Abstract
Background
GTP cyclohydrolase 1 (GTP-CH1), the rate-limiting enzyme in the synthesis of tetrahydrobiopterin (BH4), encoded by the GCH1 gene, has been implicated in the development and maintenance of inflammatory pain in rats. In humans, homozygous carriers of a “pain-protective” (PP) haplotype of the GCH1 gene have been identified exhibiting lower pain sensitivity, but only following pain sensitisation. Ex vivo, the PP GCH1 haplotype is associated with decreased induction of GCH1 after stimulation, whereas the baseline BH4 production is not affected. Contrary, loss of function mutations in the GCH1 gene results in decreased basal GCH1 expression, and is associated with DOPA-responsive dystonia (DRD). So far it is unknown if such mutations affect acute and inflammatory pain.
Results
In the current study, we examined the involvement of the GCH1 gene in pain models using the hyperphenylalaninemia 1 (hph-1) mouse, a genetic model for DRD, with only 10% basal GTP-CH1 activity compared to wild type mice. The study included assays for determination of acute nociception as well as models for pain after sensitisation. Pain behavioural analysis of the hph-1 mice showed reduced pain-like responses following intraplantar injection of CFA, formalin and capsaicin; whereas decreased basal level of GTP-CH1 activity had no influence in naïve hph-1 mice on acute mechanical and heat pain thresholds. Moreover, the hph-1 mice showed no signs of motor impairment or dystonia-like symptoms.
Conclusions
In this study, we demonstrate novel evidence that genetic mutations in the GCH1 gene modulate pain-like hypersensitivity. Together, the present data suggest that BH4 is not important for basal heat and mechanical pain, but they support the hypothesis that BH4 plays a role in inflammation-induced hypersensitivity. Our studies suggest that the BH4 pathway could be a therapeutic target for the treatment of inflammatory pain conditions. Moreover, the hph-1 mice provide a valid model to study the consequence of congenital deficiency of GCH1 in painful conditions.
Keywords: GTP-cyclohydrolase, Tetrahydrobiopterin, hph-1, Inflammatory pain, Mechanical hypersensitivity, CFA, Formalin, Capsaicin, Dystonia
Background
Chronic pain is a severe and common healthcare problem that affects millions of people worldwide. In spite of a number of analgesics available for pain therapy, pain often remains inadequately treated in many patients and represents a major cause of suffering and reduced quality of life [1]. One of the reasons for the difference in success of pharmacologic pain treatment rely on the different genetic disposition of patients to develop pain or to respond to analgesics [2].
The GCH1 gene is one of few genes reported to be involved in the modulation of pain sensitivity in humans [3-5]. The gene codes for the enzyme guanosine triphosphate cyclohydrolase 1 (GTP-CH1), the first and rate-limiting enzyme in the de novo synthesis of 5,6,7,8-tetrahydrobiopterin (BH4) (see Additional file 1). BH4 is an essential cofactor for phenylalanine, tyrosine and tryptophan hydroxylases and for all isoforms of nitric oxide synthase (NOS). Hence, BH4 regulates the synthesis of catecholamines, serotonin and nitric oxide (NO) (see Additional file 1), all involved in pain signalling [6]. In 2002, Costigan and colleagues made a systematic search for pain-related genes using microarray based gene expression analysis [7]. This led to the discovery of two genes both involved in BH4 biosynthesis; the GCH1 gene and the gene for sepiapterin reductase (SR) (see Additional file 1). Both genes were upregulated in dorsal root ganglion (DRG) following peripheral axotomy in rats. In a follow-up study, BH4 synthesis was found to be upregulated in primary sensory neurons after peripheral inflammation and nerve injury [4]. Inhibition of BH4 synthesis with the prototypic GTP-CH1 inhibitor 2,4-diamino-6-hydroxypyrimidine (DAHP) reduced the nociceptive responses in rodent models of neuropathic and inflammatory pain accompanied by reduced BH4 concentrations in DRGs, whereas intrathecal injection of BH4 induced pain in naïve rats [4]. Furthermore, in humans a particular haplotype of the GCH1 gene identified as “pain protective”(PP) was found to be associated with reduced pain sensitivity in healthy subjects as well as persistent low back pain in patients after discectomy [3,4], though other research did not confirm this association [8]. Recently, downregulation of the GCH1 gene by adeno-associated virus mediated expression of small hairpin RNA against GCH1 was shown to reduce neuropathic pain hypersensitivity in rats [9]. Also, inhibition of GTP-CH1 was found to reduce cancer-induced pain in mice [10].
In contrast to the GCH1 PP haplotype leading to moderate reduction of BH4 availability and only after stimulation, loss of function mutations in the GCH1 gene causes reduced basal concentrations of BH4 and is associated with DOPA-responsive Dystonia (DRD). DRD is a rare movement disorder that manifest without hyperphenylalaninemia and the classical clinical characterisation are gait problems due to dystonia, mostly in the lower extremities with onset in childhood. The patients respond well to treatment with L-DOPA [11].
The hyperphenylalaninemia 1 (hph-1) mouse is a genetic model for DRD. The mutant mouse was originally generated by mutagenesis using the sperm mutagen N-ethyl-N´-nitrosourea [12], and is characterised by a marked decrease in baseline GCH1 mRNA expression accompanied by a large decrease in both GTP-CH1 activity and BH4 synthesis. The hph-1 mutation has been localised to an interval of 1.6-2.8 Mb on chromosome 14, containing the murine GCH1 gene [13]. However, the exact location of the mutation is still undefined.
To the best of our knowledge it has not yet been reported whether mutations in the GCH1 gene, leading to profound decrease in basal gene expression, affects pain sensitivity. Therefore, in the current study we investigated the hph-1 mice in animal models of acute and inflammatory pain. We demonstrate that the mutant mice exhibited reduced inflammatory hypersensitivity, whereas acute responses to mechanical and heat stimuli were normal compared to wild type (WT) controls.
