Abstract
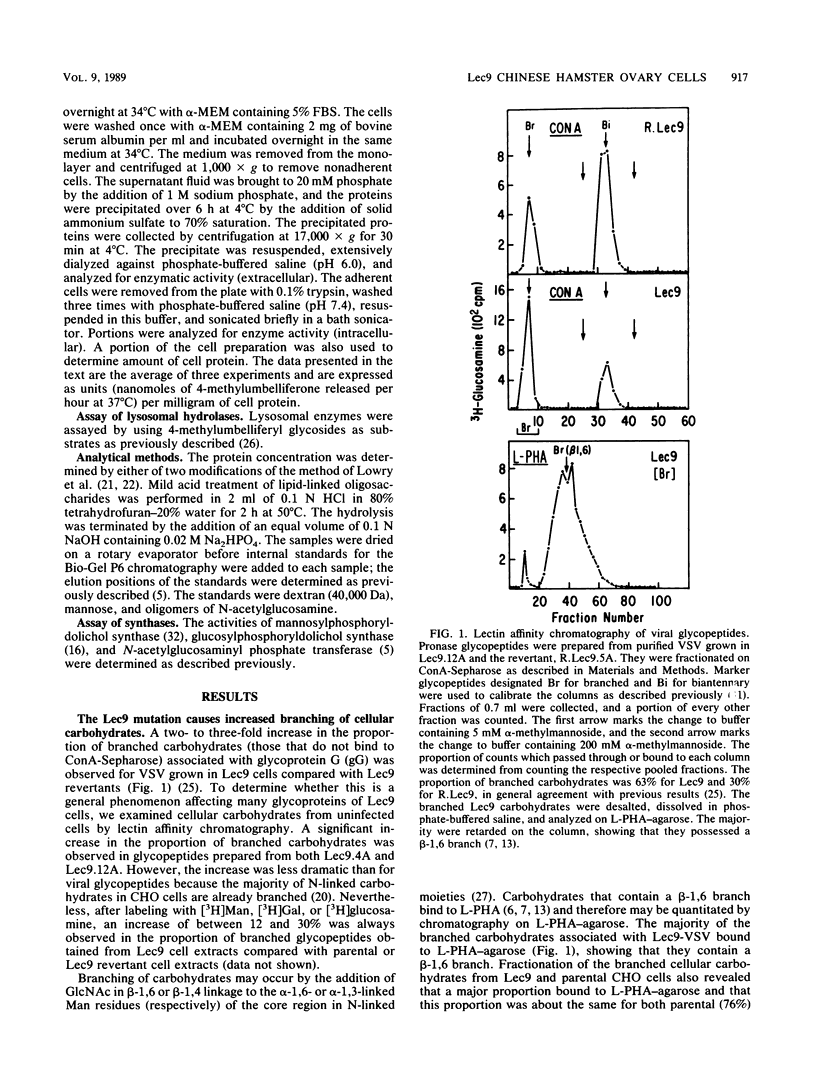
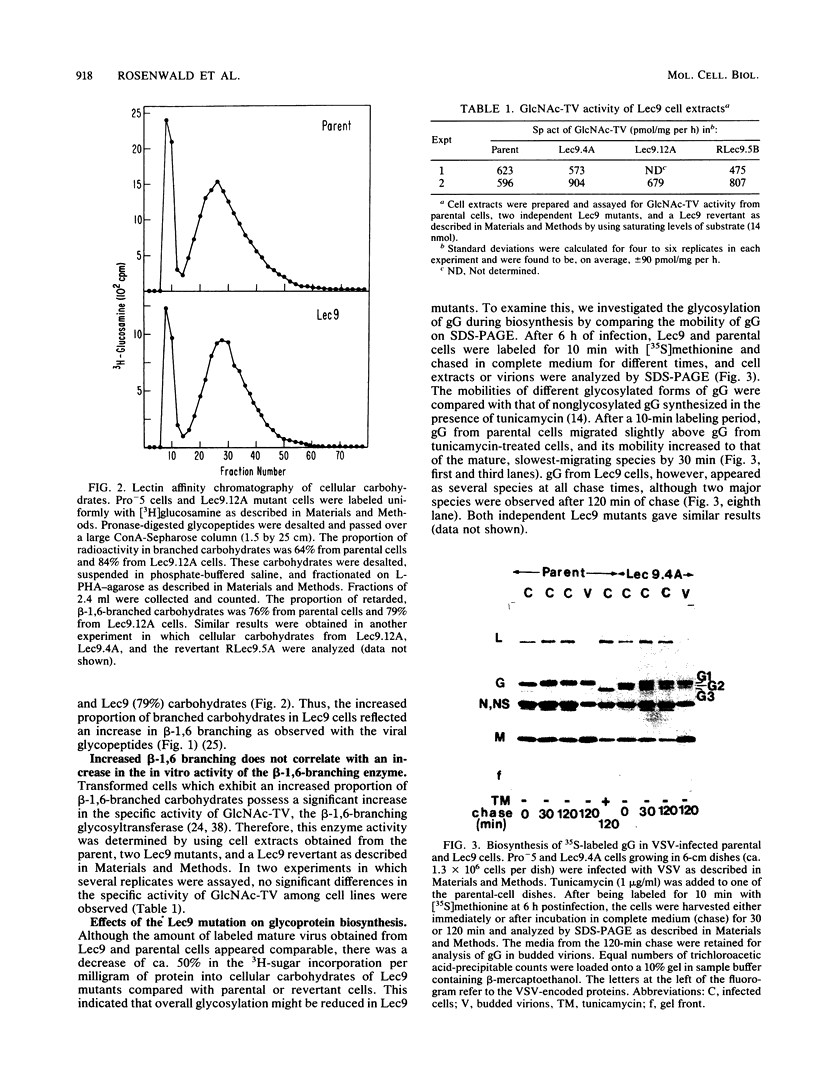
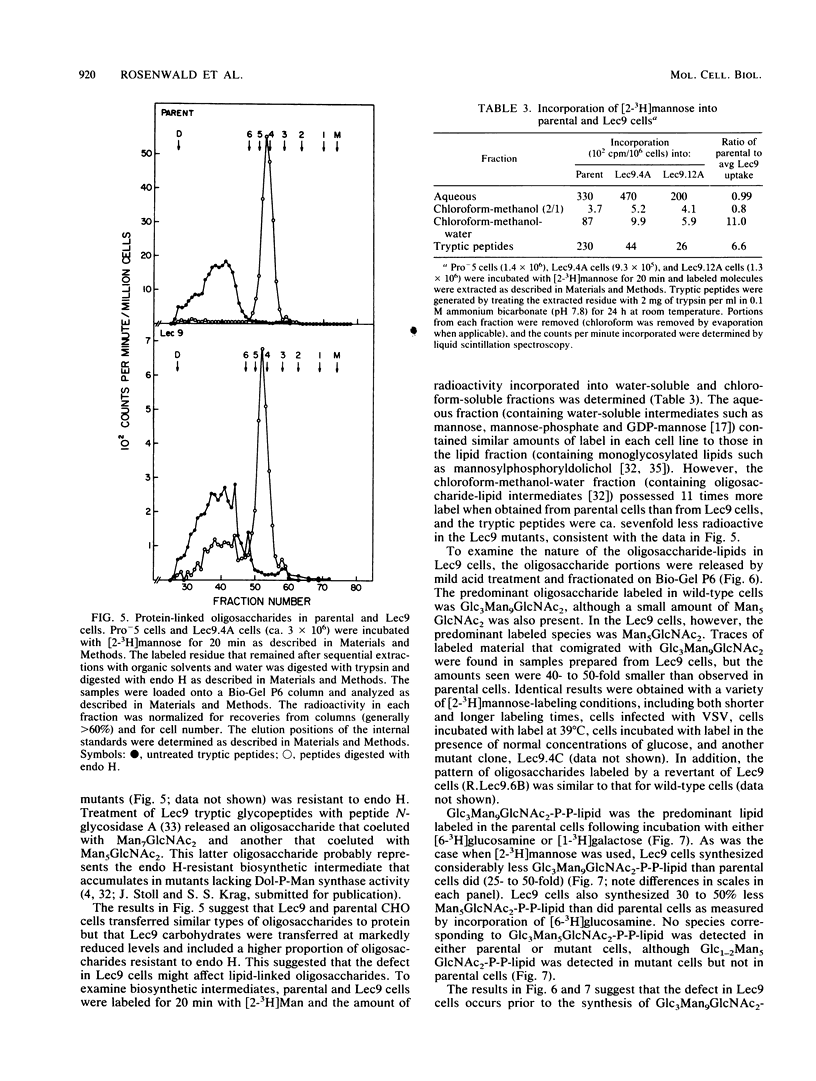
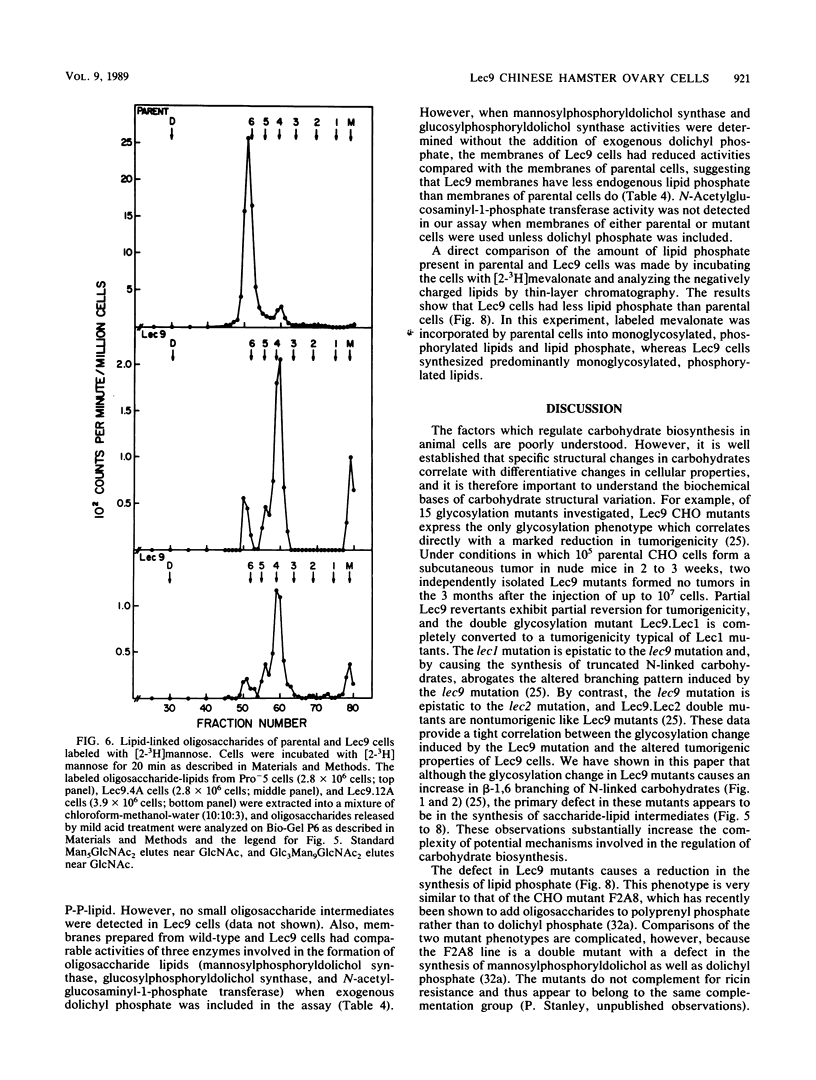
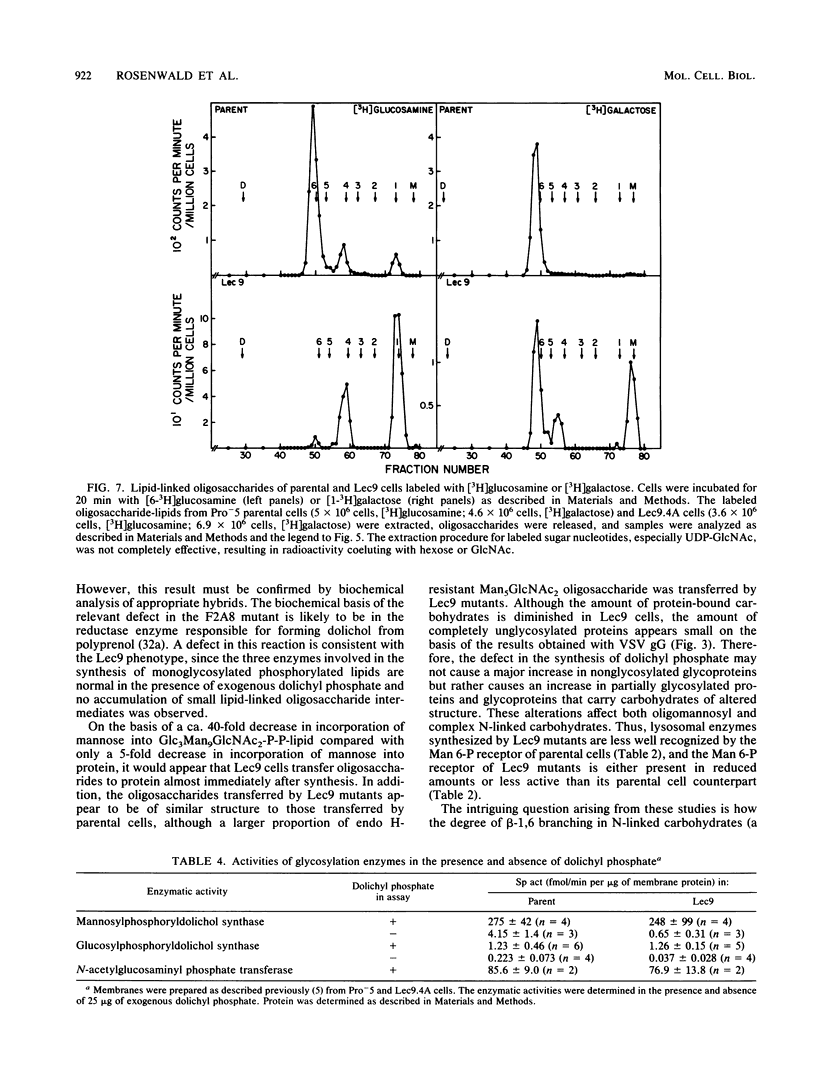
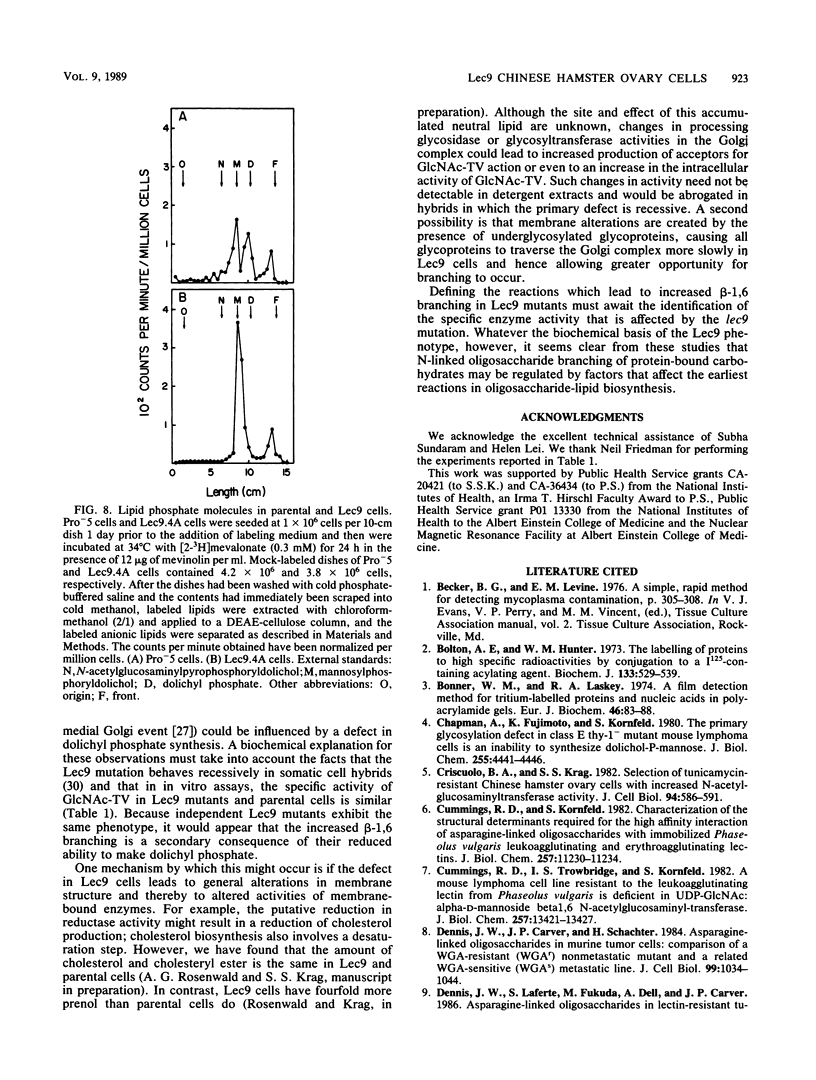
A correlation between increased beta-1,6 branching of N-linked carbohydrates and the ability of a cell to metastasize or to form a tumor has been observed in several experimental models. Lec9 Chinese hamster ovary (CHO) mutants exhibit a drastic reduction in tumorigenicity in nude mice, and this phenotype directly correlates with their ability to attach an increased proportion of beta-1,6-branched carbohydrates to the G glycoprotein of vesicular stomatitis virus (J. Ripka, S. Shin, and P. Stanley, Mol. Cell. Biol. 6:1268-1275, 1986). In this paper we provide evidence that cellular carbohydrates from Lec9 cells also contain an increased proportion of beta-1,6-branched carbohydrates, although they do not possess significantly increased activity of the beta-1,6 branching enzyme (GlcNAc-transferase V). Biosynthetic labeling experiments show that a substantial degree of underglycosylation occurs in Lec9 cells and that this affects several classes of glycoproteins. Lec9 cells synthesize ca. 40-fold less Glc3Man9GlcNAc2-P-P-lipid and