Abstract
Objective
We tested the hypothesis that the matricellular protein thrombospondin-1 (TSP1), through binding to and activation of the cell receptor CD47, inhibits basal and thermal-mediated cutaneous blood flow.
Background Data
Abnormal and decreased cutaneous blood flow in response to temperature changes or vasoactive agents is a feature of cardiovascular disease and aging. The reasons for decreased cutaneous blood flow remain incompletely understood. Further, a role for matricellular proteins in the regulation skin blood flow has never been proposed.
Methods
C57BL/6 wild type, TSP1- and CD47-null 12 and 72 week old male mice underwent analysis of skin blood flow (SkBF) via laser Doppler in response to thermal stress and vasoactive challenge.
Results
Young and aged TSP1- and CD47-null mice displayed enhanced basal and thermal sensitive SkFB changes compared to age matched wild type controls. Nitric oxide-mediated increases in SkBF were also greater in null mice. TSP1 and CD47 were expressed in skin from young wild type mice, and both were significantly upregulated in aged animals. Tissue 3',5'-cyclic guanosine monophosphate (cGMP), a potent vasodilator, was greater in skin samples from null mice compared to wild type regardless of age. Finally, treating wild type animals with a CD47 monoclonal antibody, that inhibits TSP1 activation of CD47, enhanced SkBF in both young and aged animals.
Conclusions
The above results suggest that secreted TSP1, via its cognate receptor CD47, acutely modulates SkBF. These data further support therapeutically targeting CD47 to mitigate age-associated loss of SkBF and maximize wound healing.
Introduction
Adequate skin blood flow (SkBF) is necessary for wound healing and to modulate core body temperature1. The processes that regulate cutaneous blood flow are complex and include input from the neural system2. Additionally local factors at the level of blood vessels and vascular cells contribute to regulate cutaneous flow3,4. Decreased or abnormal SkBF has been demonstrated in patients with diabetes, peripheral vascular disease5, scleroderma6, thromboangiitis obliterans (Buerger's disease)4, Raynaud's phenomenon7 and in the elderly8,9. Among these patient groups, abnormal and decreased SkBF is a major contributor to the pathogenesis and chronicity of soft tissue wounds, though the reasons for altered SkBF remain incompletely defined. Conversely, the goal of enhancing SkBF to increase wound healing has met with limited experimental and clinical success10,11.
The biogas nitric oxide (NO) participates in the regulation of SkBF12,13 and wound healing14,15. Loss of NO bioavailability and sensitivity contributes to abnormal SkBF16–18, whereas therapeutic enhancement of NO signaling increases SkBF in pre-clinical models19–21. Studies in human subjects also suggest a role for NO in the regulation of SkBF22, with local thermally-induced cutaneous vasodilation mediated substantially through NO signaling23. Loss of cutaneous NO and decreased cutaneous vasodilatory response24 are associated with decreased healing capacity in the elderly25,26. Yet it is not known what factors account for the age-associated loss of cutaneous NO signaling.
The secreted matricellular protein thrombospondin-1 (TSP1) is unregulated in several disease states that are associated with loss of SkBF and impaired wound healing including diabetes, scleroderma27 and systemic sclerosis28, and has recently been postulated to account for the loss of cutaneous blood flow in these individuals29. In pre-clinical models of cutaneous wound healing TSP1 antisense oligomers delayed wound healing30 and overexpression of TSP1 in the skin of mice greatly slowed wound closure and wound-associated angiogenesis31. We have reported that TSP1-null and CD47-null mice demonstrated enhanced ischemic wound healing in aged animals compared to wild type controls21. Herein then we tested the hypothesis that temperature- and age-associated changes in sKBF are limited by TSP1-activation of the cell receptor CD47. TSP1- and CD47-null mice displayed enhanced basal cutaneous blood flow and a greater dynamic response in flow to both core temperature changes and pharmacologic activation of the NO pathway compared to wild type controls at any age. These findings were associated with enhanced levels of the NO second messenger 3',5'-cyclic guanosine monophosphate (cGMP) in skin from null mice regardless of age. In wild type murine skin TSP1 and CD47 expression increased with age and were paralleled by a concurrent drop in NO signaling and SkBF. Finally, blocking CD47 activation in wild type mice with an antibody that prevents TSP1 binding to CD47 increased cutaneous flow in young and aged mice. Together these data suggest (1) that induction of the TSP1-CD47 signaling axis may account, in part, for age-associated decreases in cutaneous NO signaling and SkBF, and (2) that CD47 targeting enhances skin blood flow and may promote wound healing.