Results
Reduced BH4 concentrations in hph-1 mice compared to WT mice
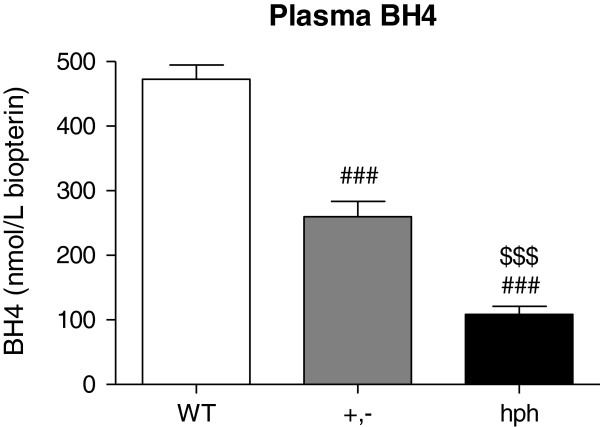
Plasma BH4 concentrations were examined by high performance liquid chromatography (HPLC) analysis to evaluate if the hph-1 mice used in this study had the expected phenotype of reduced BH4 synthesis [14]. The data showed that heterozygous hph-1 (+,-) mice and homozygous hph-1 (hph) mice had significantly lower BH4 concentrations compared to WT controls (###p < 0.001, Figure 1), with +,- mice having intermediate amounts of BH4. The plasma BH4 values were 472.8 ± 22.14 nmol/L, 259.6 ± 23.92 nmol/L and 108.5 ± 12.42 nmol/L for WT, +,- and hph mice, respectively. Hence, in plasma, heterozygous and homozygous animals have approximately 55% and 20% of normal BH4 concentrations, respectively.
Figure 1.
Plasma concentrations of BH4 determined as biopterin (nmol/L) by reversed-phase HPLC. The BH4 concentration was significantly reduced in both +,- and hph mice compared to WT mice (###p < 0.001) (n = 6 for WT and +,- mice, and n = 7 for hph mice). Moreover, hph mice displayed significantly lower BH4 concentrations compared to +,- mice ($$$p < 0.001). One-way ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean + SEM.
Lack of motor impairment and dystonia-like symptoms in hph-1 mice
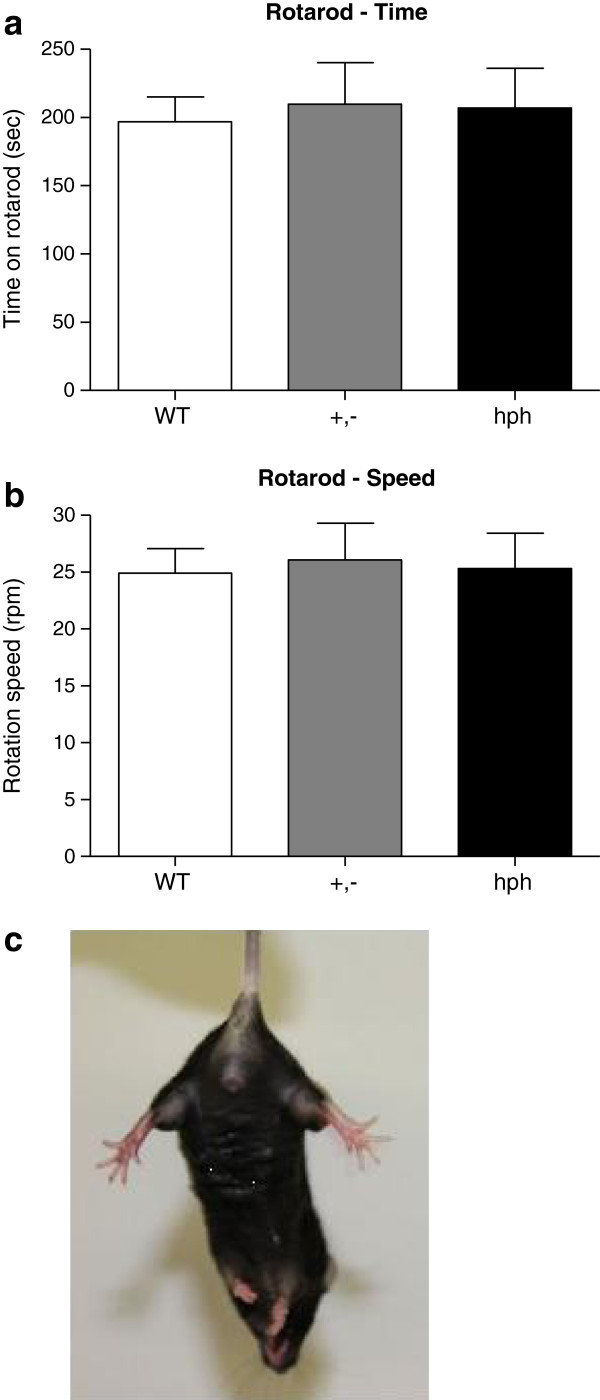
As the hph-1 mouse is biochemically a model for DOPA-responsive dystonia (DRD) [15], the motor behaviour was examined in this strain. We found that the hph-1 mice showed no signs of motor impairment nor any abnormal behaviour when carefully observing the animals. Also in the wire hanging test, both hph-1 and WT mice were able to hang upside down for 120 sec, indicating normal neuromuscular function.
In the rotarod test, +,- and hph mice had no difficulty maintaining balance on the rod compared to WT mice (Figure 2a-b). Both the time on rotarod (sec) and rotation speed (rpm) were not significantly different among genotypes (p = 0.92 and p = 0.95). The WT, +,- and hph mice stayed on the rotating rod for 197 ± 29 sec, 210 ± 31 sec and 207 ± 18 sec, respectively. In addition, dystonia-like symptoms were examined by suspending the animals by their tail for 15 sec, and this revealed that the mutant mice did not display any hind-paw clasping (Figure 2c). Overall, these observations demonstrate that hph-1 mice are not associated with reduced motor function nor dystonia-like symptoms.
Figure 2.
No motor impairment nor dystonia-like symptoms were seen in +,- and hph mice. (a) time walking on the rotarod (sec) and (b) rotation speed (rpm). n = 10, n = 8 and n = 6 for WT, +,- and hph mice, respectively. No difference in rotarod performance was found between genotypes (p = 0.92 and p = 0.95, respectively). One-way ANOVA with pair-wise comparisons using the Fisher’s LSD test. (c) hind-paw clasping; normal splaying of hind-paws was observed in hph mice as well as WT mice (not shown). Data are presented as mean + SEM.