ca. 2-fold less Man5GlcNAc2-P-P-lipid than parental cells do. In addition, Lec9 cells possess ca. fivefold less protein-bound oligosaccharide intermediates, and one major species is resistant to release by endo-beta-N-acetylglucosaminidase H (endo H). Membranes of Lec9 cells exhibit normal mannosylphosphoryldolichol synthase, glucosylphosphoryldolichol synthase, and N-acetylglucosaminylphosphate transferase activities in the presence of exogenous dolichyl phosphate. However, in the absence of exogenous dolichyl phosphate, mannosylphosphoryldolichol synthase and glucosylphosphoryldolichol synthase activities are reduced in membranes of Lec9 cells, indicating that membranes of Lec9 cells are deficient in lipid phosphate. This was confirmed by analysis of lipids labeled by [3H]mevalonate, which showed that Lec9 cells have less lipid phosphate than parental CHO cells. Mechanisms by which a defect in the synthesis of dolichol-oligosaccharides might alter the degree of beta-1,6 branching in N-linked carbohydrates are discussed.
Full text
PDF




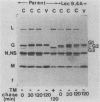
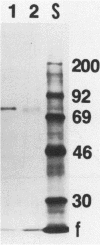
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Criscuolo B. A., Krag S. S. Selection of tunicamycin-resistant Chinese hamster ovary cells with increased N-acetylglucosaminyltransferase activity. J Cell Biol. 1982 Sep;94(3):586–591. doi: 10.1083/jcb.94.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Cummings R. D., Trowbridge I. S., Kornfeld S. A mouse lymphoma cell line resistant to the leukoagglutinating lectin from Phaseolus vulgaris is deficient in UDP-GlcNAc: alpha-D-mannoside beta 1,6 N-acetylglucosaminyltransferase. J Biol Chem. 1982 Nov 25;257(22):13421–13427. [PubMed] [Google Scholar]
- Dennis J. W., Carver J. P., Schachter H. Asparagine-linked oligosaccharides in murine tumor cells: comparison of a WGA-resistant (WGAr) nonmetastatic mutant and a related WGA-sensitive (WGAs) metastatic line. J Cell Biol. 1984 Sep;99(3):1034–1044. doi: 10.1083/jcb.99.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Hall C. W., Robbins A. R., Krag S. S. Preliminary characterization of a Chinese hamster ovary cell glycosylation mutant isolated by screening for low intracellular lysosomal enzyme activity. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):35–45. doi: 10.1007/BF00230634. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. K., Boon D. Y., Crum F. C. N-Acetylglucosamine- 1 -phosphate transferase from hen oviduct: solubilization, characterization, and inhibition by tunicamycin. Biochemistry. 