Materials and Methods
Animals
C57BL/6 male wild type, TSP1-null and CD47-null mice (Jackson Lab stock numbers 000664, 006141 and 003173 respectively) were maintained in a pathogen-free environment with ad libitum access to standard rat chow and water. Animal ages at the time of use were 12 or 72 weeks as indicated. Care and handling of animals was in accordance with the Institutional Animal Care and Use Committees of the National Institutes of Health and of the University of Pittsburgh School of Medicine. Hair from the dorsum of the animals was removed with electric sheers followed by a depilatory lotion (Nair®) prior to laser Doppler flow measurements.
Laser Doppler analysis of skin blood flow
Core temperature was monitored via rectal probe and maintained at 37.5°C by a heating pad and warming lamp. In experiments were core temperature was altered the heating lamp and pad were adjusted to raise or lower core temperature by 0.5°C intervals. Animals were acclimated at new core temperatures for 15m prior to laser Doppler analysis. Anesthesia was obtained with isoflurane (1.5% wild type and TSP1 null mice; 1.2% CD47 null mice) with a 50:50 ration of oxygen to room air via nose cone inhalation. (See below for an explanation concerning the variation in anesthesia dosing between mouse strains.) A MoorLDλ 1–2 laser Doppler scanner (Moor Instruments, Devon, England) was used to acquire real time cutaneous blood flow data with the following parameters: scan area, 1.6× 2.5 cm; scan speed, 4 ms per pixel; scan time, 1 minute 54 sec; override distance, 25 cm. The override distance was 20 cm.
Blood pressure measurement
We have published that CD47-null mice display decreased mean arterial blood pressure (MAP) at rest compared to wild type and TSP1-null animals32. For this reason, we placed femoral arterial catheters (Millar Mikro-Tip pressure Catheter) in age matched wild type, TSP1- and CD47-null mice and monitored MAP under inhalation isoflurane anesthesia. Though MAP at a concentration of 1.5% isoflurane trended slightly lower in CD47-null mice, decreasing the concentration of anesthetic to 1.2% brought MAP pressure in CD47-null animals to within 3–5 mm Hg of values recorded in wild type and TSP1-null animals.
Vasoactive challenge experiments
Baseline SkBF data was obtained in animals (n = 6 per strain/treatment group). Animals then received a vasoactive challenge with the primary NO donor (DEA-NO, 100 nmol/g body weight in 100 μl pre-warmed normal saline via rectal installation) or the endothelial nitric oxide synthase (eNOS) activator acetylcholine (ACh, 0.08 μg/gram weight in 100 μl pre-warmed normal saline via i.p. injection) and 60s later cutaneous blood flow was measured via laser Doppler.
Therapeutic blockade of TSP1-CD47 signaling
Age matched male C57BL/6 wild type mice were randomized into one of four groups: no treatment, vehicle (sterile phosphate-buffered saline), CD47 blocking antibody (clone 301), or isotype matched control antibody. Treatments were administered via intraperitoneal injection. Blood flow analysis was performed via laser Doppler 3h later. Antibody dose (0.4 μg/g weight i.p. in 100 μL sterile phosphate-buffered saline) was based on our prior published data showing therapeutic efficacy33.
Protein expression by western blot
Sections of dorsal skin were homogenized in ice-cold lysis buffer containing NP-40, protease inhibitor cocktail (Sigma), sodium fluoride, sodium orthovanadate and PhosStop (Roche), centrifuged at 12,000 rpm for 20 min at 4°C, supernatants collected and lysates stored at −20°C. Protein was quantified using a Bradford assay (BioRad). Thirty micrograms (30 μg) of total protein was boiled, resolved by SDS-page and transferred onto nitrocellulose (BioRad). In blots for CD47, non-reducing Laemmli buffer was used with 8% SDS-PAGE as previously published34. Blots were probed with primary antibody to the respective proteins and were visualized after 1 h incubation in secondary antibody on an Odyssey Imaging System (Licor). The following antibodies were employed - mouse anti-thrombospondin-1 (Abcam, 1:500 dilution, Cat.No. ab1823); goat anti-CD47 C-18 (Santa Cruz, 1:500 dilution) and rabbit anti-β-actin (Cell Signaling, dilution 1:5000, Cat. No. 4967). The intensity of the bands was quantified using the Odyssey software or Image J (rsbweb.nih.gov/ij/).
Determination of mRNA transcript
TSP1 and CD47 mRNA levels in skin samples from 12 and 72 week old C57BL/6 wild type mice were determined by qtPCR. Specific Taqman primers and probes for HPRT1 (Mm_01545399_m1), CD47 (Mm_00495005_m1) and TSP1 (Mm_01335418_m1) were obtained from Applied Biosystems (Carlsbad, CA). Total RNA was extracted using Qiagen RNeasy® Mini Kits (Qiagen, Hilden, Germany) and Proteinase K digestion as per the manufacturer instructions. RNA was quantified using the Take3 Gen5 spectrophotometer (BioTek, Winooski, VT). One microgram (1 μg) of RNA was treated with DNase I (amplification grade, Invitrogen, Grand Island, NY) and then reverse-transcribed using the Superscript III First Strand Synthesis Supermix (Invitrogen). cDNA was amplified using Platinum® Quantitative PCR SuperMix-UDG (Invitrogen) in 20μl volumes in triplicate with gene specific primers and probe on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems), according to manufacturer instructions. Thermal cycling conditions were 50°C for 2 minutes, 95°C for 2 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data were analyzed using the ΔΔCt method with expression normalized to the housekeeping gene.