The hph-1 mutants exhibit normal pain behavioural responses to heat and mechanical stimuli
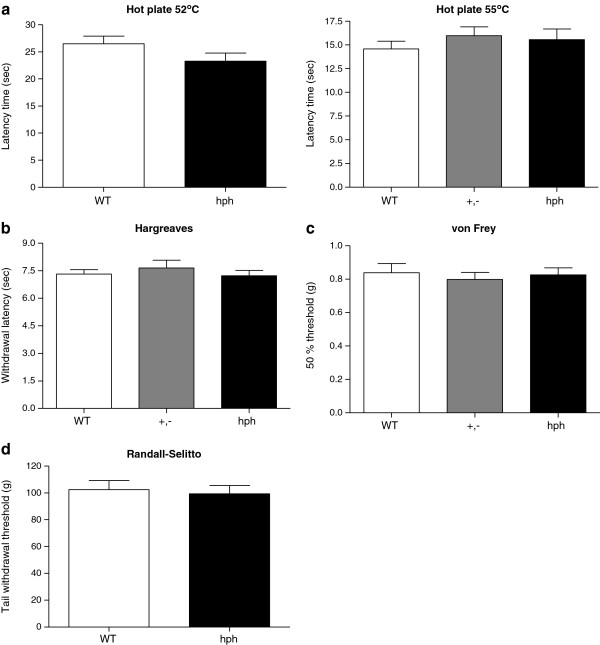
Acute heat sensitivity was assessed by two different assays; the hot plate test (Figure 3a) and the Hargreaves test (Figure 3b). In the hot plate test, the latency time to respond to noxious heat stimulation was not significantly different between hph-1 mice and WT mice at neither 52°C (WT: 27 ± 1 sec and hph: 23 ± 2 sec, p = 0.13) nor 55°C (WT: 14.6 ± 0.8 sec, +,-: 15.9 ± 0.9 sec and hph: 15.5 ± 1.1 sec, p = 0.54). In the Hargreaves test, the paw withdrawal latencies of mutant mice to heat stimulation was also not different from WT responses (WT: 7.3 ± 0.2 sec, +,-: 7.6 ± 0.4 sec and hph: 7.0 ± 0.3 sec, p = 0.65).
Figure 3.
Normal behavioural responses to heat and mechanical stimuli were observed in +,- and hph mice. (a) Hot Plate; latency time to respond by hind-paw licking, lifting or flinching at a plate temperature of 52°C (n = 19) or 55°C (n = 12–17). No significant change was observed between genotypes (p = 0.13 and p = 0.54, respectively). (b) Hargreaves Test; withdrawal latencies to noxious heat stimuli (n = 7–15). No change in latency time was found among genotypes (p = 0.65). (c,d) von Frey and Randall Selitto; withdrawal threshold to mechanical stimulus (n = 10–16 and n = 13–15, respectively). Hph-1 mice exhibited similar mechanical thresholds as WT mice (p = 0.92 and p = 0.68). Mann–Whitney t-test, two-tailed or one-way ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean + SEM.
Mechanical sensitivity was examined using the von Frey and Randall Selitto test (Figure 3c-d). In the von Frey test, the withdrawal threshold to mechanical stimulation was not significantly different between genotypes (WT: 0.84 ± 0.06 g, +,-: 0.79 ± 0.04 g and hph: 0.83 ± 0.04 g, p = 0.92). Similarly, the mean weight resulting in tail withdrawal in the Randall Selitto test was 102.4 ± 6.8 g for WT mice and 99.4 ± 6.2 g for hph mice, representing a non-significant difference between the groups (p = 0.68). Together, these behavioural observations suggest that reduction of BH4 synthesis in hph-1 mice does not seem to change sensitivity to peripherally applied heat and mechanical stimuli in naïve mice.
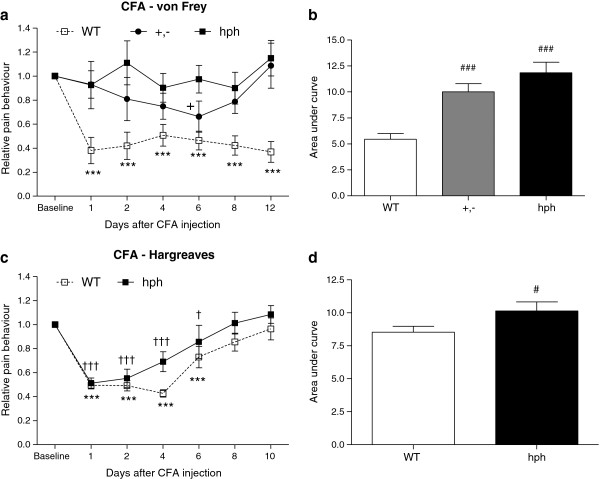
Reduced CFA-induced mechanical and heat hypersensitivity in hph-1 mice compared to WT mice
In the complete Freund’s adjuvant (CFA) model, the baseline withdrawal thresholds to mechanical stimuli of naïve mice were not significantly different between genotypes (0.8 ± 0.07 g, 0.74 ± 0.03 g and 0.65 ± 0.06 g for WT, +,- and hph mice, respectively. p > 0.05). Moreover, the mean withdrawal latencies to heat stimuli were similar in hph mice and WT mice (9 ± 1 sec and 9 ± 1 sec, respectively).
Intraplantar injection of CFA into the hind-paw of WT mice induced a long-term mechanical hypersensitivity, starting on day 1 and persisting on day 12 (Figure 4a). The latter is indicated by reduced withdrawal thresholds to innocuous mechanical stimulation as compared to baseline values (***p < 0.001). In mutant mice, CFA injection evoked reduced withdrawal thresholds of the affected paw in +,- mice (+p < 0.05, Figure 4a), whereas almost no effect was observed in homozygous mice. Comparison of overall area under the curves (AUCs) revealed a significantly lower mechanical hypersensitivity of +,- and hph mice as compared to WT controls (###p < 0.001, Figure 4b). In addition, no statistical significant difference in pain-like responses was found between +,- and hph mice (p > 0.05). These results show that in homozygous mice, mechanical hypersensitivity was almost completely suppressed throughout the observation period compared to WT controls, whereas heterozygous mice displayed intermediate responses to CFA injection (Figure 4a). This suggests a genotype-dependent anti-hypersensitive effect, with the largest effect in the homozygous hph-1 mice. Therefore, in subsequent experiments only WT and hph mice were tested.
Figure 4.
Reduced hypersensitivity after CFA injection in +,- and hph mice. (a,c) Time course of mechanical and heat hypersensitivity. CFA injection induced mechanical hypersensitivity in +,- and WT mice, but not in hph mice (n = 10–14). In the Hargreaves test, both WT mice and hph mice exhibited heat hypersensitivity after peripheral inflammation (n = 11–14). WT: ***p < 0.001, +,-: +p < 0.05 and hph: †p < 0.05 and †††p < 0.001 versus baseline values (two-way RM-ANOVA with Fisher’s LSD test). (b,d) Area under curve analysis. A statistical significant difference in mechanical and heat hypersensitivity was found between hph-1 mice and WT mice. ###p < 0.001, one-way ANOVA with pair-wise comparisons using the Fisher’s LSD test (log transformed data). #p = 0.04, unpaired t-test, two-tailed (log transformed data). Data are presented as mean ± or + SEM.