1979 Sep 4;18(18):3946–3952. doi: 10.1021/bi00585a016. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S. Immunologic studies of membrane mutants of a highly metastatic murine tumor. Am J Pathol. 1979 Dec;97(3):609–622. [PMC free article] [PubMed] [Google Scholar]
- Krag S. S. A concanavalin A-resistant Chinese hamster ovary cell line is deficient in the synthesis of [3H]glucosyl oligosaccharide-lipid. J Biol Chem. 1979 Sep 25;254(18):9167–9177. [PubMed] [Google Scholar]
- Krag S. S., Robbins A. R. A Chinese hamster ovary cell mutant deficient in glucosylation of lipid-linked oligosaccharide synthesizes lysosomal enzymes of altered structure and function. J Biol Chem. 1982 Jul 25;257(14):8424–8431. [PubMed] [Google Scholar]
- Krag S. S., Robbins P. W. Sindbis envelope proteins as endogenous acceptors in reactions of guanosine diphosphate-[14C]Mannose with preparations of infected chicken embryo fibroblasts. J Biol Chem. 1977 Apr 25;252(8):2621–2629. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li E., Gibson R., Kornfeld S. Structure of an unusual complex-type oligosaccharide isolated from Chinese hamster ovary cells. Arch Biochem Biophys. 