Determination of tissue cGMP
Skin cGMP was measured as we have published35 with slight modification. Briefly, skin biopsies of equal wet weight were excised, flash frozen in liquid nitrogen and pulverized in a mortar and pestle. Homogenates of the pulverized tissue were prepared lysis buffer chilled to 4°C, centrifuged at 4°C and supernatants used for analysis via immunoassay (Amersham, GE Healthcare) as per the manufacture instructions.
Statistics
Significance was calculated with Student's t test and 1-way or 2-way ANOVA as appropriate, with a Bonferroni post test using a soft ware package (GraphPad Prism 5, La Jolla, CA) with p < 0.05 taken as significant.
Results
Cutaneous TSP1 and CD47 expression increases with age
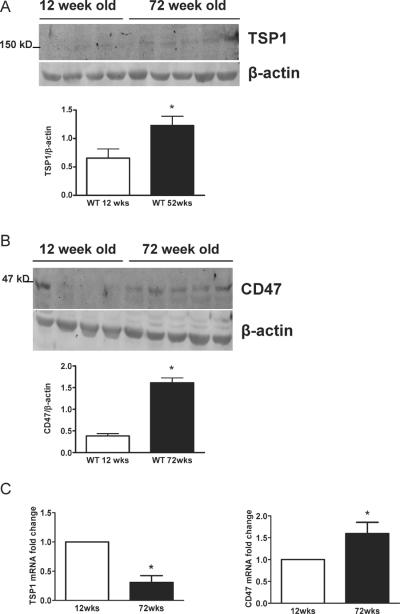
In the absence of injury or disease TSP1 expression is minimal29,36. However, with acute injury TSP1 expression increases rapidly37. In pre-clinical models age-associated induction of TSP1 has been described in the kidney38 and heart39. To date little is know about CD47 expression in health or disease. Analysis of cutaneous biopsies from wild type male mice at 12 weeks demonstrated expression of both TSP1 and CD47 protein and mRNA (Figs. 1A–C). In cutaneous samples from aged 72 week old mice both TSP1 and CD47 protein and CD47 transcript increased significantly (Figs. 1A–C). Interestingly though in aged skin TSP1 mRNA was significantly decreased. These data provide the first evidence of aged-associated induction of tissue CD47.
Figure 1. Cutaneous TSP1 and CD47 protein and mRNA are increased with age.
Biopsies of skin from the dorsum of 12 and 72 week old male C57BL/6 mice were collected, tissue lysates prepared, protein separated by SDS-PAGE and Western blotted for TSP1 and CD47 (A). Densitometry represents the mean ± SD of blots prepared from distinct tissue samples from individual mice (n = 4 12 week old mice and n = 5 72 week old mice). * = p < 0.05 compared to 12 week old animals. (B). q-PCR analysis of TSP1 and CD47 mRNA (C). Results normalized to HPRT are the mean ± SD of material prepared from animal cohorts described in A. * = p < 0.05 compared to 12 week old animals.
TSP1 and CD47 limit basal cutaneous blood flow
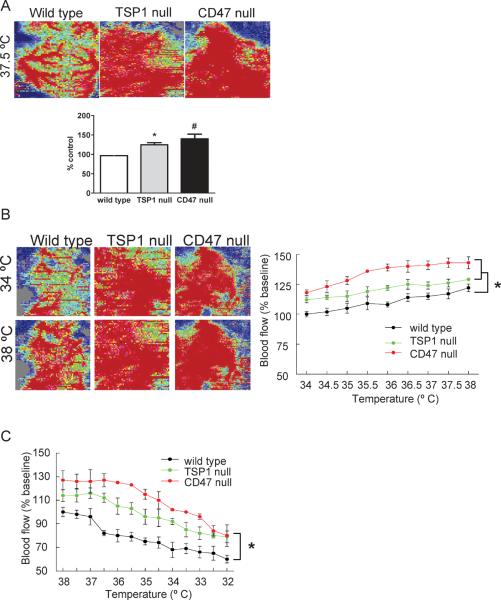
We have published that TSP1 and CD47 are hypertensive and support blood pressure32,40. To control for this in the present work we measured blood pressure in age matched wild type, TSP1- and CD47-null mice and adjusted the concentration of inhalation anesthesia accordingly to achieve parity in MAP between strains (see Methods Section). We now show in 12 week old mice, at a constant core temperature of 37.5 °C, that SkBF is significantly greater in both TSP1- and CD47-null mice compared to wild type C57BL/6 controls (Fig. 2A). These findings are important in light of our recent report that physiologically relevant TSP1 signaling occurs through binding to and activation of cell receptor CD4741.