Hind-paw injection of CFA also induced a significant reduction in paw withdrawal latencies in response to noxious heat stimuli in WT mice as compared to baseline values (***p < 0.001, Figure 4c). The heat hypersensitivity began at day 1 and lasted for 6 days after paw injection. In contrast to mechanical hypersensitivity, heat hypersensitivity was present in hph mice from 1 to 6 days after CFA injection (†††p < 0.001 and †p < 0.05). However, a trend towards faster recovery and less heat hypersensitivity was seen in mutant mice compared to WT mice by day 4 (Figure 4c). Comparison of AUCs showed that hph mice exhibited significantly lower heat hypersensitivity than WT controls (#p = 0.04, Figure 4d).
To determine the degree of inflammation, the dorsal-plantar paw thickness was measured 24 hours after CFA inoculation (see Additional file 2). Injection of CFA increased the paw thickness in both genotypes as compared with baseline values (p < 0.001), and the data showed no difference in paw thickness between WT and hph mice (p > 0.05), indicating similar peripheral inflammation.
These data show that BH4 plays an anti-hypersensitive role in inflammatory pain in particular mechanical sensitivity following peripheral inflammation. For the heat hypersensitivity, the data suggest that BH4 may modulate pain sensitivity. However, this effect does not seem to be robust.
Reduced acute nociceptive behaviour in response to formalin injection in hph mice
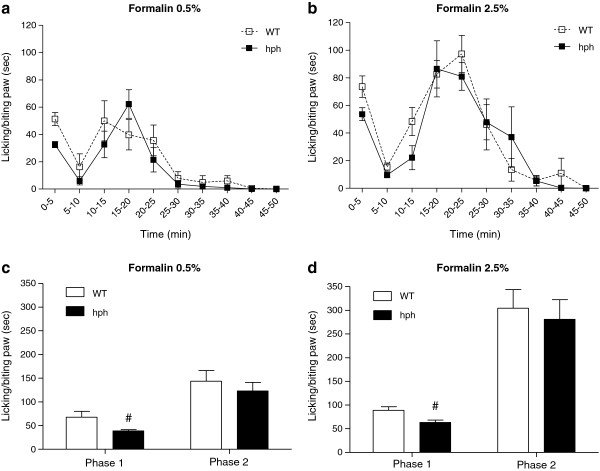
Spontaneous nociceptive pain behaviours due to chemical stimuli were tested using the formalin test (Figure 5). Intraplantar injection of 0.5 and 2.5% formalin produced a biphasic response in both WT mice and hph mice, characterised by licking and biting the paw, with a first phase starting immediately after formalin injection and a second phase that began after 10 min and lasted for 50 min (Figure 5a-b).
Figure 5.
Reduced formalin-induced pain behaviour in hph mice. (a) and (b) time course after paw injection of 0.5 and 2.5% formalin. (c) and (d) total time licking and biting the paw during 0–10 min (phase 1) and 10–50 min (phase 2). A statistical significant reduction in spontaneous pain activity of hph mice was seen at both formalin-concentrations in the first phase compared to WT mice (0.5%: #p = 0.014, n = 9 and 2.5%: #p = 0.012, n = 9). Mann–Whitney t-test, two-tailed. No significant change in pain behaviour between genotypes was found in the second phase (0.5%: p = 0.48 and 2.5%: p = 0.69). Unpaired t-test, two-tailed. Data are presented as mean ± or + SEM.
Following injection of 0.5% formalin, a significant decrease in total time licking and biting the paw was seen in hph mice in the first phase compared to WT controls (hph: 39 ± 3 sec, WT: 68 ± 12 sec, #p = 0.014, Figure 5c). On administration of 2.5% formalin a similar reduction in spontaneous pain behaviour was seen in the first phase (hph: 63 ± 5 sec, WT: 89 ± 7 sec, #p = 0.012, Figure 5d). Regardless of the intensity of noxious chemical stimulus, no significant difference in spontaneous pain activity was found in the second phase between homozygous mutant mice and WT mice (0.5%: 123 ± 18 sec vs. 144 ± 22 sec, p = 0.48 and 2.5%: 281 ± 41 sec vs. 304 ± 39 sec, p = 0.69, Figure 5c-d).
These data demonstrate a significant effect of inherited BH4 reduction on formalin-evoked spontaneous pain responses. This was specifically observed in the first phase, suggesting that BH4 might modulate acute nociception induced by formalin injection.
Reduced capsaicin-induced mechanical hypersensitivity but not spontaneous pain behaviour in hph mice compared to WT mice
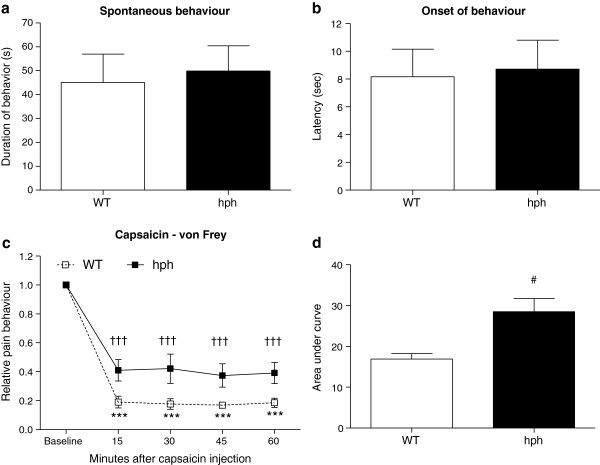
Intraplantar injections of capsaicin into the hind-paw evoked an immediate nocifensive behaviour characterised by licking, biting and lifting of the paw (Figure 6a). The duration of the spontaneous pain activity was not significantly different between hph mice and WT mice (45 ± 12 sec vs. 50 ± 11 sec, p = 0.46). In addition, the latency time to respond in hph mice was also similar to that observed in WT controls (8 ± 2 sec vs. 9 ± 2 sec, p = 1.00).
Figure 6.
