Abstract
Non-mammalian vertebrates have a remarkable capacity to regenerate brain tissue in response to CNS injury. Nevertheless, it is not clear whether animals recover lost function after injury or whether injury-induced cell proliferation mediates recovery. We address these questions using the visual system and visually-guided behavior in Xenopus laevis tadpoles. We established a reproducible means to produce a unilateral focal injury to optic tectal neurons without damaging retinotectal axons. We then assayed a tectally-mediated visual avoidance behavior to evaluable behavioral impairment and recovery. Focal ablation of part of the optic tectum prevents the visual avoidance response to moving stimuli. Animals recover the behavior over the week following injury. Injury induces a burst of proliferation of tectal progenitor cells based on phospho-histone H3 immunolabeling and experiments showing that Musashi-immunoreactive tectal progenitors incorporate the thymidine analog iododeoxyuridine after injury. Pulse chase experiments indicate that the newly-generated cells differentiate into N-β–tubulin-immunoreactive neurons. Furthermore, in vivo time-lapse imaging shows that Sox2-expressing neural progenitors divide in response to injury and generate neurons with elaborate dendritic arbors. These experiments indicate that new neurons are generated in response to injury. To test if neurogenesis is necessary for recovery from injury, we blocked cell proliferation in vivo and found that recovery of the visual avoidance behavior is inhibited by drugs that block cell proliferation. Moreover, behavioral recovery is facilitated by changes in visual experience that increase tectal progenitor cell proliferation. Our data indicate that neurogenesis in the optic tectum is critical for recovery of visually-guided behavior after injury.
Keywords: retinotectal system, optic tectum, brain damage, visual avoidance behavior, cell proliferation, stem cell, neural progenitor cell, musashi, pH3, N-β-tubulin, injury response
Introduction
Pediatric brain injuries can have catastrophic effects depending on the type and location of the injury and the age at which it occurs. Although the developing nervous system displays a greater capacity for recovery after penetrating injury than the adult CNS (Kazim et al., 2011; Mackerle and Gal, 2009; Plantman et al., 2011), the cellular mechanisms contributing to recovery are not clear. Few experimental models exist to study penetrating brain injury in developing systems (Cernak, 2005; Statler et al., 2011), particularly those that assess a functional behavioral recovery after injury. We were interested in establishing a model to study cellular mechanisms of response to brain injury which would also assess recovery of behavior in intact animals.
The developing nervous system can respond to injury with an increase in endogenous neurogenesis, however the ability to engage endogenous neurogenesis to regenerate lost brain tissue after damage depends upon the type of injury, species, and the age at injury (Endo et al., 2007; Ferretti, 2011; Liu and Guthrie, 2011; Magavi et al., 2000). Studies in developing mammalian systems indicate that brain injury can result in increased cell proliferation near the injury site and migration of progenitors and new neurons into the injury site (Kim and Szele, 2008; Sundholm-Peters et al., 2005; Thored et al., 2007), however survival of newly generated neurons in the mammalian CNS is extremely low (Thored et al., 2007). Studies on recovery from brain injury have benefited from classical work on non-mammalian vertebrate species, which are capable of axon regeneration, synaptogenesis and, in some species, the regeneration of brain tissue in response to injury (Endo et al., 2007; Zupanc, 2009). In anuran amphibians, such as Xenopus, surgical brain lesions trigger proliferation of cells in the ventricular zone (Endo et al., 2007).The proliferative response to injury varies across brain regions and decreases with development from tadpoles to froglets (Filoni et al., 1995). Despite evidence for CNS regenerative capacity in amphibian, it is still not clear whether animals recover lost function after injury or whether injury-induced cell proliferation mediates recovery. We set out to address these questions using the visual system and visually-guided behavior in the Xenopus laevis tadpole.
Work in frogs has revealed many mechanisms fundamental to brain development, neuronal plasticity, and learning & memory, which subsequently have been shown to function in mammalian systems, including humans. The frog tadpole offers many experimental advantages for studying mechanisms underlying recovery from developmental brain injury. In particular, tadpoles exhibit optic tectum-dependent visually-guided behavior (Dong et al., 2009; Shen et al., 2011), which we use to assess behavioral recovery from injury. The optic tectum is the primary visual center in non-mammalian vertebrates, integrating multisensory information and governing motor output. Previous work has shown that the optic tectum mediates visual avoidance responses postulated to be required for tadpole survival (Dong et al., 2009; Shen et al., 2011), however it is not yet clear whether tectal damage in Xenopus tadpoles results in a deficit of visual avoidance behavior, whether tadpoles recover the visual avoidance behavior after damage, or whether damage to the tectum induces neurogenesis that is required for recovery of function.
Neurogenesis in the optic tectum occurs in the ventricular proliferative zone throughout larval stages in tadpoles (Straznicky and Gaze, 1972). Newly generated cells differentiate into neurons and are incorporated into the retinotectal circuit (Gaze et al., 1979). More recent work in our lab using incorporation of thymidine analogs such as bromodeoxyuridine (BrdU) (Sharma and Cline, 2010) or in vivo time lapse analysis of neural cell lineage (Bestman et al., 2012) has shown that cell proliferation and differentiation of progenitor cells in the optic tectal are regulated by visual system input to the tectum. Specifically, we showed that 2 days of visual deprivation causes neural progenitor cells to continue dividing and therefore expands the neural progenitor pool in the optic tectum, whereas visual experience promotes the differentiation of progenitors into neurons (Sharma and Cline, 2010) (Bestman et al., 2012). Here, we tested whether manipulating neurogenesis through visual experience might affect recovery of visual system function following injury.
Materials and Methods
Animals
Xenopus laevis tadpoles of either sex (bred in house or purchased from either Nasco, Fort Atkinson, WI or Xenopus Express, Brooksville, FL) were reared in 0.1X Steinberg’s Solution at 22°C with a 12hr light/12hr dark cycle, unless otherwise noted. All animal protocols were approved by the Institutional Animal Use and Care Committee of The Scripps Research Institute. For visual deprivation experiments, animals were housed in a light-impermeable compartment at 22°C immediately following surgery. After 48 hours, animals were tested for visual avoidance behavior, as described below, then housed in standard 12hr light/12hr dark conditions until the end of the experiment. All animals were anesthetized in 0.02% MS222 (3-aminobenzoic acid ethyl ester, Sigma) before surgical procedures, and were terminally anesthetized in 0.2% MS222 at the end of the experiment.
Visual Avoidance Behavior
We assessed visual avoidance behavior using an assay modified from Dong et al (2009), as described in Shen et al (2011). Stage 47 (Nieuwkoop and Faber, 1956) animals were screened for the optomotor response (OMR) to evaluate general health (Dong et al., 2009; Portugues and Engert, 2009; Roeser and Baier, 2003; Shen et al., 2011). Only animals that exhibited a normal OMR were included in visual avoidance assays. Four to five tadpoles were placed in a clear Plexiglas tank fitted with a translucent sheet of 3M projector screen. Animals were given 1 minute to distribute within the tank and then visual stimuli were presented for 1 minute using a microprojector (3M, MPro110) positioned below the tank. Tadpoles were visualized with infrared (IR) LEDs and videos of the tadpole movements were captured with a Hamamatsu ORCA-ER digital camera. The entire setup is enclosed within a light-tight compartment. Visual stimuli were generated and presented by a custom-written MATLAB code (The MathWorks and Psychophysics Toolbox extensions). Behavioral testing was performed at the same time each day. Videos were analyzed post hoc, frame-by-frame, for encounter events and avoidance responses. Data acquisition and analysis were conducted blind to treatment. An avoidance response was scored when a tadpole displayed a sharp turn within 500ms of an encounter with a dot moving perpendicularly (within a range of 90° ± 15°) toward the eye. Data are presented as an Avoidance Index, or the fraction of the first ten encounters with moving dots that result in an avoidance response. The Avoidance Index of trials throughout each experiment was normalized to the Avoidance Index determined before treatment on Day 0, to allow comparison of behavioral results across experiments. Every experiment included intact control animals to control for batch-to-batch variation and overall clutch health. Raw data (non-normalized values) are presented in Table 2. In most cases, 1 minute of recording an animal’s responses to the moving spot stimulus was sufficient to identify 10 encounters, however a few animals did not have 10 encounters within the 1 minute test period. For cases with more than 5 but fewer than 10 encounters, the Avoidance Index was calculated as the number of avoidance responses divided by the number of encounters within 1 minute. Animals with fewer than 5 encounter events were excluded from the analysis. Out of the 1458 behavioral trials from the 261 animals analyzed in this study, only 99 trials showed fewer than 10 encounters during the 1 minute recording period (<7%). Because the stimulus could appear distorted by the edges of the chamber, animals swimming along the edge were not analyzed until they were one body width away from the edge. The Wilcoxon signed-rank test was used to test whether behavior was significantly different from random baseline non-stimulus turning. Each experiment was repeated 3 times. Data were determined to be normally distributed, so a Student’s T-test (2 sample, 2 tailed) was used to test for significant differences between experimental groups. Because the number of surviving animals can change over the course of the experiment, the data are presented as the group average ± standard error of the mean (SEM).
Table 2. Avoidance Behavior Raw Data.
Avoidance behavior data for each timepoint and each experiment presented. Data are presented as the average percent avoidance ± standard error of the mean with animal number (n).
| Group | Day 0 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Intact | 74.1±1.5 (53) | 73±1.1 (42) | 74±1.7 (40) | 76±1.0 (42) | 76.4±1.7 (39) | 75±1.8 (33) | 76±1.5 (32) | 77.5±1.1 (29) |
| Sham | 75±3.4 (6) | 78.3±2.6 (7) | 78±3.1 (6) | 78±3.7 (5) | 75±2.2 (5) | 75±2.9 (4) | 75±5 (3) | ||
| Injured | 22±1.9 (20) | 29.1±3.4 (20) | 45.4±3.6 (21) | 56.4±3.2 (14) | 66.4±2.4 (11) | 76±5.4 (10) | 77.8±4.9 (9) | ||
| 9A | Intact | 71±1.4 (60) | 66.2±2.4 (13) | 69.6±2 (12) | 66.2±2.4 (13) | 66.7±2.6 (12) | n.t. | 67.7±1.6 (11) | n.t. |
| Intact +DMSO | 72.9±1.7 (41) | 70.2±1.3 (19) | n.t. | 69.8±2.1 (16) | n.t. | 70.5±1.7 (13) | n.t. | ||
| Intact +CDBs | 71.2±1.5 (17) | n.t. | 71±1.4 (18) | n.t. | 71.3±2.1 (9) | n.t. | |||
| Injured +DMSO | 23.9±1.1 (50) | 48.5±1.9 (26) | 54.7±1.6 (10) | 66.8±1.6 (28) | n.t. | 68.2±1.5 (17) | n.t. | ||
| Injured +CDBs | 39.2±1.9 (19) | n.t. | 46.3±2.2 (16) | n.t. | 62.4±2.5 (17) | n.t. | |||
| Injured | 24.6±1.4 (12) | 37±2.4 (9) | 48.4±3.4 (8) | 57±2.1 (10) | n.t. | 66.9±3.4 (4) | n.t. | ||
| 9B | Intact | 74.7±1.6 (47) | 77.1±1.7 (16) | 76.4±1.3 (32) | 76.9±2.8 (8) | 77±2.7 (11) | 77.1±3.6 (7) | 74.7±1.9 (19) | n.t. |
| Injured | 25.6±1.5 (30) | 29.9±1.6 (23) | 43.3±3.7 (9) | 54.5±2.3 (10) | 63.7±2.7 (10) | 67.8±1.6 (14) | n.t. | ||
| Injured +Dark | 28±1.7 (31) | 42.6±18 (38) | 59.6±3.6 (9) | 63.4±2.2 (9) | 66.2±1.7 (17) | 71.1±1.8 (14) | n.t. |
Microscopy and Presentation
For live animal imaging and whole-mount immunofluorescence, confocal stacks were collected on an Ultraview VOX spinning-disk system with Volocity 5 software (Perkin-Elmer, Foster City, CA). The system has a Yokagowa CSU-X1 spinning disk confocal attachment mounted on either a Olympus BX61WI microscope equipped with a 20X 0.95 NA water immersion objective (Figures 2B–F, 4, 5), or on a Nikon Eclipse FN1 microscope equipped with a 25X 1.1NA water immersion objective (Figures 2G–H, 8). Green and red fluorescent signals were excited with 488nm and 561nm laser lines and differentiated with 515(30) and 615(75) filters, respectively. Vibratome sections were imaged with an Olympus FluoView FV500 laser-scanning confocal microscope equipped with a 20X 0.8 NA oil-immersion objective (Figures 6, 7). Green and red fluorescent signals were excited with 488nm and 546nm laser lines and distinguished with 505–525 and 550–600 bandpass filters, respectively. Care was taken to minimize pixel saturation during image acquisition. Single optical sections in Figures 2E–F and 3D confocal projections in Figures 4, 5, and 8 were made using Volocity 3D imaging software. The single optical sections in Figures 6 and 7 were made using Metamorph imaging software. Adobe Photoshop was used to adjust contrast uniformly across time-points within experiments for Figures 2, 4, and 5. In Figure 8 the brightness of Day 7 image was increased compared to the images from previous days to enhance the appearance of fainter processes at that timepoint. Figures were compiled using Adobe Illustrator.
