women, 65% for men).
At the end of Phase 1, 5.4% of women and 7.4% of men exhibited a successful opioid use outcome (ns). At the end of treatment in Phase 2, 51.7% of women and 47.4% of men exhibited a successful outcome (ns) and 7.9% of women and 9.1% of men exhibited a successful outcome at 8 weeks post-treatment (ns). There were no significant gender differences in any of these response rates (ps ranged from .31-.70)
4. Discussion
In this large, treatment-seeking sample, several previous findings of gender differences common to a number of substances were replicated. Women reported greater psychiatric severity, higher rates of both major depressive disorder and posttraumatic stress disorder, and greater functional interference in employment and family/social domains. Women also were more likely to describe a pattern of use motivated by a desire to modulate negative affective and somatic (pain) states, even when controlling for the severity of emotional distress and pain.
This study extends previous work on gender differences in substance dependence by highlighting differences specific to prescription drug abusing populations. First, women were more likely than men to have first used opioids via a legitimate prescription. Second, women were more likely to use opioids orally or sublingually (i.e., via the intended route of administration) relative to men. Finally, women were more likely than men to use opioids consistent with their indicated use to manage pain. Thus, women appear to use prescription opioids in a pattern that more closely approximates medication adherence.
Consistent with previous studies (Smyth, Fagan, & Kernan, 2012; Ziedonis et al., 2009) there were no significant gender differences in treatment outcome indicators, including maximum daily dose of buprenorphine-naloxone, achievement of a successful opioid treatment outcome, or treatment retention. Of note, as reported earlier (Weiss et al., 2011), there were no statistically significant gender X treatment interaction effects (ps ranged from .12-.83). This suggests that once women in this sample entered treatment for prescription opioid dependence, they achieved outcomes similar to men, despite the presence of worse functioning in several domains prior to treatment initiation. However, as the rates of treatment success were low for both men and women, we cannot rule out that the lack of differences was attributable to a limited range of scores (i.e., a floor effect).
There are several clinical implications of these findings. First, these results underscore the importance of careful assessment of prescription opioid misuse in women, as traditional markers of opioid misuse and need for treatment (e.g., using the drug via a non-recommended route) were less common in women. The replication of this finding in samples not presenting for substance dependence treatment (e.g., individuals receiving treatment in primary care or chronic pain clinics) will be important to informing the identification of prescription opioid use disorders outside of SUD treatment settings. Likewise, the finding that woman are more likely to use opioids as intended (e.g., orally) has implications for the utilization of abuse-deterrent opioid formulations. For example, a formulation that is more difficult to modify (e.g., crush to use intranasally) may be less of an opioid abuse deterrent for women. Additionally, these results contribute further to the extant literature highlighting the importance of clinicians being particularly alert to psychiatric problems in women presenting for the treatment of opioid dependence. Women in the current sample had a higher rate of co-occurring psychiatric disorders, including major depressive disorder and posttraumatic stress disorder, and were more likely to use opioids to cope with negative affect. Thus, interventions that target co-occurring psychiatric disorders and maladaptive responses to negative affective states may be particularly indicated for women. Due to the greater family, employment, and social functional impairment experienced by women, barriers to treatment and financial and social concerns may be especially relevant when working with female patients. Interventions aimed to target these other domains of functioning may be needed for women in particular. Given that women were more likely to use opioids to cope with pain and to have initially received opioids from a legitimate prescriber, collaboration with primary care and pain management providers may be important to monitor misuse patterns and to facilitate adequate treatment of pain. Moreover, given the low treatment response rates overall, the development of novel treatment strategies for prescription opioid dependence for both men and women is of particular importance.
There are several limitations to the current study. The study sample reflected patients seeking detoxification who were enrolled in a clinical trial of treatment for prescription opioid dependence, and thus the findings are not necessarily generalizable to non-treatment-seeking patients. The sample of this study was predominantly White, consistent with epidemiologic data on the prevalence of prescription opioid dependence (Wu, Woody, Yang, & Blazer, 2010). The generalizability of these data to other racial groups will require additional study. Data available were limited to those assessments implemented in the parent study (e.g., not all Axis I disorders were assessed). Patients reporting a major pain event in the 6 months prior to enrollment or chronic pain requiring ongoing management were excluded; thus, limiting generalizability to those with more significant pain-related problems. Nonetheless, 42% of the sample endorsed current chronic pain and included participants with mild and moderate pain. Moreover, the presence of chronic pain was not associated with differences in treatment outcome (Weiss et al., 2011). This study examined gender differences cross-sectionally and thus the nature of interactions among these variables and their impact on long-term prescription opioid abuse and treatment outcome cannot be determined. Due to the lack of available data on the timing of opioid use onset and onset of opioid dependence, we were unable to examine whether women exhibited a telescoping course of illness relative to men in this study. In addition, more detailed information on the nature of opioid prescriptions (e.g., duration of opioid prescriptions) were not available, thus there is no clinical data to complement the self-report of receiving prescriptions from a legitimate source. Future studies using longitudinal and experimental designs are needed to better understand the association of these gender differences with the development and maintenance of prescription opioid dependence.