Reduced capsaicin-induced mechanical hypersensitivity in hph mice, but normal spontaneous pain behaviours. (a) Spontaneous pain responses; total time licking, biting and lifting the paw during 10 min after capsaicin injection (1 μg/paw). No difference was found between hph mice and WT mice (p = 0.46, n = 7–8). (b) Onset of pain behaviour. No differences in latency time was seen among genotypes (p = 1.00, n = 7–8). Mann–Whitney t-test, two-tailed. (c) von Frey time course; after capsaicin injection (1 μg/paw) both WT mice and hph mice developed mechanical hypersensitivity (n = 6–10). WT: ***p < 0.001 and hph: †††p < 0.001 versus baseline values. Two-way RM-ANOVA with pair-wise comparisons using the Fisher’s LSD test. (d) Area under curve analysis. A statistical significant difference in mechanical hypersensitivity between hph mice and WT controls was found (#p = 0.04). Mann–Whitney t-test, two-tailed. Data are presented as mean ± or + SEM.
In the capsaicin test, the baseline withdrawal thresholds to mechanical stimulation of naïve mice were also similar between genotypes (p = 0.78). The mean withdrawal thresholds were 1.03 ± 0.09 g and 0.99 ± 0.04 g for WT mice and hph mice, respectively. Following capsaicin injection, mechanical sensitivity was evaluated within a 60 min observation period. Intraplantar injection of capsaicin (1 μg/paw) into the hind-paw induced a substantial mechanical hypersensitivity in both groups at all time points (WT: ***p < 0.001, hph: †††p < 0.001, Figure 6c). When comparing the overall AUCs, a significant reduction in mechanical hypersensitivity was found in hph mice compare to WT mice (#p = 0.04, Figure 6d). At lower concentrations of capsaicin (0.25 and 0.5 μg/paw) both groups exhibited a clear mechanical hypersensitivity in the injected paw, however at these concentrations no difference in mechanical sensitivity was found between genotypes (p > 0.05, Table 1). Heat sensitivity following capsaicin injection was also examined, but in this case it was not possible to demonstrate presence of heat hypersensitivity in neither WT mice nor hph mice (see Additional file 3).
Table 1.
Mechanical hypersensitivity following intraplantar injection of 0.25-1 μg/paw capsaicin in WT mice and hph mice
|
Time of testing (min) |
|
|
|
|
|---|---|---|---|---|
| Concentration | von Frey (g) | Baseline | 30 | 60 |
|
0.25 μg/paw |
|
|
|
|
| WT mice |
0.81±0.04 |
1.00±0.00 |
0.49±0.15b |
0.39±0.07b |
| hph mice |
0.78±0.04a |
1.00±0.00 |
0.44±0.10a,b |
0.47±0.07a,b |
|
0.5 μg/paw |
|
|
|
|
| WT mice |
0.81±0.08 |
1.00±0.00 |
0.26±0.03b |
0.28±0.11b |
| hph mice |
0.80±0.10a |
1.00±0.00 |
0.28±0.11a,b |
0.26±0.03a,b |
|
1 μg/paw |
|
|
|
|
| WT mice |
1.03 ± 0.09 |
1.00±0.00 |
0.18±0.04b |
0.19±0.03b |
| hph mice | 0.99 ± 0.04a | 1.00±0.00 | 0.42±0.10b,c | 0.39±0.07b,c |
The results are expressed as relative pain behaviour (normalised to the baseline response of each mouse). For 0.25, 0.5 and 1 μg/paw n = 3–6, n = 4 and n = 6–10, respectively. The letter a, indicates non-significant differences when compared to WT mice (p > 0.05), the letter b, indicates significant differences compared to baseline, and the letter c, indicates significant differences relative to WT (p < 0.05). Unpaired t-test (two-tailed) or two-way RM-ANOVA with Fisher’s LSD test. Data is shown as mean ± SEM.
These data suggest that the effect of the mutation in hph mice on mechanical sensitivity is dependent on the intensity of the noxious stimulus induced by capsaicin. Moreover, the results revealed that reduced BH4 availability impaired capsaicin-evoked mechanical hypersensitivity, but not the immediate response induced by capsaicin itself.
Discussion
In the current study, we used the hph-1 mouse model deficient in GTP-CH1 to study the involvement of the GCH1 gene in acute and inflammatory pain models. This included assays to determine mechanical and heat nociception as well as pain-like responses to intraplantar injection of chemical and inflammatory substances. We demonstrated that hph-1 mice exhibited reduced mechanical and heat hypersensitivity following CFA, and reduced mechanical hypersensitivity after capsaicin injection compared to WT mice, whereas pain behavioural responses to heat and mechanical stimuli were normal in naïve mice.
GCH1 in mechano-and thermosensation
The present findings suggest that processes involved in the transmission of mechanical and heat stimuli in the non-sensitised organism are independent of GTP-CH1. This is consistent with previous studies in human carriers of the GCH1 PP haplotype, and in rats following pharmacological inhibition with the GTP-CH1 inhibitor DAHP [4,5]. In rats, intraperitoneal administration of DAHP attenuated inflammatory pain behaviours, but it did not change mechanical and heat pain sensitivity in naïve animals [4]. Furthermore, differences in pain sensitivity between carriers and non-carriers of the GCH1 PP haplotype were only observed after local skin inflammation or capsaicin sensitisation [3,5]. It was proposed that the reason for this observation is that differences in GTP-CH1 activity in carriers and non-carriers of the GCH1 PP haplotype were only seen after stimulation with LPS [5]. In contrast to the human GCH1 PP haplotype, the hph-1 mice have significantly lower GCH1 expression at baseline compared to WT mice. Baseline GTP-CH1 activity in homozygous hph-1 mice is only about 10% of the WT mice [16] and in agreement with this a reduced BH4 concentration in blood was found in this study (Figure 1). Hence, our results indicate that even though baseline GTP-CH1 activity is reduced in hph-1 mice it did not appear to influence mechanical and heat pain thresholds in naïve mice.
GCH1 in acute and inflammatory pain
After CFA injection, the homozygous mice did not develop mechanical hypersensitivity, while heat hypersensitivity was present in the mutant mice. This may suggest differential contribution of GTP-CH1 in the maintenance of mechanical and heat hypersensitivity. Several studies point to the involvement of different mechanisms of mechanical and heat hypersensitivity. Heat hypersensitivity is thought to be mediated by afferent C-fibers, whereas mechanical hypersensitivity involves activation of afferent Aβ-fibers [17]. Also studies using neuronal NOS knock-out mice reported profound loss of mechanical hypersensitivity, with no change in sensitivity to noxious heat stimulation [18]. Finally, the lack of correlation between mechanical and heat hypersensitivity in several pain models suggest that these are mediated by different neural mechanisms [19].