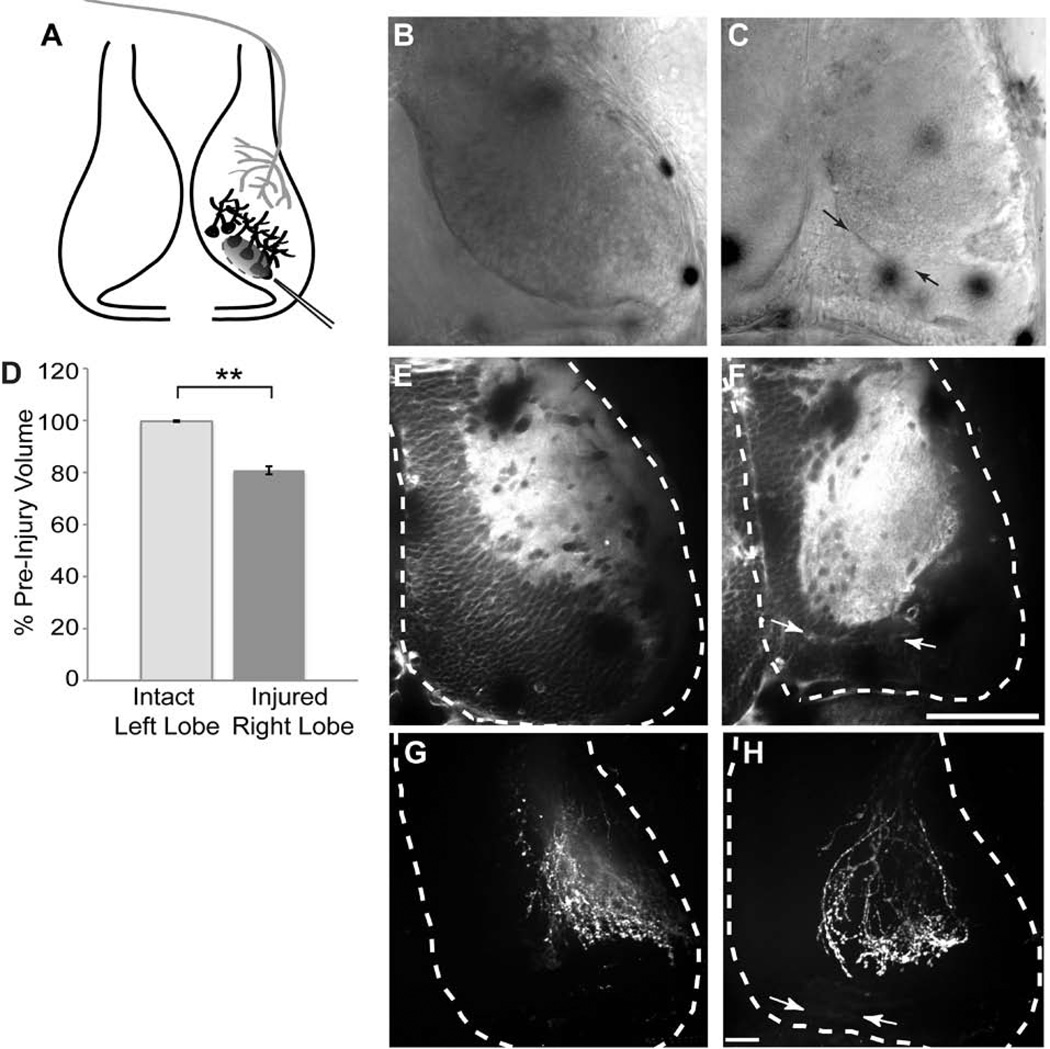
Figure 2. Unilateral injury to the optic tectum.
A. Schematic of the injury paradigm. Stage 47 tadpoles were surgically injured by aspirating a focal region of optic tectal cells (black) from the caudal right tectal lobe, caudomedial to the region of retinal axon innervation (gray). B, C. DIC images show the tectal lobe before surgery (B) and after surgery (C). Arrows in C indicate wound area. D. Quantification of the tectal volumes before and after surgery. Surgery decreases the volume of the right tectal lobe without affecting the volume of the left tectal lobe. N = 16 animals, p<0.01. E, F. Single optical sections of fluorescent FM4-64 membrane dye labeling before (E) and after surgery (F). The tectal lobes are outlined in white. The injury site in the right lobe was between the arrows. G, H. DiI labeling of the RGC axons in intact (G) and injured (H) tecta. Injury does not alter gross morphology of the RGC axon arbors. Scale bars, B–F 100µm, G–H 30µm.
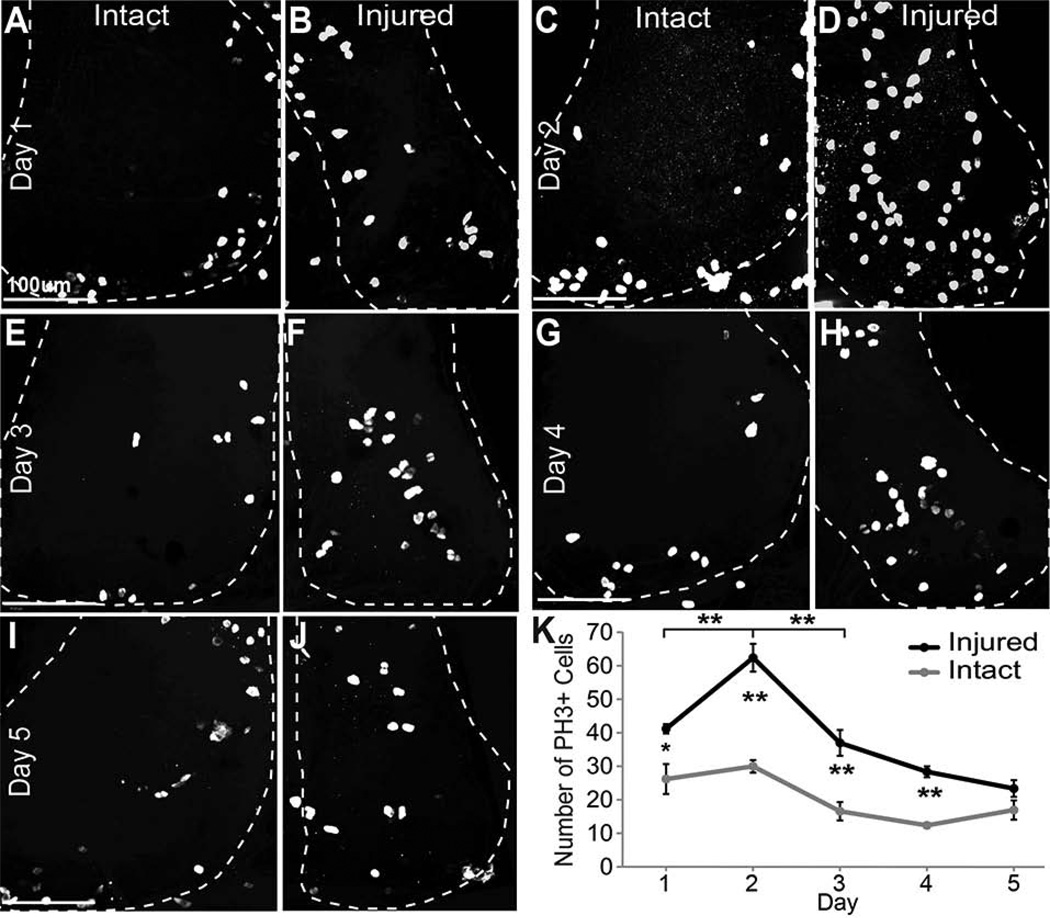
Figure 4. Phospho-Histone 3 immunoreactivity increases after injury.
The right tectal lobe of stage 47 tadpoles was injured and changes in cell division were assessed in the right and left tectal lobes by immunolabeling for phospho-histone H3 (PH3) to identify dividing cells in M phase of the cell cycle over the course of 5 days. Data were analyzed as individual optical sections, but are presented as confocal Z-projections of the tectal lobes, which are outlined. A–J. PH3 labeling of dividing cells in the tectal lobes 24hrs (A,B), 48 hrs (C,D), 3 days (E,F), 4 days (G,H), and 5 days (I,J) after injury in the intact left tectal lobe (A,C,E,G,I) and in the injured right tectal lobe (B,D,F,H,J) of the same animals. K. Total counts of PH3-labeled nuclei in the injured (black line) and intact (gray line) tecta over a 5-day period after injury. N=25 animals total (5 for each time point). *p<0.05, **p<0.01, n.s. = not significant. Scale bars, 100 µm. Data are average ± SEM.
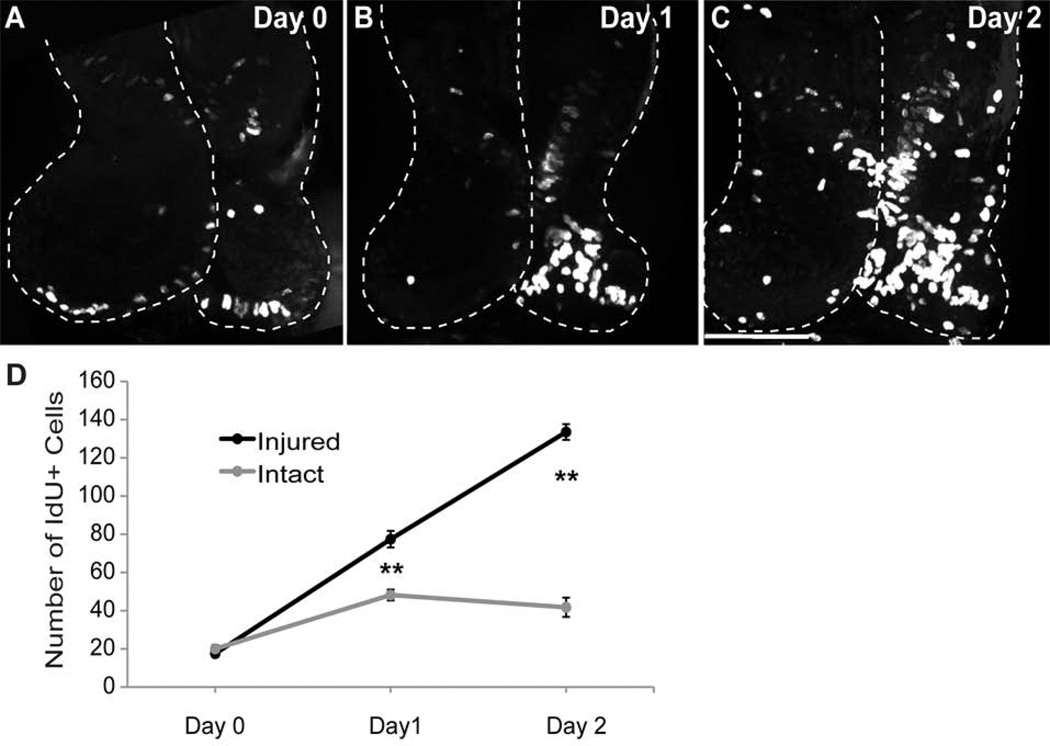
Figure 5. Cell Proliferation increases after injury.