Although the prevalence and severity of prescription opioid dependence was similar among men and women in this sample, the functional impairment associated with this disorder appears to be more severe for women, which is consistent with findings across other substances, such as alcohol and cannabis (Hernandez-Avila et al., 2004). Given that women exhibit a pattern of behavior characterized by fewer typical markers of problematic prescription use (e.g., use via non-intended route), identifying prescription opioid dependence may be more challenging among women relative to men. In addition, women in the current study were more likely to use opioids to modulate negative affective and somatic states and demonstrated greater psychiatric comorbidity, highlighting the importance of assessing and treating these co-occurring symptoms. Nonetheless, women and men exhibited similar outcomes in treatment for prescription opioid dependence. Rates of treatment success were low regardless of gender. Therefore, effort to improve overall outcome are needed. Improving treatment outcome among women may require particular attention to thorough assessment for prescription opioid dependence and the treatment of co-occurring psychiatric and pain symptoms.
Acknowledgments
Support in part by U10DA015831-09 (Carroll/Weiss); K24 DA022288 ( Weiss); K24 DA019855-06 (Greenfield); K12 DA031050 (Devito; Mazure, PI). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest related to this manuscript.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Social Psychiatry and Psychiatric Epidemiology. 1998;33:80–88. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addictive Behaviors. 2011;36:829–834. doi: 10.1016/j.addbeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addictive Behaviors. 2010;35:1001–1007. doi: 10.1016/j.addbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Ling W. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. American Journal of Drug and Alcohol Abuse. 2011;37:313–323. doi: 10.3109/00952990.2011.596982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Manual for the Beck Depression Inventory II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Medicine. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;139:127–135. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Courvoisier DS, Etter JF. Comparing the predictive validity of five cigarette dependence questionnaires. Drug and Alcohol Dependence. 2010;107:128–133. doi: 10.1016/j.drugalcdep.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Gjeilo KH, Stenseth R, Wahba A, Lydersen S, Klepstad P. Validation of the brief pain inventory in patients six months after cardiac surgery. Journal of Pain and Symptom Management. 2007;34:648–656. doi: 10.1016/j.jpainsymman.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Green TC, Grimes Serrano JM, Licari A, Budman SH, Butler SF. Women who abuse prescription opioids: findings from the Addiction Severity Index-Multimedia Version Connect prescription opioid database. Drug and Alcohol Dependence. 2009;103:65–73. doi: 10.1016/j.drugalcdep.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Miele GM. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug and Alcohol Dependence. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Karno MP, Warda US, Niv N, Moore AA. Gender and comorbidity among individuals with opioid use disorders in the NESARC study. Addictive Behaviors. 2009;34:498–504. doi: 10.1016/j.addbeh.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Joranson DE, Ryan KM, Gilson AM, Dahl J. Trends in medical use and abuse of opioid analgesics. Journal of the American Medical Association. 2000;283:1710–1714. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. American Journal of Psychiatry. 2010;168:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke J, Hesse M, Austin SF, Oestrich I. Validity of the BPRS, the BDI and the BAI in dual diagnosis patients. Addictive Behaviors. 2008;33:292–300. doi: 10.1016/j.addbeh.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Stefania C, Pacini M, Maremmani AG, Carlini M, Golia F, Dell’Osso L. Differential substance abuse patterns distribute according to gender in heroin addicts. Journal of Psychoactive Drugs. 2010;42:89–95. doi: 10.1080/02791072.2010.10399789. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: Origins, contributions and transitions. American Journal on Addictions. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies, and policy. In: Fertig JB, Allen JP, editors. Alcohol and tobacco: From basic science to clinical practice. National Institute of Alcohol Abuse and Addiction; Bethesda, MD: 1995. [Google Scholar]
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138:507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiology and Drug Safety. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Sidak Z. Rectangular confidence region for the means of multivariate normal distributions. Journal of the American Statistical Association. 1967;62:626–633. [Google Scholar]
- Smyth BP, Fagan J, Kernan K. Outcome of heroin-dependent adolescents presenting for opiate substitution treatment. Journal of Substance Abuse Treatment. 2012;42:35–44. doi: 10.1016/j.jsat.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Sordo L, Chahua M, Bravo MJ, Barrio G, Brugal MT, Domingo-Salvany A, De la Fuente L. Depression among regular heroin users: the influence of gender. Addictive Behaviors. 2012;37:148–152. doi: 10.1016/j.addbeh.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2010 National Survey on Drug Use and Health: Summary of Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. Journal of Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE. Gender and non-medical use of prescription opioids: Results from a national US survey. Addiction. 2008;103:258–268. doi: 10.1111/j.1360-0443.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug and Alcohol Dependence. 2009;105:154–159. doi: 10.1016/j.drugalcdep.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clinical Journal of Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. American Journal of Drug and Alcohol Abuse. 1995;21:289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodology. Contemporary Clinical Trials. 2010;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Composite International Diagnostic Interview (CIDI): Core Version 2.1. World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]
- Wu L, Woody GE, Yang C, Blazer DG. Subtypes of nonmedical opioid users: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2010;112:69–80. doi: 10.1016/j.drugalcdep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Ling W, Burchett B, Blazer DG, Shostak J, Woody GE. Gender and racial/ethnic differences in addiction severity, HIV risk, and quality of life among adults in opioid detoxification: results from the National Drug Abuse Treatment Clinical Trials Network. Substance Abuse and Rehabilitation. 2010;1:13–22. doi: 10.2147/SAR.S15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis DM, Amass L, Steinberg M, Woody G, Krejci J, Annon JJ, Ling W. Predictors of outcome for short-term medically supervised opioid withdrawal during a randomized, multicenter trial of buprenorphine-naloxone and clonidine in the NIDA clinical trials network drug and alcohol dependence. Drug and Alcohol Dependence. 2009;99:28–36. doi: 10.1016/j.drugalcdep.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]