1980 Feb;199(2):393–399. doi: 10.1016/0003-9861(80)90295-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Pierce M., Arango J., Tahir S. H., Hindsgaul O. Activity of UDP-GlcNAc:alpha-mannoside beta(1,6)N-acetylglucosaminyltransferase (GnT V) in cultured cells using a synthetic trisaccharide acceptor. Biochem Biophys Res Commun. 1987 Jul 31;146(2):679–684. doi: 10.1016/0006-291x(87)90582-1. [DOI] [PubMed] [Google Scholar]
- Ripka J., Shin S., Stanley P. Decreased tumorigenicity correlates with expression of altered cell surface carbohydrates in Lec9 CHO cells. Mol Cell Biol. 1986 Apr;6(4):1268–1275. doi: 10.1128/mcb.6.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R. The mannose 6-phosphate receptor of Chinese hamster ovary cells. Compartmentalization of acid hydrolases in mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10623–10627. [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Stanley P. Carbohydrate heterogeneity of vesicular stomatitis virus G glycoprotein allows localization of the defect in a glycosylation mutant of CHO cells. Arch Biochem Biophys. 1982 Nov;219(1):128–139. doi: 10.1016/0003-9861(82)90141-2. [DOI] [PubMed] [Google Scholar]
- Stanley P. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet. 1983 Sep;9(5):593–608. doi: 10.1007/BF01574260. [DOI] [PubMed] [Google Scholar]