Following intraplantar formalin injection a significant reduction in spontaneous pain behaviour in hph mice was only seen during the first phase. Phase 1 is postulated to involve direct activation of nociceptors by formalin, probably mediated through chemical stimulation of the transient receptor potential cation channel subfamily A member 1 (TRPA1) [20,21], whereas phase 2 depends on inflammatory reactions in the peripheral tissue and/or central sensitisation of spinal cord neurons [22]. It has been proposed that the second phase results from the barrage of nociceptor inputs during phase 1 [23], hence in the mutant mice a reduction in pain behaviour during phase 2 was expected. In addition, our findings contrast with results from rats following pharmacological inhibition of GTP-CH1 activity, where attenuated pain behaviour was seen in both phases [4]. The reason for this discrepancy is not immediately apparent but it may relate to (i) difference in animal species and (ii) difference in the mechanisms following pharmacological inhibition versus genetic inhibition of GTP-CH1 activity. It is possible that although the nociceptive inputs from peripheral nerves are attenuated during phase 1 in the mutant mice, there is an increase or no change in excitability of spinal cord neurons in the second phase. Another potential explanation for the present observation is that sustained peripheral nerve activity rather than spinal sensitisation drives the nociceptive responses in phase 2. This is supported by previous studies showing that inhibition of pain responses during phase 1 with anaesthetics or opioids does not change the magnitude of nociceptive responses during phase 2 [24,25]. Nevertheless, the finding that spontaneous activity in hph-1 mice was not influenced in the second phase after injection of both low and high formalin concentrations suggests that at least in hph-1 mice, changes in GTP-CH1 activity is not critical for the processes driving the second phase of the formalin test.
Tegeder et al., 2006 showed that peripheral inflammation increased the BH4 concentrations in DRG compared to that of naïve rats, and that administration of DAHP reduced this excess BH4 production [4]. Reductions in BH4 synthesis in hph-1 mice have been shown in several tissues, including brain, liver and lung [13,26,27], and GTP-CH1 activity is probably reduced in all tissues where it is normally expressed. Although the reduction of GCH1 in hph-1 mice have been shown to vary in different brain structures including dorsal raphe (51%), locus coeruleus (30%) and substantia nigra (39%), the GCH1 expression was substantially reduced in all brain areas [28], consistent with decreased BH4 synthesis in the whole brain [26]. Furthermore, stimulation of cultured mast cells from hph-1 mice with cytokines and administration of LPS increased the GTP-CH1 activity and BH4 synthesis, respectively. This indicates that enzyme activity can be induced in mutant mice, but absolute values were always markedly lower in hph-1 mice than in WT mice [27,29]. Taken together, it is likely that the reduction of hypersensitivity following sensitisation is due to lower induction of GCH1 and hence BH4 in DRG of hph-1 mice compared to WT mice.
It is known that BH4 also contributes to the development of other types of pain conditions such as neuropathic pain and cancer-induced pain. Previous studies have demonstrated that systemic administration of DAHP has analgesic properties in three models of neuropathic pain and in two models of cancer-induced pain [4,10]. In humans, the GCH1 PP haplotype has been associated with reduced risk of chronic pain after discectomy as well as a slow progression of pain in cancer patients [4,30]. The findings that inhibition of BH4 synthesis modulates pain sensitivity following inflammation, nerve injury and cancer imply a common mechanism of action of BH4 in these different pain states.
Possible mechanisms of action
The mechanisms leading to pain and the sites of action of BH4 are still unknown. BH4 is not a neurotransmitter but a cofactor required for activity of a number of enzymes, including tyrosine and tryptophan hydroxylases and all isoforms of NOS. Accordingly, BH4 regulates the synthesis of serotonin, NO and catecholamines, all playing a complex role in nociceptive signalling. BH4 may therefore mediate noxious pain responses through mechanisms involving NO and/or biogenic amines [4,31]. Previous studies imply that BH4 acts partly through NO mediated mechanisms as injection of the GTP-CH1 inhibitor DAHP impaired excess NO production following nerve injury [4]. The implication of NO in development and maintenance of hypersensitivity in response to inflammation is well documented [18,32,33], and it has been shown that NO plays an essential role in both peripheral [33-36] and central [18] mechanisms of inflammatory pain. Reduced NOS activity and NO generation have previously been reported in hph-1 mice determined in the brain as well as astrocytes and plasma [26,29,37]. Therefore, the alleviated mechanical hypersensitivity following CFA and capsaicin injection may possibly result from (i) decreased spinal neuronal NOS activity followed by decreased excess NO production and/or (ii) reduced NO-mediated peripheral sensitisation.
Caveats of genetic mouse models
Although the use of genetic mouse models has their advantages, they are associated with important inherited caveats that need to be considered when interpreting the data [38]. As the hph-1 mouse is a mutant model, having decreased GTP-CH1 activity during the development and throughout life, the role of BH4 in pain might be different from the ongoing role in the normal adult mouse. Therefore, genetic based studies might have different outcomes compared with pharmacological downregulation of GTP-CH1 in normal animals. However, it should be emphasised that this caveat also applies to humans carrying the GCH1 PP haplotype.
The GCH1 gene may also have multiple roles since it influences many biological systems. Consequently, disruption of this gene may have multiple effects unrelated to nociception such as change in motor function. The mutation in the hph-1 mice share similar biochemical features as an autosomal dominantly inherited mutation in the human GCH1 gene manifested as DRD [15]. DRD results from a reduction in BH4 biosynthesis and the prominent phenotype is gait problems due to dystonia [39]. Therefore, reduced BH4 in mice may also yield effects on motor behaviour, which can confound the pain behaviours in these animals. However, hph-1 mice did not demonstrate any signs of motor impairment nor dystonia-like symptoms. Considering these observations, the differences in pain-related behaviours observed between hph-1 mice and WT mice are not related to effects on motor function.
Conclusions
In conclusion, our study supports previous findings that genetic functionally variants of the GCH1 gene influence pain sensitivity after sensitisation. Furthermore, it revealed that even profound reduction in basal levels of GCH1 activity did not affect nociceptive pain in naïve animals. The hph-1 mice with profound decrease in GTP-CH1 activity displayed reduced pain-like responses after peripheral inflammation and sensitisation. As GTP-CH1 is the rate-limiting enzyme in the synthesis of BH4, these findings suggests that BH4 plays a role in modulating inflammatory pain that could be a therapeutic target to treat inflammatory pain conditions. Moreover, the hph-1 mouse represents a new model to investigate the role of congenital GCH1 deficiency in pain.