Figure 2. Basal and thermal-mediated SkBF is increased in the absence of TSP1 and CD47.
Under general anesthesia 12 week old male wild type, TSP1- and CD47-null mice underwent SkBF analysis with core temperature maintained at 37°C (A). Typical images of real time laser Doppler analysis are presented for wild type, TSP1- and CD47-null mice. Results represent the mean ± SD of 6 animals of each strain. * and # = p < 0.05 compared to wild type. Under general anesthesia 12 week old male C57BL/6 wild type, TSP1- and CD47-null mice under went SkBF analysis with laser Doppler beginning at 37°C, followed by controlled elevation of core temperature by 0.5°C with SkBF determined at each new core temperature, proceeding to a maximum core temperature of 38°C (B). Typical images of real time laser Doppler analysis are presented for wild type, TSP1- and CD47-null mice at the indicated core temperatures. Results represent the mean ± SD of 6 mice of each strain. * = p < 0.05 compared to wild type. Under general anesthesia 12 week old male C57BL/6 wild type, TSP1- and CD47-null mice under went SkBF analysis with laser Doppler beginning at 38°C, followed by controlled lowering of core temperature by 0.5°C with SkBF determined at each new core temperature, proceeding to a core temperature of 32°C (C). Results represent the mean ± SD of 6 mice of each strain. * = p < 0.05 compared to wild type.
Temperature-induced changes in blood flow are limited by TSP1 and CD47
Thermal stress alters SkBF and this process is both directly and indirectly mediated via NO42,43. We reported that blood vessels from TSP1 and CD47 null mice demonstrate enhanced vasodilatation in ex vivo myography bioassays compared to wild type controls40. Considering these findings, we tested the hypothesis that SkBF changes to thermal stress are limited by TSP1 and CD47. Beginning with animals at a core temperature of 34°C, we increased core body temperature by 0.5°C to a maximum of 38°C and measured cutaneous blood flow after each thermal adjustment. Interestingly, both TSP1- and CD47-null mice showed increased SkBF under basal conditions that persisted throughout the period of controlled elevation of core body temperature as compared to wild type (Fig. 2B). Likewise, TSP1- and CD47-null mice also showed maintenance of greater cutaneous perfusion during periods of controlled cooling (Fig. 2C). Null mice did experienced a drop in SkBF with cooling, however at the lowest core temperature achieved TSP1- and CD47-null mice still demonstrated approximately 25% greater SkBF compared to wild type animals, suggesting a possible primary deficiency in reflex protection of core temperature in null animals.
Vasoactive alterations in SkBF are regulated by TSP1 and CD47
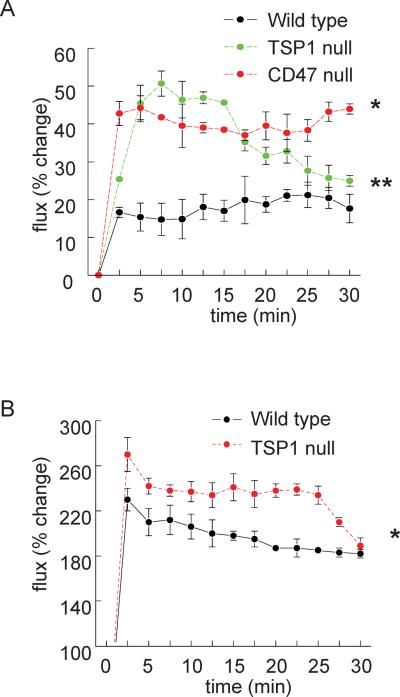
We treated mice with the primary NO-donor diethylamine NONOate (DEA-NO, t½=30s), to assess endothelial-independent effects on SkBF, and acetylcholine (ACh) (a physiologic activator of endothelial nitric oxide synthase (eNOS) that has been reported to alter SkBF44) to test endothelial-dependent blood flow effects. Consistent with enhanced basal cutaneous blood flow, SkBF in TSP1- and CD47-null animals was greater following treatment with DEA-NO (Fig. 3A) and also greater following treatment with ACh in TSP1-null animals (Fig. 3B) compared to controls. To further assess the role of NO in the above results we treated mice with the eNOS inhibitor L-nitro-L-arginine methyl ester (L-NAME). Basal wild type and TSP1- and CD47-null SkBF normalized following L-NAME treatment (data not shown).
Figure 3. NO-mediated effects on SkBF are limited by TSP1 and CD47.