The right tectal lobe of stage 47 tadpoles was injured and changes in cell proliferation were assessed by exposing animals to IdU for 2 hrs. Data were analyzed as individual optical sections, but are presented as confocal Z-projections of the entire tectum from whole-mount brains. A-C. IdU incorporation in the tectum 2 hrs after injury (A), 24hrs after injury (B), and 48 hrs after injury (C). D. Total cell counts of IdU-positive cells per tectal lobe in the injured vs. intact tectum over a 48 hour period after injury. N=15 animals total (5 for each time point), **p<0.01. Scale bar, 100 µm. Data are average ± SEM.
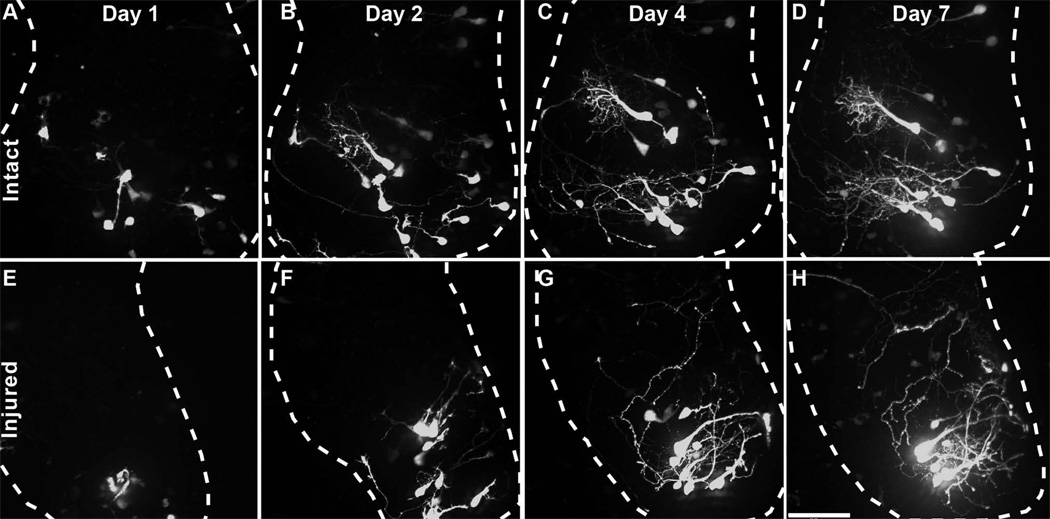
Figure 8. Tectal cells generated after injury differentiate into neurons.
In vivo time-lapse images of tectal cells labeled by expression of turboGFP in Sox2-expressing neural progenitors cells at the time of injury. Time-lapse images of the uninjured (A–D) and injured (E–H) tecta collected 1, 2, 4, and 7 days after injury are shown. Scale bar is 70µm.
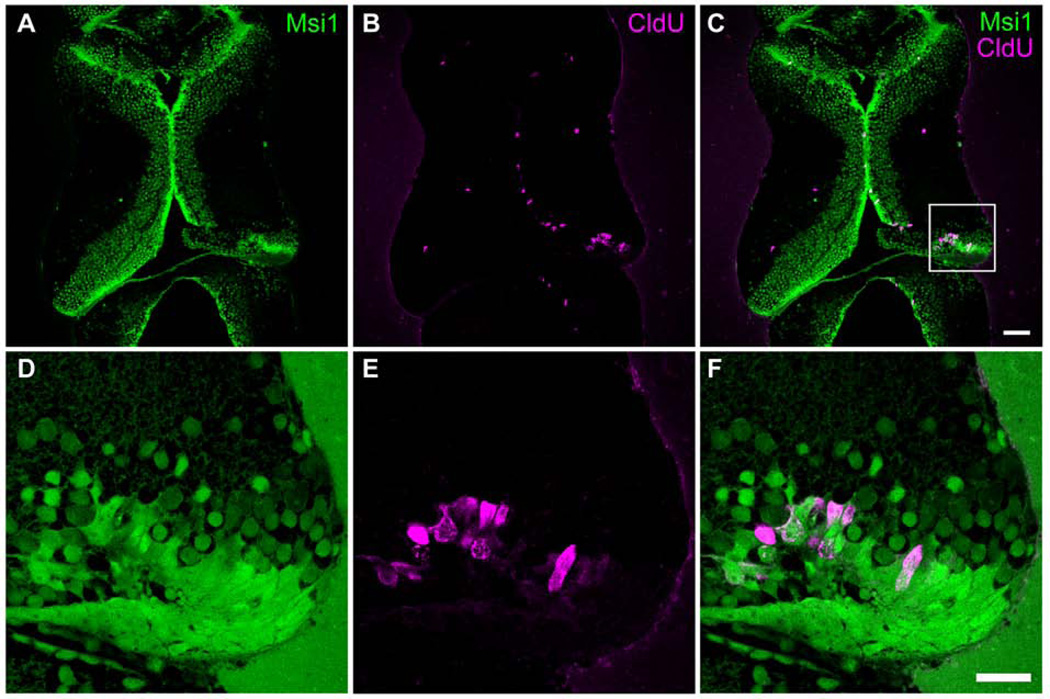
Figure 6. Proliferating cells are musashi1-expressing neural progenitor cells.
Stage 47 tadpoles were injured in the right tectum. Two days later animals were exposed to CldU for 2hrs and processed immediately for CldU immunolabeling. A–F. Representative single optical sections from confocal images of 30 µm sections through the optic tectum of tadpoles labeled with antibodies to musashi1 (Msi1, green) and CldU (red). D–F show enlargements of the boxed area marked in C. 93.3 ± 2.5% of CldU-positive cells in injured tectal lobes were double-labeled with musashi1 antibodies (n=7 animals). Note that the injury is restricted to the tectal cell body layer and the neuropil remains intact.
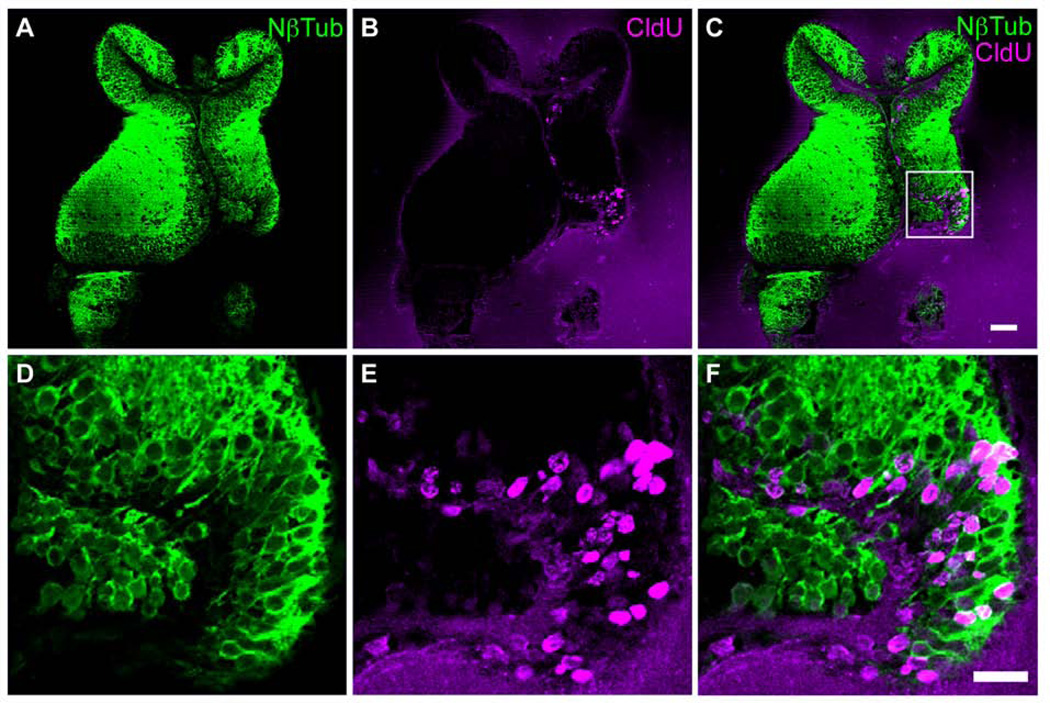
Figure 7. Injury increases the generation of new neurons.
The right tectal lobe of stage 47 tadpoles was injured. Two days later tadpoles were then exposed to CldU for 2hrs in rearing solution and were allowed to develop in the absence of CldU for 2 days. A–F. Representative images of single optical sections of N-β-tubulin-(green; A,C,D,F) and CldU- (red; B,C,E,F) immunoreactivity in 30 µm vibratome sections. D–F show enlargements of boxed area in C. Scale bar in C = 50 µm, E = 20 µm. Note that the injury is restricted to the tectal cell body layer and the neuropil remains intact.
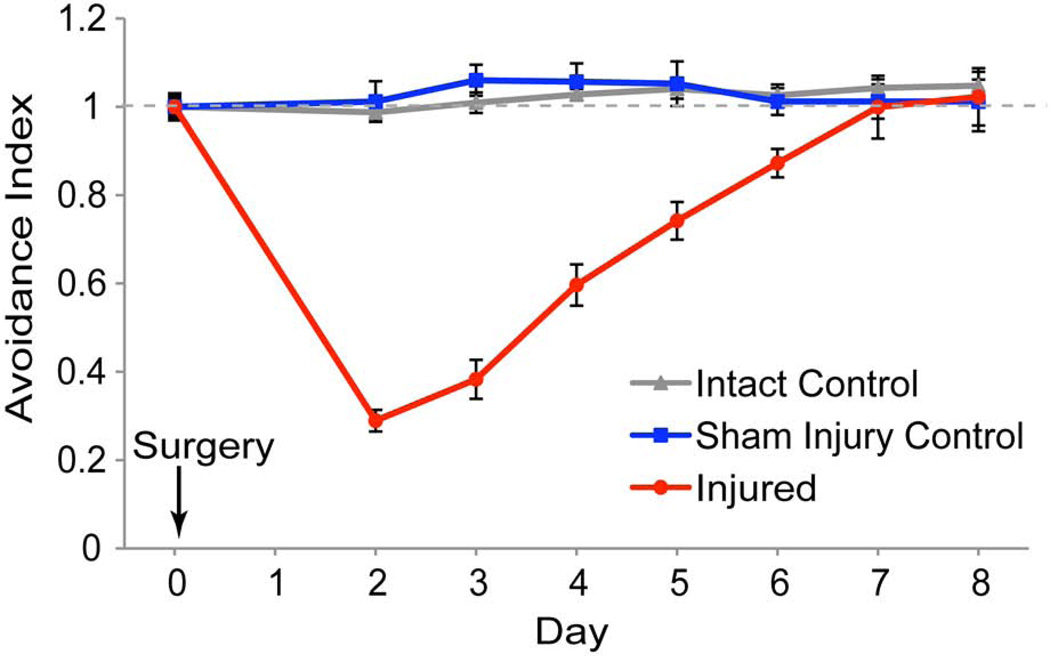
Figure 3. Recovery of visual avoidance behavior after local injury to the optic tectum.
Time-course of recovery of visual avoidance response. Stage 47 tadpoles were assayed for baseline avoidance behavior (Day 0), subjected to injury, and assayed for recovery of the visual avoidance behavior every day starting 2 days after surgery (red line). Animals gradually recover the visual avoidance behavior over 6–8 days. Sham injured animals (blue line) were subjected to anesthesia and to needle penetration without vacuum aspiration. Control animals (grey line) were not subjected to injury and serve as a control for overall clutch health. Data are average ± SEM. ** p<0.01.