Methods
Animals
The hph-1 mice have previously been generated and backcrossed for more than 20 generations into the C57BL/6JOlaHsd background (Harlan Laboratories, UK) [13]. Heterozygous male hph-1 (+,-) mice from the Welcome Trust Center for Human Genetics were shipped to Taconic Denmark (Ejby, Denmark), where breeding took place. Initially, male heterozygous mice were mated with female C57BL/6JOlaHsd mice to produce heterozygous progeny, and these were then mated to generate WT, heterozygous and homozygous hph-1 littermates. Later, animals were generated by homozygous breeding, no more than 4 generations. In this case control mice came from WT breeding pairs from the same colony as the hph-1 mice. Genotyping was conducted as previously described [13]. Homozygous and heterozygous progeny were fertile in both sexes with a normal litter size. Moreover, a gross preliminary examination of general health revealed that hph-1 mice mutants were healthy animals with a normal body weight compared to WT mice (Table 2).
Table 2.
Average weight of WT, heterozygous and homozygous mice (gram)
| Genotype | Weight | SEM |
|---|---|---|
| WT |
24.49 |
0.51 |
| +,- |
24.00 |
0.33 |
| hph | 23.09 | 0.55 |
n = 29.
Experiments were performed on 7–12 weeks-old male mice, housed in colony cages in temperature-controlled environments, with unrestricted access to standard diet and tap water, and kept on a 12:12 h light–dark cycle. The behavioural tests were conducted during the daylight hours. Experiments were performed according to the ethical guidelines for the investigation of experimental pain with conscious animals [40], and were approved by the Danish Animal Experiments Inspectorate, Ministry of Food, Agriculture and Fisheries (No. 2009/561-1622). The experimenter was blinded to the animal genotypes during all experimental procedures.
Determination of BH4 in plasma
BH4 was determined by isocratic HPLC analysis as the difference in biopterin (stable oxidation product of BH4) after acidic and basic oxidation with iodine [41]. Orbital blood was collected in tubes containing 10 μl of 180 mg/ml K2-EDTA (Sigma-aldrich) and 25 μl of 4% (w/v) dithiothreitol (Sigma-aldrich). Samples were then stored at room temperature for 3 h and protected from light before centrifugation at 2650 × g for 20 min [41]. The plasma samples were stored at – 80°C until analysis. Samples were then oxidised with a mixture of 1% (w/v) I2 (AppliChem, Kongens Lyngby, Denmark) and 2% (w/v) KI (Merck KGaA, Copenhagen, Denmark) in either 1 M HCl or 1 M NaOH for 1 h in the dark and at room temperature. Excess iodine was reduced by addition of 5% (w/v) ascorbic acid (Sigma-aldrich). The oxidised samples were centrifuged in Amicon Ultra Centrifugal filters (Ultracel –10 k; Millipore) at 5000 × g for 30 min. Biopterin content was determined in duplicate by reverse phase HPLC with fluorescence (Waters 474 Scanning Fluorescence Detector, Milford Massachusetts, USA) using a Nucleosil 100 C18 analytical column (5 μm, L × ID 250 × 4.6 mm; Varian) in conjunction with a pre-column (Nucleosil 100 C18 5 μm, ID 4.6 mm; Varian). The mobile phase consisted of 10 mM KH2PO4 in 5% (v/v) methanol (HPLC gradient grade; Sigma-aldrich) with pH adjusted to 4.5. The sample was injected in a volume of 20 μL and the analysis was run at a flow of 1 ml/min at ambient temperature. The peak area was used to quantify biopterin in comparison with external standards.
Motor behavioural tests
The hanging wire test was performed using a wire cage lid, and the latency (sec) to fall off the wire lid was determined during 120 sec. Hind-paw clasping was tested to evaluate whether the mutation exhibited dystonic posture of the hind-paws. Each mouse was picked up by its tail and suspended for 15 sec to observe hind-paw clasping as previously described [42]. The rotarod test was used to test motor coordination and balance using an ENV-575 M Five Station RotaRod Treamill USB-Mouse (Med Associates inc., St. Albans, Vermont, USA). Mice were placed on an elevated rod (3.2 cm diameter) beginning its acceleration at 3.5 rpm and ending at 35 rpm over 5 min. Prior to testing, mice were trained for at least three times. Between trials animals were allowed to rest for 15 min. The time in sec on the rotarod and the rotation speed was recorded. Time periods where mice were passively rotating with the rod was subtracted.
Hot plate test
Acute heat sensitivity was tested using a Hot Plate Analgesia Meter (Harvard Apparatus, Edenbridge, UK) as described by Eddy and Leimbach with few modifications [43]. On the day of testing, mice were allowed to habituate in their home cages for 60 min in the test room. After habituation, each mouse was placed one at a time in a transparent Plexiglas cylinder (13 cm high; diameter 19 cm) on a metal plate preheated to 52 or 55 ± 0.1°C, and observed until they responded by either hind-paw licking, lifting or flinching (whichever came first). Forepaw licking and lifting are common grooming responses and therefore not defined as nociceptive behaviour. The animals were removed immediately after showing a nociceptive response and were only tested once. A cut-off time of 40 sec was used to prevent tissue damage. The latencies to respond were taken for statistical analysis.
Hargreaves test
Heat sensitivity to noxious stimuli was assessed with a plantar test device (Model 400 Heated Base from IITC Inc, Woodland Hills, Ca, USA) using the method of Hargreaves et al. [44]. Each mouse was placed in a square opaque Plexiglas chamber (12 cm high; 10 × 10 cm) on a glass surface (1.2 cm thick) at room temperature. The animals were first allowed to habituate for at least 60 min before measurements began. A mobile radiant heat source (located approximately 6 cm below the glass surface) was then aimed at the middle plantar surface of the hind-paw, and the heat stimulus was applied until the mouse made a clear nocifensive withdrawal of the paw. Testing during grooming or exploratory behaviour was avoided [45]. The intensity of the heat source was adjusted to 22% of maximal intensity ≈ 80.89 ± 0.6 mW/cm2. Each mouse was tested three times, and at least 1 min was allowed between consecutive measurements in the same paw. The heat sensitivity is expressed as the mean withdrawal latency time (sec). A cut-off latency time of 20 sec was used. Baseline measurements were obtained from each animal for at least 2 consecutive days prior to inflammation or sensitisation.