Under general anesthesia 12 week old male C57BL/6 wild type, TSP1- and CD47-null mice underwent SkBF analysis with laser Doppler with core temperature maintained constant at 37°C. After a 30 minute stabilization interval basal SkBF was determined, and animals were challenged with either vehicle (normal saline) or the primary NO donor DEA-NO (100 nmol/g body weight via rectal installation) (A), or the eNOS activator acetylcholine (ACh, 0.08 μg/gram weight via intravenous injection; wild type and TSP1-null mice) (B) and SkBF determined. Results are the mean ± SD of 6 animals of each strain. * and ** = p < 0.05 compared to wild type.
Age-associated alterations in SkBF are less prominent in TSP1- and CD47-null mice
Loss of cutaneous blood flow, associated tissue necrosis and poor wound healing are known consequences of age and certain vasculopathies27,28,45. We have reported that skeletal muscle blood flow is maintained in old TSP1- and CD47-null mice compared to old wild type animals21. These findings suggested that TSP1-CD47 signaling may also limit SkBF in aged animals. We tested this hypothesis in 72 week old wild type and null mice. Both basal and thermal associated changes in SkBF were greater in 72 week old TSP1- and CD47-null mice compared to wild type controls, though statistical significance was only obtained in CD47-null mice (Fig. 4A).
Figure 4. Aged TSP1- and CD47-null mice maintain SkBF and NO signaling.
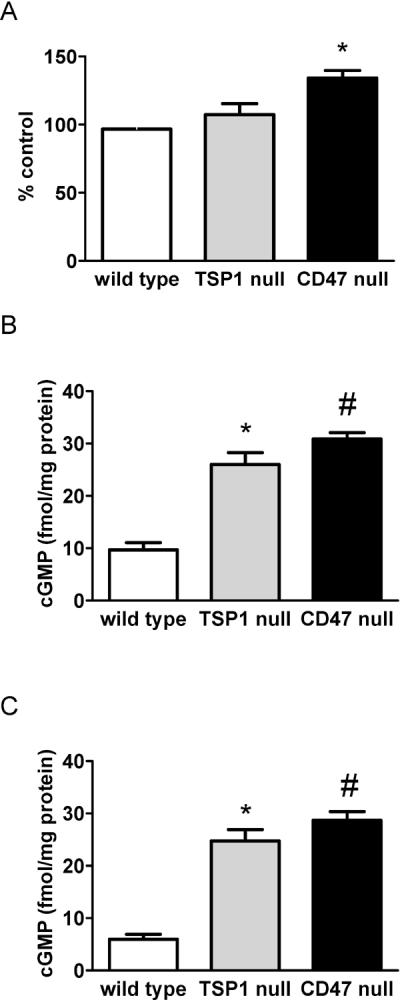
Under general anesthesia 72 week old male wild type, TSP1- and CD47-null mice with core temperature maintained at 37°C underwent SkBF determination via laser Doppler. Results are the mean ± SD of 6 animals of each strain. * = p < 0.05 compared to wild type. Dorsal cutaneous skin samples from 12 week (B) or 72 week (C) old male wild type, TSP1- and CD47-null mice were harvested, snap frozen in liquid nitrogen, pulverized, homogenized in lysis buffer and assayed for tissue cGMP as via ELISA (Amersham, GE Healthcare). Results presented are the mean ± SD of 4 animals of each strain. * and # = p < 0.05 compared to wild type.
Tissue cGMP is elevated in young and old TSP1- and CD47-null skin samples
Nitric oxide activation of soluble guanylyl cyclase (sGC) results in rapid production of cGMP and subsequent vasodilation46,47. Analysis of tissue cGMP levels in freshly harvested skin biopsies demonstrated increased cGMP in samples from 12 week old TSP1- and CD47-null mice compared to wild type (Fig. 4B). Surprisingly, analysis of cGMP levels in skin from 72 week old TSP1- and CD47-null mice confirmed persistent elevation of cutaneous cGMP (Fig. 4C). In contrast cutaneous cGMP levels decreased with age in wild type mice (Fig. 4C). These findings demonstrate persistence of NO signaling in null animals independent of the aging process and suggest age-associated loss of NO signaling may be secondary, in part, to induction of activated CD47 axis.