Surgical Injury
Stage 47 tadpoles were screened for the OMR as an indicator of general health (Dong et al., 2009; Portugues and Engert, 2009; Roeser and Baier, 2003; Shen et al., 2011) and then screened for visual avoidance behavior. Only animals that exhibited both behaviors were used in experiments. Animals were first tested on Day 0 for a baseline Avoidance Index (Supplementary Movie 1). For surgery, tadpoles were anesthetized with 0.02% MS222, and placed on a Kimwipe moistened with MS222 on a stage of a dissecting microscope. A 1mm diameter glass micropipette pulled into a fine tipped needle with a 30–40 µm diameter opening was connected to a vacuum with a mild suction and mounted in a micromanipulator. The micropipette tip was positioned perpendicular to the surface of the caudal region of the right tectal lobe and was advanced into the cell body layer, deep to the neuropil to avoid the retinal ganglion cell (RGC) axons which laminate the superficial layers of the tectum. We applied a brief pulse of vacuum aspiration to remove neuronal cells from the tectum, taking care not to disrupt either the ventricular layer of cells or the RGC axons. Injuries were inflicted only to the right tectal lobe. The extent of injury was determined by imaging the dorsoventral extent of both tectal lobes labeled with a lipophilic dye (1mM FM4–64, Invitrogen) injected into the brain ventricle. Confocal stacks were collected before and after surgery and the tectal volume was determined using Volocity software. We determined the tectal volumes before and after surgery for a subset of animals in each experiment. After a 2-day rest period, animals were re-screened for the OMR to assess general health, visual response, and motor function. Animals that failed to exhibit an OMR were not used. Injured animals with a normal OMR were then tested with the visual avoidance assay as described above (Supplementary Movies 2 and 3). Note that we designed the analysis such that animals are scored for their first 10 encounters with the visual stimulus, thereby making healthy swimming a requirement for animals to be included in the analysis.
Drug Teatments
Cell proliferation was blocked with a bath solution containing 20 mM hydroxyurea and 150 µM aphidicolin (Sigma-Aldrich, St. Louis, MO) (Harris and Hartenstein, 1991). Animals were treated for a 24-hour time period from 24–48hrs after surgery. Control animals were treated similarly with drug vehicle, 0.5% DMSO.
Cell Proliferation Immunolabeling, and Imaging
Proliferating cells were labeled with the thymidine analogues iododeoxyuridine (IdU) for whole-mount single-label, or chlorodeoxyuridine (CldU) for double-labeled sections. Cells were labeled by transferring animals to rearing solution containing 3.8 mM IdU or CldU for 2hrs (Sharma and Cline, 2010). For immunolabeling, tadpoles were terminally anesthetized in 0.2% MS222 solution and fixed with 4% paraformaldehyde in PBS (pH 7.4) overnight at 4°C. Brains were dissected, rinsed, blocked, incubated with antibodies, described below, and analyzed in wholemount or 30 µm horizontal vibratome sections. The antibodies used in this study are listed in Table 1.
Table 1. Primary Antibodies Used in the Study.
| Antigen | Immunogen | Species, type |
Manufacturer, catalog number |
Dilution |
|---|---|---|---|---|
| Phosph-Histone H3 | KLH-conjugated peptide (ARK[pS]TGGKAPRKQLC) corresponding to amino acids 7–20 of human Histone H3 phosphorylated at serine 10 | Rabbit Polyclonal | EMD/Milipore #06-570 | 1:1000 |
| IdU | 5-iodo-2′-deoxyuridine conjugated to ovalbumin | Mouse monoclonal | BD Biosciences #347580 | 1:500 |
| CldU | 5-bromo-2′-deoxyuridine conjugated to ovalbumin | Rat | Accurate/AbSerotec OBT0030G | 1:400 |
| Musashi1 | First 25 aa of human Musashi1 | Rabbit polyclonal | Abcam ab21628 | 1:250 |
| β-tubulin I+II | Carboxy-teminal sequence (EEEGEEDEA) of the human β-tubulin isotype II coupled to BSA | Mouse monoclonal | Sigma T-8535 | 1:200 |
For both IdU and CldU labeling, brains were treated with 2N HCl for 1 hr at 37°C, rinsed in PBS-Tween 20 (0.3%) and blocked in 5% normal goat serum in PBS-Tween 20 (0.3%) for 1 hr before incubating overnight at 4°C with antibody. For CldU labeled sections, dissected brains were embedded in gelatin and cut into 30 μm horizontal sections with a vibratome. Sections were blocked in 5% goat serum and 0.3% Tween 20 in PBS for 1 hr before incubating overnight with antibodies. Detection was performed using Alexa-fluor-tagged secondary antibodies (1:200, Molecular Probes/Invitrogen, Eugene, OR). Sections were mounted in ProLong™ Gold (Molecular Probes/Invitrogen, Eugene, OR) and imaged with an Olympus FluoView FV500 laser-scanning confocal microscope as described above. Whole-mount brains labeled with IdU were mounted in Vectashield (Vector Labs, Burlingame, CA) and imaged with an Olympus BX61WI fitted with a Perkin-Elmer Ultraview VOX spinning disk confocal attachment as described above. All samples were prepared, photographed and analyzed in parallel using the same acquisition and analysis settings. Image analysis and fluorescently-labeled cell counts for the whole-mount data (Figures 4 and 5) were done in 3D using Volocity (Perkin-Elmer, Waltham, MA) image processing software to mark cells in optical sections, ensuring each cell was counted only once. Image analysis and cell counts for the CldU double-label experiments in vibratome sections (Figure 6 and 7) were performed using Metamorph (Universal Imaging Corporation/Molecular Devices, Downingtown, PA). Images were background subtracted and cells were counted in single optical sections the using the “manually count object” feature of Metamorph as described (Sharma and Cline, 2010). For N-beta tubulin analysis, counts were made in single optical sections from the top and bottom of vibratome section due to poor antibody penetration specific to this antibody. Two non-neighboring horizontal sections through each optic tectum were used for analysis. Data were determined to be normally distributed. Either the Student’s T-test (2 sample, 2 tailed) or the non-parametric Mann-Whitney-Wilcoxon test was used to compare between groups as stated. Data are represented as mean ± SEM.
Antibody Characterization
A list of primary antibodies used in this study is provided in Table 1.
Anti-PH3
The antibody against phospho-histone H3 (PH3) was obtained from Millipore (Billerica, MA, Catalog # 06-570; Journal of Comparative Neurology Database and Neuroinformatics Framework antibody registry # 310177). This antibody was raised against the synthetic phosphorylated peptide ARK[pS]TGGKAPRKQLC coupled to keyhole limpet hemocyanin, according to the manufacturer's information. This sequence corresponds to the N-terminus of human histone H3 (aa7-20) phosphorylated at serine 10. The manufacturer tested the specificity of this antibody by western blot and found that it recognized a single band of 17KDa in colchemid-arrested HeLa cells as predicted. This antibody has been reported to detect mitotic cells in several species, including the optic tectum of chick, fish, and xenopus where staining is concentrated in mitotically active nuclei that line the ventricular layer of the tectum, similar to the expression we report (Schmidt and Derby, 2011; Tibber et al., 2006; Wirsching et al., 2012). We found that the antibody labels select nuclei in the Xenopus tadpole tectal proliferative zone that display morphological features of nuclei in various phases of mitosis.
Anti-IdU
The mouse monoclonal antibody against IdU was obtained from BD Biosciences (San Jose, CA, Catalog # 347580; Journal of Comparative Neurology Database and Neuroinformatics Framework antibody registry # 400326). This antibody was raised against 5-iodo-2′-deoxyuridine conjugated to ovalbumin and binds to 5-bromo-2′-deoxyuridine as well as 5-iodo-2′-deoxyuridine, according to the manufacturer's information. Several studies have shown that this antibody specifically labels nuclei that have incorporated BrdU or IdU into their newly synthesized DNA during the S-phase of the cell cycle (Schmidt and Derby, 2011). We have previously shown that this antibody labels cells in the proliferative layer of the Xenopus optic tectum in animals that have been exposed to BrdU or IdU, and that no labeling is present in the brains of animals that have not been exposed to BrdU or IdU (Sharma and Cline, 2010).
Anti-CldU
The rat antibody against CldU was obtained from Accurate Chemical (Westbury, NY, Catalog # OBT0030G (also available from Ab Serotec, Oxford, UK); Journal of Comparative Neurology Database and Neuroinformatics Framework antibody registry # 609568). This antibody was raised against 5-bromo-2′-deoxyuridine conjugated to ovalbumin and binds to 5-bromo-2′-deoxyuridine incorporated into single stranded DNA, attached to a protein carrier and free BrdU, as well as 5-chloro-2′-deoxyuridine but does not cross-react with 5-iodo-2′-deoxyuridine, according to the manufacturer's information. We have previously shown that this antibody labels cells in the proliferative layer of the Xenopus optic tectum in animals that have been exposed to BrdU or CldU (Sharma and Cline, 2010). Additionally, we demonstrated anti-CldU specificity by showing that this antibody shows no labeling in Xenopus tadpoles exposed to IdU (Sharma and Cline, 2010), consistent with other studies in the literature (Sullivan et al., 2007).
Anti-musashi1
The rabbit antibody against Musashi1 (Msi1) was obtained from Abcam (Cambridge, MA, Catalog # ab21628). This antibody was generated against a KLH-conjugated synthetic peptide corresponding to a region within the first 25 amino acids of the human Msi1 protein. On immunoblots of mouse embryonic brain tissues, human neuroblastoma cell lines, and mouse neural progenitor cells, the antibody recognizes a 39KDa band corresponding to the expected size of Msi1, which can be blocked with the immunizing peptide, according to the manufacturer. In tissues, the antibody shows greater labeling in proliferative cells and less labeling as cells differentiate and mature, and has similar localization pattern to the 14H1 anti-Msi1 antibody used in Kaneko et al (Kaneko et al., 2000). The Xenopus homolog of musashi1 is 84% identical and 92% similar to the human musashi1 within the first 25 amino acids against which the antibody used here was generated. Our previous work demonstrates that musashi1-immunoreactive cells are present in Xenopus in the proliferative layer lining the ventricle of the optic tectum (Sharma and Cline, 2010). Moreover, we demonstrated that morpholino-mediated knockdown of the Xenopus homolog of Msi1 results in a significant decrease in Msi1-immunoreactivity compared to controls (Sharma and Cline, 2010). Our current data show an expression pattern similar to what we had previously reported.
Anti-β-tubulin I+II
The mouse monoclonal antibody against β-tubulin I+II was obtained from Sigma-Aldrich (St. Louis, MO, Catalog # T8535), derived from the JDR.3B8 hybridoma cell line originally generated by Banerjee et al (Banerjee et al., 1988). The antibody was generated against a chemically synthesized peptide corresponding to the carboxy-terminal sequence of the human β-tubulin isotype II (EEEGEEDEA) coupled to BSA, which specifically recognizes an epitope located on the isotypes I and II of human β-tubulin and cross-react with bovine, chicken, mouse, pig, rat, and frog, according to the manufacturer. The antibodies have been used to affinity purify β-tubulin isotypes I+II from chick brain extracts (Banerjee et al., 1988). Immunoblotting shows a band of the predicted size of 53KDa in rat brain extracts, bovine brain MAPs extracts, chicken brain preps, and chicken fibroblasts cell lines, and immunofluorescent staining localizes isotypes I and II in human and chicken fibroblasts, according the manufacturer. The Xenopus Class II β-tubulin is 99% identical to the chicken β2-tubulin over the length of the protein and highly conserved at the JDR.3B8 epitope site (Good et al., 1989; Moody et al., 1996). On immunoblots of Xenopus extracts from stages 37, 43, 47, and adult brain, this antibody gives a single band of the expected 53KDa molecular weight (Moody et al., 1996). This antibody has been used in Xenopus to label mature neurons (Huang et al., 2007) and we have previously shown that labeling with this N-β-tubulin antibody was enriched in the layers of the tadpole optic tectum occupied by neurons, but not detected in the proliferative layer (Sharma and Cline, 2010). The staining pattern shown in the current study is consistent with expected results based on previously published reports.
In Vivo Time-lapse Imaging of Optic Tectal Neurons
Stage 47 animals were anesthetized in 0.02% MS222, and injured in the right tectum, as described above, and then immediately electroporated with the fluorescent protein (FP) expression construct, pSox2bd::gal4UASturboGFP, described in detail (Bestman et al., 2012). The pSox2bd::FP construct drives gal4UASturboGFP expression in Sox2-expressing neural progenitor cells because protein expression requires endogenous Sox2/Oct3-4 transcription factors to bind to Sox2/Oct3-4 binding site in the miniFGF4 promotor (Bestman et al., 2012). For electroporation, plasmids (0.02µg/µl) were injected into the midbrain ventricle, and voltage pulses were applied with a Grass SD9 stimulator across the midbrain using platinum electrodes (4 pulses of 35V in each polarity) to electroporate cells lining the tectal ventricle (Bestman et al., 2012; Haas et al., 2002). Starting the next day, animals were anesthetized and the left and right optic tectal lobes were imaged once a day over the next 7 days with a Perkin Elmer spinning disk confocal as described above (Bestman et al., 2012).