- Stanley P., Vivona G., Atkinson P. H. 1H NMR spectroscopy of carbohydrates from the G glycoprotein of vesicular stomatitis virus grown in parental and Lec4 Chinese hamster ovary cells. Arch Biochem Biophys. 1984 Apr;230(1):363–374. doi: 10.1016/0003-9861(84)90119-x. [DOI] [PubMed] [Google Scholar]
- Stoll J., Robbins A. R., Krag S. S. Mutant of Chinese hamster ovary cells with altered mannose 6-phosphate receptor activity is unable to synthesize mannosylphosphoryldolichol. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2296–2300. doi: 10.1073/pnas.79.7.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J., Rosenwald A. G., Krag S. S. A Chinese hamster ovary cell mutant F2A8 utilizes polyprenol rather than dolichol for its lipid-dependent asparagine-linked glycosylation reactions. J Biol Chem. 1988 Aug 5;263(22):10774–10782. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr Oligosaccharide accessibility to peptide:N-glycosidase as promoted by protein-unfolding reagents. J Biol Chem. 1982 Sep 25;257(18):10776–10780. [PubMed] [Google Scholar]
- Waldman B. C., Oliver C., Krag S. S. A clonal derivative of tunicamycin-resistant Chinese hamster ovary cells with increased N-acetylglucosamine-phosphate transferase activity has altered asparagine-linked glycosylation. J Cell Physiol. 1987 Jun;131(3):302–317. doi: 10.1002/jcp.1041310303. [DOI] [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]