Interruption of CD47 activation enhances SkBF
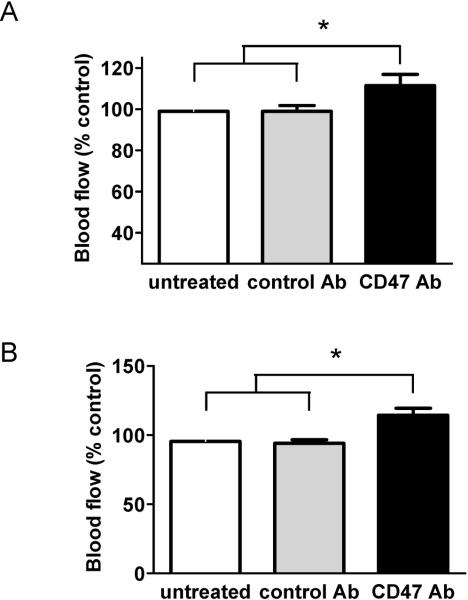
Loss of adequate skin blood flow contributes to delayed healing and wound chronicity in the elderly8,48,49. We have identified several therapeutic agents that effectively block the TSP1-CD47 signaling axis through preventing TSP1 binding to and activation of CD4733,35,50. We tested the potential for one of these therapeutic agents, by blocking TSP1-CD47 signaling, to enhance SkBF. A CD47 monoclonal antibody (clone 301) was given to 12 week old wild type mice prior to laser Doppler assessment of SkBF. Interestingly, wild type animals demonstrated enhanced SkBF under basal conditions (Fig. 5A) compared to animals treated with an isotype IgG2α control antibody. Importantly, treating aged 72 week old wild type mice with the CD47 blocking antibody also increased SkBF (Fig. 5B).
Figure 5. Therapeutic blockade of CD47 activation increases SkBF.
12 week (A) or 72 week (B) old male wild type C57BL/6 mice were treated with a CD47 blocking antibody (clone 301, 40 μg delivered as 10 μL of a 4 mg/mL stock in 100 μL of PBS in injected in the skin) or an isotype IgG2α control antibody as we had previously published53. Three hours later animals underwent general anesthesia with their core temperature maintained at 37°C for 30 minutes prior to assessment of SkBF via laser Doppler. Results presented are the mean ± SD of 4 animals in each treatment group. * = p < 0.05 compared to isotype control antibody treated and untreated.
Discussion
To our knowledge, this is the first report to demonstrate in pre-clinical studies that matricellular TSP1 is an immediate regulator of cutaneous blood flow via its activation of CD47 signaling. SkBF was greater under basal conditions in TSP1- and CD47-null animals and in response to both vasoactive and thermal challenge, suggesting a global upregulation in flow in the absence of activated CD47. Aged null animals lacking activated CD47 demonstrated more robust SkBF than wild type controls and minimal change in SkBF compared to young CD47 null animals, suggesting a primary role for activated CD47 in age-associated deficiencies in SkBF.
Autonomic pathways are known to control SkBF and we have reported that TSP1-null mice display greater shifts in blood flow after post-ganglionic autonomic blockade with hexamethonium chloride32. It is possible that differences in autonomic signaling may play a role SKBF flux measured in null mice and future studies will addresses this.
The acute effects of TSP1 on SkBF identified here are distinct from its previously reported chronic effects on tissue perfusion via its anti-angiogenic activity. Cutaneous TSP1 limits ultraviolet light-mediated angiogenesis in part through limiting VEGF signaling to decrease cutaneous vascularity51. We have shown activated CD47 inhibits both NO- and VEGF-driven angiogenesis in endothelial cells34,52. Also antibody blockade of TSP135 or gene suppression of CD4753 can increase cutaneous vascularity and subsequent blood flow. However, we have reported comparable vascular density under basal conditions in skin in 12 week old wild type and null mice, though in wound and ischemic models TSP1- and CD47-null cutaneous tissue units show enhanced vascularity at 3 and 7 day intervals53,54. Nonetheless young TSP1-and CD47-null mice have more basal cutaneous blood flow and show persistence of this advantage in SkBF after core temperature changes or NO pathway activation supporting a role for activated CD47 in the acute regulation of SkBF. However, it is not clear why SkBF is not more responsive to changes in core temperature alterations in TSP1-and CD47-null mice. Particularly unexpected was our finding that at a core temperature of 32°C TSP1- and CD47-null mice still maintained significantly increased SkBF compared to wild type. Such a result predicts a difficulty in null mice in maintaining core temperature in response to environmental challenge. Though the present work can not address this, these data are consistent with previous findings by our group of limited homeostasis in TSP1- and CD47-null mice to several stresses including anesthetic agents, vasodilators and sympathetic tone blockade32.
Interestingly, cutaneous TSP1 and CD47 levels increase with age. It is not clear if age-associated induction represents altered production, degradation or stability of these proteins. Given the identified role of TSP1 to inhibit angiogenesis, the decreased SkBF in older wild type mice may in part be secondary to decreased cutaneous vascular density. However, our data reveal that in aged animals activated CD47 also limits SkBF through an acute regulatory mechanism. Findings of increased expression of ligand TSP1 and cell receptor CD47 in aged skin are in agreement with our recent report of concurrent upregulation of both TSP1 and CD47 protein and mRNA in human and pre-clinical models of pulmonary arterial hypertension 55, suggesting linked gene regulation of these proteins.