Retinotectal Axon Imaging
Animals were subjected to unilateral injury in the right tectal lobe and then euthanized and fixed within 1 hour of injury in 4% paraformaldehyde in PBS. After 24hr of fixation, retinal ganglion cells were labeled with DiI by pressure injecting DiI solution (Vybrant DiI from Invitrogen/Life Technologies, V-22885, used according to manufacturers recommendations) into the contralateral retinas of stage 47 tadpoles, as described (Witte et al., 1996). Control animals were uninjured. After 3 weeks tecta were imaged as described above.
Results
Xenopus tadpoles exhibit an avoidance response to moving stimuli
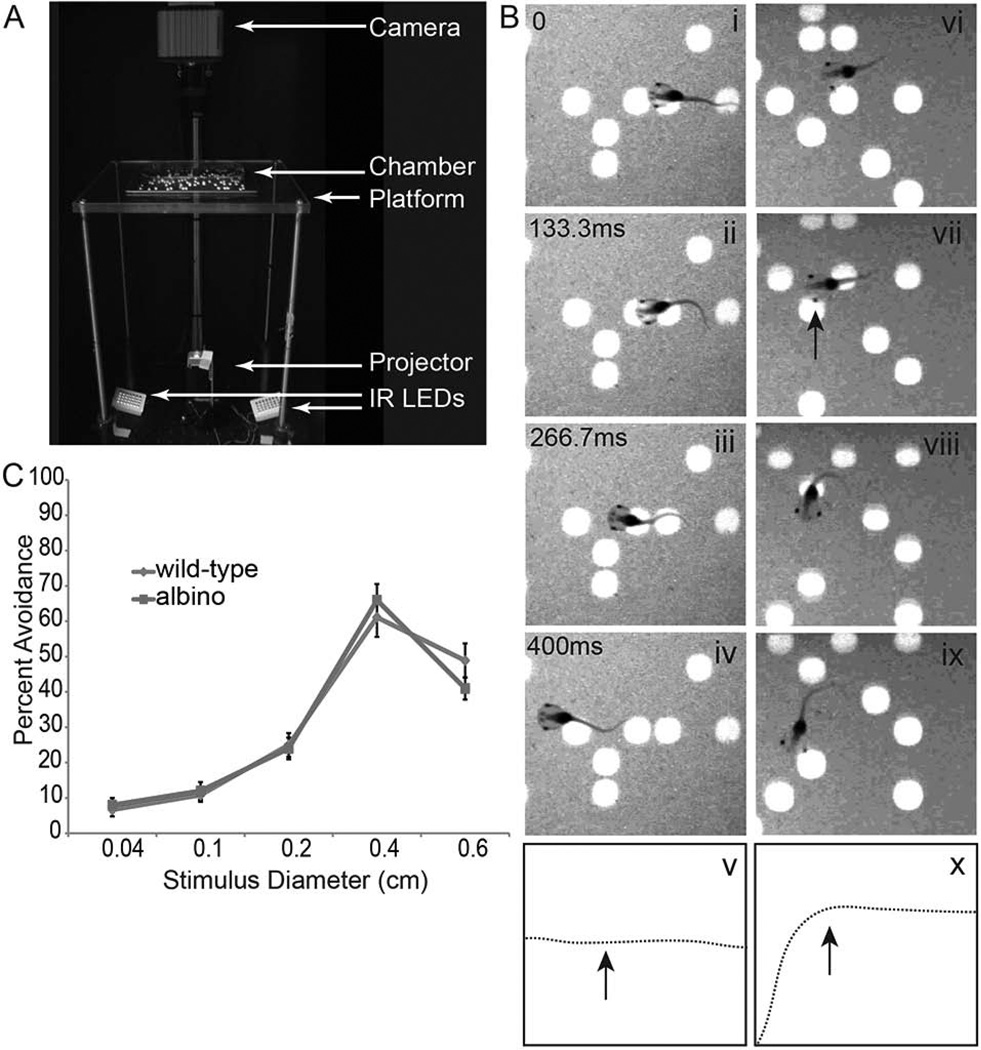
Xenopus laevis tadpoles display an innate avoidance behavior to moving objects. This behavior is mediated by the optic tectum, a region of the midbrain that receives and processes visual input. We used a behavior assay modified from Aizenman and colleagues (Dong et al., 2009), described in detail in Shen, et al (Shen et al., 2011). Briefly, animals are placed in a clear Plexiglass chamber fitted with a piece of projection film; a projector below the chamber displays a stimulus and a camera above the chamber records both the movement of the animals and the movement of the stimuli (Figure 1A). The behavior assay tests whether swimming animals avoid visual stimuli by determining their swimming trajectory in response to visual stimuli. Animals turn in response to moving spots of certain sizes that approach their eyes at approximately a right angle. Our analysis scores the first 10 encounters that swimming animals have with the visual stimuli, thereby making healthy swimming a requirement to be included in the analysis. Data are presented as an Avoidance Index, or the fraction of times that individual animals respond to the stimulus out of 10 encounters with the moving spot stimuli. Animals do not react to stationary stimuli (Figure 1B, i–v) and only change their trajectory in response to an encounter with a moving stimulus (Figure 1B, vi–x, Movie 1). Both wild-type and albino tadpoles preferentially avoid moving spots corresponding to diameters larger than or equal to 0.4 cm (Figure 1C). We therefore presented stimuli of 0.4 cm diameter and used albino tadpoles for all subsequent experiments.
Figure 1. Xenopus laevis tadpoles exhibit an avoidance response to moving stimuli.
A. Visual avoidance behavior apparatus. Animals are placed in the chamber and a moving stimulus is presented from below. Video images are captured from above. B. Still frames of a video sequence showing stage 47 tadpoles’ behavioral response to the upward moving stimulus (66.67 ms/frame, every other frame is shown). Tadpoles do not respond to stationary dots (B, i–iv, left panels) and swim in a straight trajectory, as shown in the drawing (B, v). In contrast, tadpoles exhibit an avoidance behavior in response to moving stimuli (B, vi-ix, right panels) and abruptly change their trajectory upon an encounter with a perpendicularly approaching dot, indicated by arrow in the drawing of the swim trajectory (B, x). Time stamps shown in panels Bi-iv also correspond to Bvi-ix. C. Both pigmented and albino tadpoles similarly display a strong avoidance behavior to a stimulus size of 0.4cm in diameter (n=12 animals each).
Developing an injury paradigm in the Xenopus optic tectum
We set out to establish an injury model in which postsynaptic cells in the Xenopus optic tectum were damaged, and in which we could assess recovery of function using the visual avoidance assay. Complete ablation of the optic tectum prevents the visual avoidance behavior (Dong et al., 2009), therefore we tested whether partial damage to the tectum, inflicted by gentle vacuum aspiration to remove tectal neurons unilaterally (Figure 2A) might compromise the visual avoidance behavior. This method causes minimal bleeding, inflammation, infection, and death and results in consistent damage. After surgery, animals swim normally and display an optomotor response (Dong et al., 2009; Portugues and Engert, 2009; Roeser and Baier, 2003), indicating that they are generally in good health, that the surgery did not damage the retinal ganglion cell outputs in the non-tectally-mediated visual system and that the animals swim normally. The region of damage could be seen if the brain was imaged with DIC optics immediately after surgery (Figure 2B,C). To assess the damage in the tectal lobe and to determine whether the uninjured lobe showed volume changes in response to damage to the contralateral tectum, we labeled the brain with the fluorescent membrane dye, FM4-64 and collected confocal images of live anesthetized animals before and after surgery. The surgical injury decreased the volume of the damaged tectal lobe (18.5 ± 1.5%) without changing the volume of the undamaged tectal lobe (Figure 2D–F). DiI injections into the retina contralateral to uninjured or injured tecta show that the retinotectal axons are not damaged by the surgery (Figure 2G–H).
Xenopus tadpoles recover visual avoidance behavior
We next tested whether partial tectal injury affected the visual avoidance behavior and whether animals could recover from injury. On the first day of the experiment (Day 0), stage 47 tadpoles were tested for visual avoidance behavior (Movie 1) and then immediately subjected to unilateral tectal injury. Animals were allowed 2 days for recovery and then were subjected to an optomotor test to assess their general health and response to visual motion. Only animals that passed the optomotor test were studied further (Table 2). Animals were tested in the visual avoidance behavior assay once a day from Day 2 (Supplemental Movie 2) until Day 8 (Movie 3) and results were analyzed blind to treatment (Figure 3). For each animal, the first 10 encounters of swimming animals with a dot were scored, further ensuring that animals were healthy and actively swimming to be scored. The visual avoidance responses for Days 2–8 were normalized to the visual avoidance response on Day 0, and data are graphed as an Avoidance Index (Figure 3). Raw (non-normalized) data are shown in Table 1. Intact control animals (no injury) show no change in behavior over the course of the experiment and are used as a general indicator of clutch health (Figure 3, gray line). Two days after surgery, the avoidance response was significantly impaired in injured animals, such that the Avoidance Index was reduced by 80% (0.19 ± 0.03) of the pre-injury response (Figure 3, red line, Movie 2). Injured and control animals spent comparable times swimming. We analyzed a representative set of videos from the dataset shown in Figure 3 and found that on Day 2 (2 days after injury), intact animals spent 95.79±2.7% of their time in the assay chamber swimming, while injured animals spent 94.48±1.9% of their time swimming (n=10 for each group, p>0.5). Moreover, on Day 3 intact and injured animals were actively swimming for 95.82±3.4% and 91.22±5.2% of their time in the chamber, respectively (p>0.5), indicating that the impaired avoidance index was not due to decreased time spent swimming. As shown in figure 3, injured animals gradually recovered the visual avoidance response over the course of 7–8 days (Figure 3, Supplemental Movie 3). Sham operated animals, which were exposed to anesthesia and penetrated with a closed needle without vacuum aspiration, showed no change in Avoidance Index over the course of the experiment, indicating that neither anesthesia nor needle penetration alone affect behavior (Figure 3, blue line).
Cell proliferation increases after injury
The experiments described above show that focal tectal injury significantly impairs visual avoidance behavior and that animals recover the behavioral response over the course of a week. We hypothesized that recovery from injury is based on the generation of new neurons, which then integrate into the damaged circuit and result in recovery of function. To test this hypothesis, we first determined whether cell proliferation increases after damage to the optic tectum. Injured animals were assayed for the cell proliferation marker phospho-histone H3 (PH3), which labels actively dividing cells in the mitotic phase of the cell cycle. Surgically injured animals were fixed and their brains were processed for anti-PH3 immunofluorescence and imaged as whole-mounts.
Analysis of individual confocal sections shows that PH3-immunoreactive cells are located in the ventricular layer, as expected. Images were analyzed as individual confocal sections, but are presented in Figure 4 as Z-projections of the entire tectum. The apparent widespread distribution of PH3-positive cells in the flattened Z-projection image of the whole mount tectum is due to the curvature of the ventricular layer (see (Bestman et al., 2012)). We counted the total number of PH3-positive cells in both the injured right tectum and the intact control left tectum. Consistent with our previous data showing a developmental decrease in cell proliferation (Sharma and Cline, 2010), the number of PH3-positive cells in control animals decreases over the course of the experiment (Figure 4A–D, K). Within 1 day after injury, PH3-positive cells were already significantly increased in the injured tectal lobes compared to the intact tectal lobes (p<0.05, Figure 4A, B, K). Two days after surgery the numbers of PH3-immunoreactive cells in the injured tectum had increased further, and were significantly greater than the number of PH3-positive cells in the intact tectum (p<0.01) (Figure 4C, D, K). The significant increase in PH3 labeling in the injured tectum, compared to the intact tectum, persisted until 4 days after injury (p<0.01, Figure 4E–H, K). By 5 days after injury, the numbers of PH3-positive cells in the injured tectum had decreased and were indistinguishable from the uninjured tectum (p=0.13) (Figure 4I, J, K). Within the injured tectum, the number of proliferating cells increased significantly between days 1 and 2 (p<0.01) and decreased significantly between days 2 and 3 (p<0.01), however the number of proliferating cells are not significantly different between days 3, 4 and 5.
As an independent means to detect and quantify cell proliferation, we labeled proliferating cells in S phase by exposing injured animals to the thymidine analogue IdU for 2 hr immediately following surgery or at either 24 or 48 hr after surgery and compared the IdU labeling between injured and intact tectal lobes in the same animal. Animals were fixed after the 2hr exposure period and their brains were processed for anti-IdU immunofluorescence and imaged as whole-mounts. Images were analyzed as individual confocal sections, and are presented in Figure 5 as as Z-projections of the entire tectum. Two hours after surgery, the tectal lobes showed relatively little IdU labeling and the extent of labeling was comparable between the injured and intact tectal lobes (p=0.4, Figure 5A, D). By contrast, one day after surgery, IdU incorporation was significantly greater in the injured tectal lobe compared to the intact contralateral tectal lobe (p<0.01, Figure 5B, D), indicating that the injured tectal lobe has a higher rate of cell proliferation. The increase in cell proliferation persisted such that two days after surgery, a 2-hour exposure to IdU resulted in a significantly greater number of IdU-labeled cells in the injured tectal lobe compared to the intact tectal lobe (p<0.01, Figure 5C, D). Together with the PH3 immunolabeling data, these results show that cell proliferation increases within a distinct 1–3 day time period after damage to the optic tectum and suggests that increased cell division of tectal stem cells may play a key role in recovery from injury which occurs over the following week.
Proliferating cells are neural progenitors
To determine the identity of the cells that proliferate in response to injury, we tested whether cells that incorporate CldU are neural progenitor cells. We had previously shown that neural progenitor cells in the developing optic tectum are Musashi1- immunoreactive radial glial cells (Bestman et al., 2012; Sharma and Cline, 2010). Musashi1 (Msi1) is an RNA binding protein that is enriched in neural progenitor cells and is both necessary and sufficient for maintenance and proliferation of the neural progenitor pool (Kaneko et al., 2000; Sharma and Cline, 2010). To determine whether the proliferating cells are neural progenitor cells, we exposed injured animals to CldU 2 days after surgery, when we observe the peak increase in dividing cells (Figures 4 and 5). Following 2 hr exposure to CldU, animals were fixed, sectioned, and immunostained for CldU and Musashi1. Consistent with the data presented in Figures 4 and 5, we find a significant increase in CldU-labeled cells in the ventricular proliferative zone in the injured tectal lobe compared to the intact contralateral tectal lobe (Figure 6A–C). Sections through the injured tecta had about 4 times as many CldU-labeled cells compared to the uninjured tecta (18.5±3.4 CldU-labeled cells/section in injured tecta compared to 4.7±1.3 CldU-labeled cells/section in the contralateral tecta. p<0.01, n=21 animals). Furthermore, 93.3 ± 2.5 % of the CldU-labeled cells in the injured tectum are Musashi1-immunoreactive (12.7±1.1 CldU+Msi1+ cells/section out of 13.6±1.2 total CldU+ cells/section, n=7 animals). These experiments indicate that the majority of proliferating cells in the injured tectum at 48 hours after injury, when we observe the largest increase in proliferation, are Musashi1-immunoreactive neural progenitor cells.
Injury-responsive neural progenitor cells generate neurons
We next investigated whether the injury-induced neural progenitors differentiate into neurons. We exposed injured animals to CldU in their rearing solution for 2hrs starting 48 hours after injury and then transferred animals to fresh rearing solution for 2 days, after which they were fixed and their brains were sectioned. To identify the fate of CldU-labeled progeny of neural progenitor cells, we double labeled sections with antibodies to CldU and the neuronal marker N-β-tubulin, and counted the numbers of immunolabeled cells. We found that 74.1±4.6% of the CldU-labeled cells were also immunoreactive for N-β-tubulin (Figure 7A–C), indicating that the majority of the injury-induced proliferating cells differentiate into neurons within 2 days. In fact, significantly more of the CldU-labeled progeny in injured tectal lobes expressed the neuronal marker N-β-tubulin compared to the contralateral intact tectum (74.1±4.6% in injured tecta vs. 58.1±9.0% in contralateral tecta, p<0.01; Figure 7A–C), suggesting that injury increases the rate at which cells differentiate into neurons. Together, these data indicate that injury increases proliferation of neural progenitor cells preferentially in the injured tectum and that injury affects the fate of neural progenitor cells in the injured tectum so that they generate more neuronal progeny in response to the injury.
To test whether newly generated cells in the injured tectum differentiate into neurons that extend dendritic arbors, animals were injured in the right tectum and then both tecta were immediately electroporated with the pSox2bd::gal4UASturboGFP expression vector (Bestman et al., 2012). The pSox2bd::FP construct drives gene expression in Sox2-expressing neural progenitor cells because protein expression requires endogenous Sox2/Oct3-4 transcription factors to bind to Sox2/Oct3-4 binding site in the miniFGF4 promoter (Bestman et al 2012). Once expressed, the turboGFP persists in the cells and permits visualization of the structure of neuronal progeny (Bestman et al., 2012). Animals were imaged over 7 days after electroporation and injury. The day after injury, turboGFP- (tGFP) expressing cells can be seen in both the intact and injured tecta (Figure 8A, E). Two days after injury, labeled cells in the uninjured tecta have both radial glia morphology, characteristic of neural progenitors (Bestman et al., 2012), and neuronal morphology, identified by elaborate dendritic arbors (Figure 8B), whereas the majority of tGFP-expressing cells in the injured tectum have radial glial neural progenitor morphologies (Figure 8F). Images collected 4 days after injury show that tGFP-expressing cells have differentiated into neurons with elaborate dendritic arbors (Figure 8C, G). The same neurons can also be seen in images collected 7 days after injury (Figure 8D,H). Although the labeling method does not distinguish between injury-induced neurogenesis and ongoing neurogenesis, these data indicate that neural progenitor cells labeled at the time of injury generate mature neurons with complex dendritic arbors over the period corresponding to the timecourse of behavioral recovery.
Cell proliferation promotes recovery of visual behavior after injury
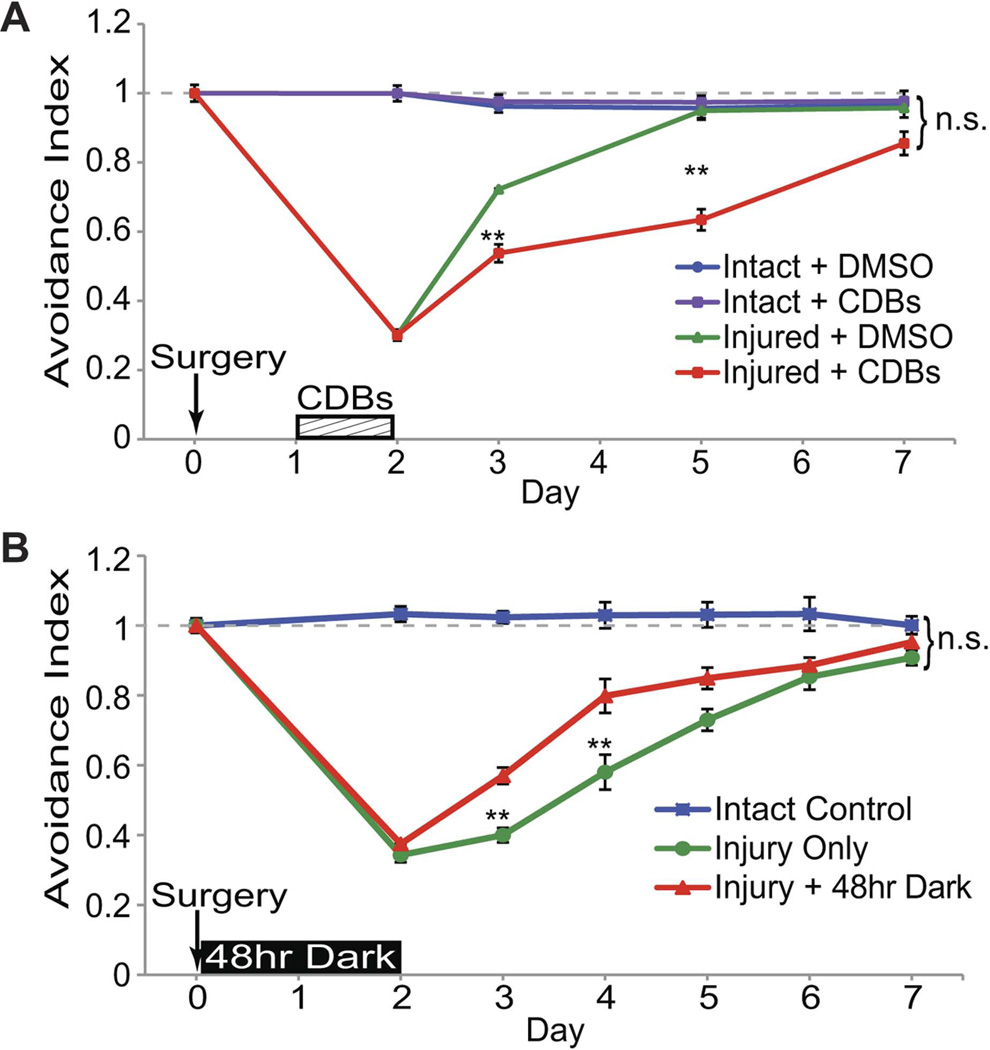
Recovery of the visual avoidance behavior after injury could result from reorganization of remaining circuitry or from the generation of new neurons and their integration into the tectal circuit. The data presented above indicate that new neurons are generated in response to injury. To test whether cell proliferation is necessary for recovery from injury, injured animals were exposed to a cell division blocker cocktail of hydroxyurea and aphidicolin (CDBs). This combination of cell division blockers has been shown to block cell proliferation in intact Xenopus tadpoles at earlier stages (Harris and Hartenstein, 1991). We find that exposure of stage 47 tadpoles to cell division blockers for 24hrs in their rearing solution decreases PH3-immunolabling by 74% on average compared to stage-matched control animals exposed to the drug vehicle, 0.5% DMSO, alone. The total number of PH3+ cells per tectal lobe is 28±3.1 for DMSO control vs. 7.2±0.73 for CDB-treated animals, p<0.01, n=8 animals (16 tectal lobes) for each condition. Animals were treated with cell division blockers for 24hrs at the peak of cell proliferation, 48–72 hrs after injury, and then tested for the visual avoidance response over the next week (Figure 9A). Exposure to cell division blockers for 24hrs had no effect on the visual avoidance response in uninjured animals, suggesting that blocking cell proliferation and the generation of new cells for a brief period does not interfere with visual circuit function with respect to the visual avoidance behavior (Figure 9A, blue line). Importantly, blocking cell division after injury resulted in a significant delay in behavioral recovery (Figure 9A, red line) compared to recovery of injured animals treated with the drug vehicle, DMSO (Figure 9A, green line). Injured animals treated with cell division blockers for 24hrs from 2–3 days after injury eventually recover the visual avoidance behavior by the end of the experiment. Because the cell division blockers act globally, either extending treatment time or delaying the period of treatment killed the animals, partially due to an effect on hematopoiesis. Nonetheless, these data indicate that tectal cell proliferation and the generation of new neurons promote behavioral recovery from injury.
Figure 9. Regulation of neural progenitor cell proliferation affects rate of recovery of visual behavior.
Stage 47 tadpoles were assayed for baseline avoidance behavior and subjected to injury (Day 0), then assayed for recovery. A. Animals were treated with cell division blockers (CDBs, red) or DMSO vehicle (green) for 24hrs from Day 2 to Day 3, then assayed for recovery of visual avoidance behavior. Injured animals treated with CDBs show a delayed recovery compared to injured animals treated with DMSO vehicle alone. CDBs have no effect on visual avoidance behavior in intact animals (purple). Control uninjured animals (blue) exposed to DMSO. B. Animals were deprived of visual experience for 48 hrs by placing them in the dark immediately after surgery (red) or were put in the standard 12hr light/dark conditions (green). Injured animals with visual deprivation show an accelerated recovery compared to injured animals housed in a normal 12hr light/dark cycle. All animals recover behavior by the end of the 7-day experiment, regardless of treatment. **p<0.01, data are average ± SEM.