Blockade of CD47 activation with a monoclonal antibody recapitulated null levels of cutaneous SkBF in wild type animals. This therapeutic advantage was enjoyed by antibody treated young and aged wild type mice. Increased cutaneous blood flow was detected shortly after a single injection of antibody. Hence, the enhanced SkBF experienced by treated wild type animals represents an acute response from existing cutaneous vascular networks rather than induction of angiogenesis. In addition, the activity of CD47 to inhibit both NO-56 and VEGF 34- mediated angiogenesis predicts that therapeutic targeting of CD47 will result in beneficial effects at the level of acute increases in blood flow and through increased angiogenesis. Thus, these data predict that drugs targeting CD47 will be multiply beneficial in enhancing blood flow and wound healing in the elderly.
Acknowledgments
Funding: This work was funded by 1RO1HL108954-01 (NIH/NHLBI), by 1P01HL103455-01 (NIH), by 11BGIA7210001 (AHA), by the Vascular Medicine Institute of the University of Pittsburgh, by the Institute for Transfusion Medicine and by the Western Pennsylvania Hemophilia Center (JSI); by the Intramural Research Program of the NCI/NIH (DDR); and by APP1016276 C.J. Martin Award (Australian NHMRC) (NAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 2.Wallin BG. Neural control of human skin blood flow. J Auton Nerv Syst. 1990;30(Suppl):S185–190. doi: 10.1016/0165-1838(90)90128-6. [DOI] [PubMed] [Google Scholar]
- 3.Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Sci Rev. 2000;28:108–112. [PubMed] [Google Scholar]
- 4.Heistad DD, Abboud FM. Factors that influence blood flow in skeletal muscle and skin. Anesthesiology. 1974;41:139–156. doi: 10.1097/00000542-197408000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Belcaro G, Vasdekis S, Rulo A, Nicolaides AN. Evaluation of skin blood flow and venoarteriolar response in patients with diabetes and peripheral vascular disease by laser Doppler flowmetry. Angiology. 1989;40:953–957. doi: 10.1177/000331978904001103. [DOI] [PubMed] [Google Scholar]
- 6.LeRoy EC, Downey JA, Cannon PJ. Skin capillary blood flow in scleroderma. J Clin Invest. 1971;50:930–939. doi: 10.1172/JCI106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salsano F, Letizia C, Proietti M, et al. Significant changes of peripheral perfusion and plasma adrenomedullin levels in N-acetylcysteine long term treatment of patients with sclerodermic Raynauds phenomenon. Int J Immunopathol Pharmacol. 2005;18:761–770. doi: 10.1177/039463200501800420. [DOI] [PubMed] [Google Scholar]
- 8.Strigini L, Ryan T. Wound healing in elderly human skin. Clin Dermatol. 1996;14:197–206. doi: 10.1016/0738-081x(95)00155-9. [DOI] [PubMed] [Google Scholar]
- 9.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 109:1538–1544. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toba K, Ouchi Y, Akishita M, et al. Improved skin blood flow and cutaneous temperature in the foot of a patient with arteriosclerosis obliterans by vasopressin V1 antagonist (OPC21268). A case report. Angiology. 1995;46:1027–1033. doi: 10.1177/000331979504601108. [DOI] [PubMed] [Google Scholar]
- 11.Herrick AL, Gush RJ, Tully M, Jayson MI. A controlled trial of the effect of topical glyceryl trinitrate on skin blood flow and skin elasticity in scleroderma. Ann Rheum Dis. 1994;53:212. doi: 10.1136/ard.53.3.212-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JB. Nitric oxide and human skin blood flow responses to acetylcholine and ultraviolet light. FASEB J. 1994;8:247–251. doi: 10.1096/fasebj.8.2.7509761. [DOI] [PubMed] [Google Scholar]
- 13.Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. 2009;106:566–570. doi: 10.1152/japplphysiol.91289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg JS, Ridnour LA, Espey MG, Wink DA, Roberts DD. Nitric oxide in woundhealing. Microsurgery. 2005;25:442–451. doi: 10.1002/micr.20168. [DOI] [PubMed] [Google Scholar]
- 15.Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin. 2005;26:259–264. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 16.Perez AC, Khawaja AM, Page CP, Paul W. Persistence of effects of nitric oxide synthase inhibitors: comparisons on blood flow and plasma exudation in guinea pig skin. Eur J Pharmacol. 1997;330:241–246. doi: 10.1016/s0014-2999(97)01018-2. [DOI] [PubMed] [Google Scholar]
- 17.Sigaudo-Roussel D, Demiot C, Fromy B, et al. Early endothelial dysfunction severely impairs skin blood flow response to local pressure application in streptozotocin-induced diabetic mice. Diabetes. 2004;53:1564–1569. doi: 10.2337/diabetes.53.6.1564. [DOI] [PubMed] [Google Scholar]
- 18.Gribbe O, Samuelson UE, Wiklund NP. Effects of nitric oxide synthase inhibition on blood flow and survival in experimental skin flaps. J Plast Reconstr Aesthet Surg. 2007;60:287–293. doi: 10.1016/j.bjps.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Zeng B, Chai Y, Luo C, Li X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in randompattern skin flap model. Ann Plast Surg. 2008;61:646–653. doi: 10.1097/SAP.0b013e318172ba1f. [DOI] [PubMed] [Google Scholar]