Previous work has shown that maintaining stage 47 tadpoles in the dark for 48h increases cell proliferation in the optic tectum and that subsequent visual experience drives newly generated cells to exit the cell cycle and differentiate into neurons (Sharma and Cline, 2010). To test whether the visual-deprivation induced increase in cell proliferation can enhance the rate of recovery from injury, injured animals were deprived of visual experience by placing them in the dark for 48 hrs immediately following surgery, and then assayed for visual avoidance behavior over the next week (Figure 9B). As for all experiments, behavioral assays and data analysis are done blind to treatment. Animals that are deprived of visual experience for 48 hrs after surgery show a significant increase in recovery of the visual avoidance behavior at 3 and 4 days after injury (Figure 9B, red line) as compared to injured animals that are housed in a normal 12hr light/dark cycle (Figure 9B, green line). These data indicate that an experience-dependent increase in endogenous cell proliferation of neural progenitors is sufficient to improve recovery of visually-guided behavior following brain injury.
Discussion
Nature of the Injury and Reactive Neurogenesis
Brain injuries induce a variety of cellular responses depending on the type of injury, the region and areal extent of injury, the age at injury and species studied (Cernak, 2005; Covey et al., 2010; Endo et al., 2007; Liu and Guthrie, 2011; Stahel et al., 1998; Xiong et al., 2010; Zupanc, 2009). Non-mammalian vertebrates, including amphibia and fish, can regenerate brain tissue after damage and in some cases can recover behavior after injury (Chernoff et al., 2003; Endo et al., 2007; Filoni and Gibertini, 1969; Filoni and Margotta, 1971; Gong and Shipley, 1995; Jordan, 1958; Minelli et al., 1990; Minelli et al., 1987; Srebro, 1957; 1959; Yoshino and Tochinai, 2004; Zhang et al., 2000), suggesting that identification of mechanisms for recovery from injury in non-mammalian species may inform studies in mammalian systems. Zebrafish have been particularly valuable for studies of regeneration in the adult CNS (Zupanc, 2009). Here we tested the potential contribution of neurogenesis and the integration of newly-generated neurons in the recovery of behavior in the developing visual system. Although neurogenesis is known to occur in response to injury in Xenopus (Endo et al., 2007; Ferretti, 2011), studies have not tested whether cell proliferation and differentiation of new neurons are required for recovery of visual function after injury. We used vacuum aspiration to remove a fraction of the tectal cells while avoiding the ventricular layer where neural progenitor cells are located. By inserting the needle perpendicular to the tectal surface, we minimized damage to the retinotectal axons which laminate the superficial layers of the tectum. This caused minimal bleeding and swelling and appears to produce injury principally by removal of tectal neurons. This injury paradigm may be analogous to penetration injuries or surgical removal of part of the brain (Kishimoto et al., 2012; Kroehne et al., 2011; Spencer and Huh, 2008; Stahel et al., 1998), for instance in treatment of epilepsy (Spencer and Huh, 2008). Animals that received closed needle penetration into the optic tectum without removal of cells, which is similar to a stab-wound injury, had no deficit in visual behavior, highlighting the value of a behavioral assessment of injury and recovery. We see no signs of “secondary injury”, where an initial injury triggers swelling, cell death and tissue deterioration, frequently seen in response to contusive or blunt force injuries (Morganti-Kossmann et al., 2001; Stahel et al., 1998). We see no evidence of a glial scar in the tectum, consistent with previous observations that little scarring occurs in Xenopus tadpoles (Gibbs et al., 2011). We also find no evidence of an immune response to injury, as reported in mammals (Davalos et al., 2005; Harry and Kraft, 2012; Nimmerjahn et al., 2005) and in Xenopus tadpoles in response to optic nerve injury (Goodbrand and Gaze, 1991), likely because of the relatively immature immune system in young Xenopus tadpoles (Hsu and Pasquier, 1984; Kim and Szele, 2008). By contrast, a penetrating injury to the telencephalon of adult Zebrafish, which produced significant axonal injury, resulted in secondary edema and reactive proliferation of immune cells at the site of injury, and neurogenic proliferation in the telecephalic ventricular zone (Kishimoto et al., 2012; Kroehne et al., 2011). Finally, the tectal injury in Xenopus tadpoles induces a local proliferative response in the injured tectum, consistent with studies demonstrating a focal signal for proliferation (Kroehne et al., 2011; Zupanc and Ott, 1999), but different from a recent study in Xenopus tadpoles where amputation of the tail induced a widespread increase in sox 2 expression in the CNS (Gaete et al., 2012).
Mechanisms for Recovery from Injury
Two types of mechanisms may contribute to recovery from brain injury: The circuit may regain function as a result of reorganization of neural components remaining after damage (Xiong et al., 2010). An alternate, but not mutually exclusive, mechanism is that newly-generated neurons integrate into the damaged circuit and result in recovery of function. Animals in which injury induces neurogenesis may allow assessment of the relative contribution of circuit reorganization versus neurogenesis for recovery of behavior. Increased cell proliferation is seen in many experimental models of brain injury (Covey et al., 2010; Kernie and Parent, 2010; Kim et al., 2011; Kleindienst et al., 2005; Xiong et al., 2010). Although the increased cell proliferation seen after injury is thought to promote recovery of function following injury (Kernie and Parent, 2010), not all conditions that increase neurogenesis restore lost brain function (Wang et al., 2010). A recent study suggested that blocking injury-induced neurogenesis in adult mouse hippocampus intensified brain-injury induced behavioral deficits in hippocampal-dependent tasks (Blaiss et al., 2011). Our studies support the idea that the generation of new neurons enhances recovery from damage. We showed that blocking cell division during the peak of the injury-induced proliferation slows the rate of recovery from injury, and that visual deprivation for 2 days, which increases proliferation of neural progenitors (Sharma and Cline, 2010), improves the rate of behavioral recovery. Animals treated with drugs to block cell proliferation are still able to recover the visual avoidance behavior, possibly through reorganization of remaining neurons in the visuomotor circuit or a delayed increase in cell proliferation after removal of the cell proliferation blockers. Similar mechanisms may operate in the spinal cord where damage-induced proliferation of Sox2-expressing progenitors appears to be required for regeneration and recovery of swimming behavior (Gaete et al., 2012).
One concern might be that the behavioral deficit arises from damage to retinotectal axons. Clearly severing RGC axons would disrupt the visual circuit and affect behavior. By inserting the needle perpendicular to the tectal surface, we minimized damage to the retinotectal axons which laminate the superficial layers of the tectum. Our imaging experiments show that retinotectal axon arbors do not appear damaged in injured tecta. Finally, cell division blockers would not be expected to affect recovery from local damage to retinotectal axons. Taken together, these data indicate that the RGC axons remain largely intact in our injury model and the injury is successfully targeted to the postsynaptic tectal cell neurons within the visual circuit.
Injured animals treated with DMSO appear to have an increased rate of recovery compared to injury only controls (Table 2). It has been reported that DMSO exhibits beneficial effects in other injury models, however the beneficial effects of DMSO are not seen consistently across studies and there is little consensus about how DMSO might act in the injured brain (Jacob and de la Torre, 2009; Julien et al., 2012). Importantly, our data show that the cell division blockers inhibit the rate of recovery despite the facilitative effects of the DMSO vehicle in which the drug is delivered (Figure 9A and Table 2).
Despite the increase in both proliferation of neural progenitors and the rate of their differentiation into neurons following injury, the injured tectum never fully recovers its original shape or volume. These data indicate that behavior can recover without complete restoration of gross brain structure that was present before injury. This suggests that the tectal circuit may not fully regenerate and furthermore that the newly generated neurons efficiently integrate into and enhance the function of the remaining tectal circuit. We have previously shown that newly-generated tectal neurons integrate into the visual circuit and participate in visual responses (Bestman et al., 2012; Chiu et al., 2008), indicating significant plasticity of the circuit. The data presented here suggest that the damaged circuit retains sufficient plasticity to integrate newly-generated neurons resulting in functional recovery of visually-guided behavior. It is also interesting to note that while the tectal injury was performed unilaterally, animals fail to avoid stimuli approaching either eye, ipsilateral or contralateral to the damage. This suggests that inter-tectal communication that is required for the behavior is disrupted by the injury and subsequently recovers.
Injury-induced Change in Fate of Neural Progenitors
Neural stem cells can divide symmetrically to generate progeny which are both progenitors, or they can divide asymmetrically to generate a progenitor and another cell that will differentiate into a neuron (Gotz and Huttner, 2005). Our in vivo time-lapse imaging of neural progenitors in the Xenopus optic tectum demonstrated that radial glial neural progenitors undergo both symmetric regenerative divisions and asymmetric neurogenic divisions (Bestman et al., 2012). Furthermore, progeny with radial glial morphology can remain proliferative or differentiate into neurons with a delay of up to 2 days before the radial glial cells show morphological evidence of neuronal differentiation (Bestman et al., 2012). This temporal delay in establishing a neuronal fate suggests that cell fate may be susceptible to extrinsic determinants for a limited time. Indeed, our previous work has shown that providing animals with visual experience promotes tectal neuronal differentiation (Bestman et al., 2012). In this study, our data demonstrate that injury produces another external trigger regulating neural progenitor fate: injury transiently increases the number of proliferating neural progenitor cells, suggesting that quiescent progenitor cells change their fate in response to an injury signal by re-entering the cell cycle. In addition, we find that a higher proportion of the CldU-labeled neural progenitor cells differentiate into neurons, suggesting that injury induces a second type of fate change in the neurogenic cells in which progeny are more likely to differentiate into neurons. The data presented here show that quiescent progenitor cells can be triggered to proliferate and differentiate in response to injury, suggesting that there is homeostatic regulation of cell number in the developing visual system. It is possible that injury induces a signal that releases the negative homeostatic control of proliferation by changing progenitor cell fate. The molecular mechanisms that negatively regulate cell proliferation in the healthy brain and then initiate it in response to injury remain largely unknown. Nitric oxide (NO) negatively regulates cell proliferation in the brain (Packer et al., 2003) and blocking NOS activity in Xenopus optic tectum increases cell proliferation (Peunova et al., 2007). We have previously shown that decreasing brain activity by visual deprivation also increases proliferation (Bestman et al., 2012; Sharma and Cline, 2010), and here we show that deprivation-induced proliferation facilitates recovery from injury. While it is not clear if the signal controlling progenitor cell fate is the same in the injury and visual deprivation paradigms, both experiments demonstrate that neuronal progenitor cells change fate in response to different physiological stimuli.
In summary, we used a visual avoidance behavior in Xenopus laevis tadpoles to assess functional recovery of the retino-tectal-spinal cord circuit following injury to the tectum. Neurogenesis in the injured tectum promoted recovery of visual behavior and increasing endogenous neurogenic activity by decreasing visual system input facilitated recovery from injury. Together, these experiments show that engaging activity-dependent mechanisms that regulate endogenous neurogenesis facilitates functional recovery from local CNS injury. Previous work showed that induced apoptosis of cortical neurons in adult mice triggers proliferation of endogenous cortical neural progenitors and differentiation of newly generated cells into neurons (Chen et al., 2004; Magavi et al., 2000), however animals did not show recovery of function. More recently, grafts of neural progenitors transplanted into a spinal cord injury site were shown to generate neurons and lead to recovery of function (Lu et al., 2012). Although neurorestorative strategies to increase neurogenesis, synaptogenesis, circuit remodeling, and angiogenesis are all promising therapies in the treatment of brain injuries (Xiong et al., 2010), understanding the unique capacity of lower vertebrates to recover from injury may assist in application of these strategies to mammalian systems that are inherently refractory to functional recovery after CNS injury. Further investigations into the mechanisms underlying the activation of quiescent neural progenitor cells and the integration of newly generated neurons into the remaining circuit may facilitate treatment of brain injury.
Supplementary Material
Acknowledgements
This work was funded by support from Dart Neuroscience, LLC, the Nancy Lurie Marks Family Foundation; the Hahn Family Foundation and the National Institutes of Health (EY011261) to HTC, a postdoctoral fellowship from the California Institute of Regenerative Medicine (CIRM) to PS, and a predoctoral fellowship from the Center for Academic Research and Training in Anthropogeny provided by the Mather's Foundation of New York to HES. We thank members of the Cline lab, both past and present, for helpful discussions.
Footnotes
Conflict of interest statement
We have no known or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, our work.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: HTC, CRM. Acquisition of data: CRM, PS, HES. Analysis and interpretation of data: CRM, PS, HES, HTC. Drafting of the manuscript: CRM, PS, HES, HTC. Critical revision of the manuscript for important intellectual content: CRM, PS, HES, WS, HTC. Statistical analysis: CRM, PS. Obtained funding: HTC. Administrative, technical, and material support: WS. Study supervision: CRM, HTC.
Literature Cited
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, Luduena RF. A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J Biol Chem. 1988;263(6):3029–3034. [PubMed] [Google Scholar]
- Bestman JE, Lee-Osbourne J, Cline HT. In vivo time-lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles. J Comp Neurol. 2012;520(2):401–433. doi: 10.1002/cne.22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31(13):4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I. Animal Models of Head Trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101(46):16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff EA, Stocum DL, Nye HL, Cameron JA. Urodele spinal cord regeneration and related processes. Dev Dyn. 2003;226(2):295–307. doi: 10.1002/dvdy.10240. [DOI] [PubMed] [Google Scholar]
- Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey MV, Jiang Y, Alli VV, Yang Z, Levison SW. Defining the critical period for neocortical neurogenesis after pediatric brain injury. Dev Neurosci. 2010;32(5–6):488–498. doi: 10.1159/000321607. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol. 2009;101(2):803–815. doi: 10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Yoshino J, Kado K, Tochinai S. Brain regeneration in anuran amphibians. Dev Growth Differ. 2007;49(2):121–129. doi: 10.1111/j.1440-169X.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Ferretti P. Is there a relationship between adult neurogenesis and neuron generation following injury across evolution? Eur J Neurosci. 2011;34(6):951–962. doi: 10.1111/j.1460-9568.2011.07833.x. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata S. Differences in the Decrease in Regenerative Capacity of Various Brain Regions of Xenopus laevis are Related to the Differences in the Undifferentiated Cell Populations. Journal of Brain Research. 1995;36(4):523–529. [PubMed] [Google Scholar]
- Filoni S, Gibertini G. A study of the regenerative capacity of the central nervous system of anuran amphibia in relation to their stage of development. I. Observations on the regeneration of the optic lobe of Xenopus laevis (Daudin) in the larval stages. Arch Biol (Liege) 1969;80(4):369–411. [PubMed] [Google Scholar]
- Filoni S, Margotta V. A study of the regeneration of the cerebellum of Xenopus laevis (Daudin) in the larval stages and after metamorphosis. Arch Biol (Liege) 1971;82(4):433–470. [PubMed] [Google Scholar]
- Gaete M, Munoz R, Sanchez N, Tampe R, Moreno M, Contreras EG, Lee-Liu D, Larrain J. Spinal cord regeneration in Xenopus tadpoles proceeds through activation of Sox2-positive cells. Neural Dev. 2012;7:13. doi: 10.1186/1749-8104-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze RM, Keating MJ, Ostberg A, Chung S-H. The relationship between retinal and tectal growth in larval Xenopus: implications for the development of the retino-tectal projection. J Embryol exp Morph. 1979;53:103–143. [PubMed] [Google Scholar]
- Gibbs K, Chittur S, Szaro B. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. Eur J Neurosci. 2011;33:9–25. doi: 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- Gong Q, Shipley MT. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron. 1995;14(1):91–101. doi: 10.1016/0896-6273(95)90243-0. [DOI] [PubMed] [Google Scholar]
- Good PJ, Richter K, Dawid IB. The sequence of a nervous system-specific, class II beta-tubulin gene from Xenopus laevis. Nucleic Acids Res. 1989;17(19):8000. doi: 10.1093/nar/17.19.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbrand IA, Gaze RM. Microglia in tadpoles of Xenopus laevis: normal distribution and the response to optic nerve injury. Anat Embryol (Berl) 1991;184(1):71–82. doi: 10.1007/BF01744263. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo- from single cells to the entire brain. Differentiation. 2002;70(4–5):148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Harris W, Hartenstein V. Neuronal Determination without Cell Division in Xenopus Embryos. Neuron. 1991;6:499–515. doi: 10.1016/0896-6273(91)90053-3. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology. 2012;33(2):191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E, Pasquier L. Ontogeny of the immune system in Xenopus. Differentiation. 1984;28(2):109–115. [Google Scholar]
- Huang JK, Dorey K, Ishibashi S, Amaya E. BDNF promotes target innervation of Xenopus mandibular trigeminal axons in vivo. BMC Dev Biol. 2007;7:59. doi: 10.1186/1471-213X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SW, de la Torre JC. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol Rep. 2009;61(2):225–235. doi: 10.1016/s1734-1140(09)70026-x. [DOI] [PubMed] [Google Scholar]
- Jordan M. Regeneration of the endbrain in postmetamorphic Xenopus laevis. Folia Biol (Krakow) 1958;6:103–115. [Google Scholar]
- Julien C, Marcouiller F, Bretteville A, El Khoury NB, Baillargeon J, Hébert SS, Planel E. Dimethyl Sulfoxide Induces Both Direct and Indirect Tau Hyperphosphorylation. PLoS ONE. 2012;7(6):e40020. doi: 10.1371/journal.pone.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Sakakibaraa S, Imaia T, Suzukia A, Nakamuraa Y, Sawamotoa K, Ogawaa Y, Toyamad Y, Miyataa T, Okanoa H. Musashi1: An Evolutionally Conserved Marker for CNS Progenitor Cells Including Neural Stem Cells. De Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- Kazim SF, Shamim MS, Tahir MZ, Enam SA, Waheed S. Management of penetrating brain injury. J Emerg Trauma Shock. 2011;4(3):395–402. doi: 10.4103/0974-2700.83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR, Chun SK, Kim TW, Kim H, Ono K, Takebayashi H, Ikenaka K, Oppenheim RW, Sun W. Evidence for the spontaneous production but massive programmed cell death of new neurons in the subcallosal zone of the postnatal mouse brain. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2010.07557.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Szele FG. Activation of subventricular zone stem cells after neuronal injury. Cell Tissue Res. 2008;331(1):337–345. doi: 10.1007/s00441-007-0451-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, Shimizu K, Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech. 2012;5(2):200–209. doi: 10.1242/dmm.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22(6):645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138(22):4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Liu H, Guthrie KM. Neuronal replacement in the injured olfactory bulb. Exp Neurol. 2011;228(2):270–282. doi: 10.1016/j.expneurol.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerle Z, Gal P. Unusual penetrating head injury in children: personal experience and review of the literature. Childs Nerv Syst. 2009;25(8):909–913. doi: 10.1007/s00381-009-0901-z. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Minelli G, del Grande P, Franceschini V, Ciani F. Proliferative response of the mesencephalic matrix areas in the reparation of the optic tectum of Triturus cristatus carnifex. Z Mikrosk Anat Forsch. 1990;104(1):17–25. [PubMed] [Google Scholar]
- Minelli G, Franceschini V, Del Grande P, Ciani F. Newly-formed neurons in the regenerating optic tectum of Triturus cristatus carnifex. Basic Appl Histochem. 1987;31(1):43–52. [PubMed] [Google Scholar]
- Moody SA, Miller V, Spanos A, Frankfurter A. Developmental expression of a neuron-specific beta-tubulin in frog (Xenopus laevis): a marker for growing axons during the embryonic period. J Comp Neurol. 1996;364(2):219–230. doi: 10.1002/(SICI)1096-9861(19960108)364:2<219::AID-CNE3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16(3):165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: Elsevier-North Holland Publishing Co.; 1956. [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100(16):9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peunova N, Scheinker V, Ravi K, Enikolopov G. Nitric oxide coordinates cell proliferation and cell movements during early development of Xenopus. Cell Cycle. 2007;6(24):3132–3144. doi: 10.4161/cc.6.24.5146. [DOI] [PubMed] [Google Scholar]
- Plantman S, Ng KC, Lu J, Davidsson J, Risling M. Characterization of a novel rat model of penetrating traumatic brain injury. J Neurotrauma. 2011 doi: 10.1089/neu.2011.2182. [DOI] [PubMed] [Google Scholar]
- Portugues R, Engert F. The neural basis of visual behaviors in the larval zebrafish. Curr Opin Neurobiol. 2009;19(6):644–647. doi: 10.1016/j.conb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser T, Baier H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. Journal of Neuroscience. 2003;23(9):3726–3734. doi: 10.1523/JNEUROSCI.23-09-03726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Derby CD. Cytoarchitecture and ultrastructure of neural stem cell niches and neurogenic complexes maintaining adult neurogenesis in the olfactory midbrain of spiny lobsters, Panulirus argus. J Comp Neurol. 2011;519(12):2283–2319. doi: 10.1002/cne.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Cline HT. Visual activity regulates neural progenitor cells in developing xenopus CNS through musashi1. Neuron. 2010;68(3):442–455. doi: 10.1016/j.neuron.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, McKeown CR, Demas JA, Cline HT. Inhibition to excitation ratio regulates visual system responses and behavior in vivo. J Neurophysiol. 2011;106(5):2285–2302. doi: 10.1152/jn.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- Srebro Z. Regeneration of the endbrain in Xenopus laevis tadpoles. Folia Biol (Krakow) 1957;5:211–231. [Google Scholar]
- Srebro Z. Investigation on the regenerative capacity of the between brain and the influence of its removal upon the development of Xenopus laevis tadpoles. Folia Biol (Krakow) 1959;7:191–202. [Google Scholar]
- Stahel PF, Morganti-Kossmann MC, Kossmann T. The role of the complement system in traumatic brain injury. Brain Res Brain Res Rev. 1998;27(3):243–256. doi: 10.1016/s0165-0173(98)00015-0. [DOI] [PubMed] [Google Scholar]
- Statler KD, Jenkins LW, Dixon CE, Clark RSB, Marion DW, Kochanek PM. The Simple Model Versus the Super Model: Translating Experimental Traumatic Brain Injury Research to the Bedside. Journal of Neurotrauma. 2011;18(11) doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Straznicky K, Gaze R. The development of the tectum in Xenopus laevis: an autoradiographic study. Journal of Embryology and Experimental Morphology. 1972;28(1):87–115. [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. J Comp Neurol. 2007;500(3):574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HKC, Goings GE, Walker AS, Francis G, Szele FG. Subventricular Zone Neuroblasts Emigrate Toward Cortical Lesions. J Neuropathol Exp Neurol. 2005;64(12):1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Whitmore AV, Jeffery G. Cell division and cleavage orientation in the developing retina are regulated by L-DOPA. J Comp Neurol. 2006;496(3):369–381. doi: 10.1002/cne.20920. [DOI] [PubMed] [Google Scholar]
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein L, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2010 doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsching HG, Kretz O, Morosan-Puopolo G, Chernogorova P, Theiss C, Brand-Saberi B. Thymosin beta4 induces folding of the developing optic tectum in the chicken (Gallus domesticus) J Comp Neurol. 2012;520(8):1650–1662. doi: 10.1002/cne.23004. [DOI] [PubMed] [Google Scholar]
- Witte S, Stier H, Cline HT. In vivo observations of timecourse and distribution of morphological dynamics in Xenopus retinotectal axon arbors. Journal of Neurobiology. 1996;31(2):219–234. doi: 10.1002/(SICI)1097-4695(199610)31:2<219::AID-NEU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11(3):298–308. [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Tochinai S. Successful reconstitution of the non-regenerating adult telencephalon by cell transplantation in Xenopus laevis. Dev Growth Differ. 2004;46(6):523–534. doi: 10.1111/j.1440-169x.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Clarke JD, Ferretti P. FGF-2 Up-regulation and proliferation of neural progenitors in the regenerating amphibian spinal cord in vivo. Dev Biol. 2000;225(2):381–391. doi: 10.1006/dbio.2000.9843. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. Towards brain repair: Insights from teleost fish. Semin Cell Dev Biol. 2009;20(6):683–690. doi: 10.1016/j.semcdb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH, Ott R. Cell Proliferation after Lesions in the Cerebellum of Adult Teleost Fish: Time Course, Origin, and Type of New Cells Produced. Experimental Neurology. 1999;160:78–87. doi: 10.1006/exnr.1999.7182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.