- 20.Gribbe O, Gustafsson LE, Wiklund NP. Transdermally administered nitric oxide by application of acidified nitrite increases blood flow in rat epigastric island skin flaps. Eur J Pharmacol. 2008;578:51–56. doi: 10.1016/j.ejphar.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Isenberg JS, Hyodo F, Pappan LK, et al. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 22.Coffman JD. Effects of endothelium-derived nitric oxide on skin and digital blood flow in humans. Am J Physiol. 1994;267:H2087–2090. doi: 10.1152/ajpheart.1994.267.6.H2087. [DOI] [PubMed] [Google Scholar]
- 23.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008;15:569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan YK, Struck BD, Foreman RD, Robinson C. Wavelet analysis of sacral skin blood flow oscillations to assess soft tissue viability in older adults. Microvasc Res. 2009;78:162–168. doi: 10.1016/j.mvr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchida Y, Fukuda O, Kamata S. The correlation of skin blood flow with age, total cholesterol, hematocrit, blood pressure, and hemoglobin. Plast Reconstr Surg. 1991;88:844–850. doi: 10.1097/00006534-199111000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchida Y. The effect of aging and arteriosclerosis on human skin blood flow. J Dermatol Sci. 1993;5:175–181. doi: 10.1016/0923-1811(93)90764-g. [DOI] [PubMed] [Google Scholar]
- 27.Macko RF, Gelber AC, Young BA, et al. Increased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activation. J Rheumatol. 2002;29:2565–2570. [PubMed] [Google Scholar]
- 28.Morgan-Rowe L, Nikitorowitcz J, Shiwen X, et al. Thrombospondin-1 in hypoxia conditioned media blocks the growth of human microvascular endothelial cells and is increased in systemic sclerosis tissues. Fibrogenesis Tissue Repair. 4:13. doi: 10.1186/1755-1536-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Leask A, Abraham DJ, et al. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogenactivated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 4:9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- 31.Streit M, Velasco P, Riccardi L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isenberg JS, Qin Y, Maxhimer JB, et al. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide. 2009;21:52–62. doi: 10.1016/j.niox.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 38.Olson BA, Day JR, Laping NJ. Age-related expression of renal thrombospondin 1 mRNA in F344 rats: resemblance to diabetes-induced expression in obese Zucker rats. Pharmacology. 1999;58:200–208. doi: 10.1159/000028282. [DOI] [PubMed] [Google Scholar]
- 39.van Almen GC, Verhesen W, van Leeuwen RE, et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer EM, Qin Y, Miller TW, et al. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isenberg JS, Annis DS, Pendrak ML, et al. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellogg DL, Jr., Zhao JL, Wu Y, Johnson JM. Antagonism of Soluble Guanylyl Cyclase Attenuates Cutaneous Vasodilation During Whole Body Heat Stress and Local Warming in Humans. J Appl Physiol. doi: 10.1152/japplphysiol.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrell DM, Bishop VS. Permissive role for nitric oxide in active thermoregulatory vasodilation in rabbit ear. Am J Physiol. 1995;269:H1613–1618. doi: 10.1152/ajpheart.1995.269.5.H1613. [DOI] [PubMed] [Google Scholar]
- 44.Brown H, Moppett IK, Mahajan RP. Transient hyperaemic response to assess vascular reactivity of skin: effect of locally iontophoresed acetylcholine, bradykinin, epinephrine and phenylephrine. Br J Anaesth. 2003;90:446–451. doi: 10.1093/bja/aeg099. [DOI] [PubMed] [Google Scholar]
- 45.Lutz J, Huwiler KG, Fedczyna T, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNF alpha-308A allele in children with juvenile dermatomyositis. Clin Immunol. 2002;103:260–263. doi: 10.1006/clim.2001.5212. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Ignarro LJ, Byrns RE, Wood KS. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987;60:82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- 48.Tepelidis NT. Wound healing in the elderly. Clin Podiatr Med Surg. 1991;8:817–826. [PubMed] [Google Scholar]
- 49.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 50.Isenberg JS, Romeo MJ, Abu-Asab M, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 51.Yano K, Oura H, Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol. 2002;118:800–805. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- 52.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isenberg JS, Pappan LK, Romeo MJ, et al. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isenberg JS, Hyodo F, Matsumoto K, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer PM, Bauer EM, Rogers NM, et al. Activated CD47 Promotes Pulmonary Arterial Hypertension Through Targeting Caveolin-1. Cardiovasc Res. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]