von Frey test
Mechanical sensitivity was measured using von Frey monofilaments (ranging from 0.008 to 2.0 g, North Coast Medical Inc., Morgan Hill, Ca, USA) by the up-and-down method described by Chaplan et al. [46]. The mechanical sensitivity is recorded as 50% threshold (in grams), the force of the monofilament to which the animal responds in 50% of the stimuli. Each mouse was placed in an individual red Plexiglas cylinder (7.3 cm high; diameter 7.5 cm) on an elevated wire mesh floor (0.65 × 0.65 cm) allowing access to the plantar surface of the hind-paws. The animals were allowed a habituation period of at least 60 min. von Frey monofilaments were then applied perpendicularly to the middle plantar surface with sufficient force to cause filament bending and held for approximately 5 sec or until the hind-paw was withdrawn, defining a positive response. Lifting the hind-paw due to normal locomotory behaviour was ignored, and testing during deep sleep, grooming and exploring was avoided [45]. Baseline measurements to mechanical stimuli were performed in each animal for at least 2 consecutive days prior to inflammation or sensitisation.
Randall Selitto test
Mechanical pain behaviour was measured using a Randall Selitto device (IITC Life Science Inc., Victory Blvd Woodland Hills, CA, USA). Briefly, mice were allowed to habituate in their home cages for 60 min in the test room. Each mouse was then placed one at a time in a transparent restrainer for at least 5 min before the application of increased pressure to the tail. Mice were tested three times on different locations starting from the middle of the tail to the root. At least 3 min was allowed between consecutive measurements. A cutoff of 300 g was used. The mechanical sensitivity is expressed as the mean withdrawal latency in grams.
CFA-induced inflammatory pain
Persistent inflammatory pain was induced by injection of CFA (1 mg/ml Mycobacterium tuberculosis, Sigma-aldrich) into the hind-paw of mice. The mice were lightly restrained using a piece of cloth and 20 μl of CFA suspension was subcutaneously (s.c.) injected into the plantar surface of the right hind-paw using a GASTIGHT® 50 μl microsyringe (Hamilton Company, VWR, Denmark) with a 301/2-gauge needle. Mechanical hypersensitivity after peripheral inflammation was then tested 1, 2, 4, 6, 8 and 12 days after injection, whereas heat hypersensitivity was tested 1, 2, 4, 6, 8 and 10 days after paw inflammation as described above. Paw thickness (mm) was measured after 24 hours using a digital caliper.
Formalin test
Spontaneous nociceptive pain behaviours in response to chemical stimulation were measured using the formalin test described by Hunskaar et al. [47], with some modifications. Mice were placed within a glass cylinder (diameter 10.5 cm), and allowed to habituate for 60 min before behavioural testing began. Angled mirrors were placed behind and beside the glass cylinder to allow for an unimpeded view of the paws. After habituation, 20 μl of 0.5 or 2.5% (v/v) formalin solution (in isotonic saline) was injected into the right hind-paw as described above. The mice were immediately returned to the glass cylinder and a timer started to mark the beginning of the observation period. The total time spend licking/biting the right hind-paw during 60 min was measured with a stopwatch and recorded to the nearest second in 5 min intervals.
Capsaicin test
Injection of capsaicin into the hind-paws of animals evokes spontaneous pain behaviour and hypersensitivity to mechanical and heat stimuli. Spontaneous nociceptive pain behaviours in response to capsaicin injection were measured as described by Sakurada et al. [48], with some modifications. The experimental procedure is similar to the formalin test described above. After habituation 10 μl of 1 μg/paw of capsaicin (Sigma-aldrich, Brøndby, Denmark) in 0.5% (v/v) ethanol and isotonic saline was injected s.c. into the plantar surface of the right hind-paw. The total time spend licking, biting and lifting the injected paw during 10 min was measured with a stopwatch and recorded to the nearest second.
Hypersensitivity to heat and mechanical stimuli was determined as described by Gilchrist et al. [49], with some modifications. Capsaicin 0.25, 0.5 or 1 μg/paw was injected s.c. into the plantar surface of the paw. Hypersensitivity was then tested at 15, 30, 45 and 60 min after capsaicin injection. Heat hypersensitivity was measured at the site of injection, whereas mechanical hypersensitivity was determined approximately 2 mm from the site of capsaicin injection.
In preliminary pilot studies (not shown), vehicle injections did not induce significant pain-like behaviours in the capsaicin test as well as the CFA and formalin pain models. Hence, in this study vehicle controls were not included.
Data analysis
The concentrations of BH4 in plasma are expressed as nmol/L biopterin. Dihydrobiopterin (BH2), involved in the regeneration of BH4 (see Additional file 1), can also be oxidised to biopterin. During acidic oxidation both BH2 and BH4 are converted to biopterin, whereas basic oxidation involves the oxidation of BH2 only. Hence, the actual levels of BH4 are calculated as the difference in biopterin levels between acidic and basic oxidation.
The CFA and capsaicin data are expressed as relative pain behaviour. Each mouse was normalised to its own baseline value, i.e. expressed as index values with the baseline value defined as 1.0 for each mouse. Data were analysed using either unpaired t-test, one-way analysis of variance (ANOVA) or two-way repeated measured analysis of variance (RM-ANOVA) followed by pair-wise comparisons on the predicted means using the Fisher’s LSD test. Area under curve analysis was conducted using the trapezoidal rule. Statistical transformations were done where appropriate to meet the requirement of normal distribution and/or equal variances. The Mann–Whitney test was performed where appropriate. Data are presented as mean ± SEM or + SEM. p values less than 0.05 were considered significant. Statistical analysis was performed using SigmaPlot 11.0 (Systat Software Inc.) or GraphPad Prism 4.03 (GraphPad Software Inc.).
Abbreviations
Hph-1: Hyperphenylalaninemia 1; BH4: Tetrahydrobiopterin; BH2: Dihydrobiopterin; GTP-CH1: Guanosine triphosphate cyclohydrolase 1; NOS: Nitric oxide synthase; NO: Nitric oxide; DRG: Dorsal root ganglion; DAHP: 2,4-diamino-6-hydroxypyrimidine; PP: “Pain-protective”; WT: Wild type; HPLC: High performance liquid chromatography; DRD: DOPA-responsive dystonia; CFA: Complete Freund’s adjuvant; TRPA1: Transient receptor potential cation channel subfamily A member 1; TRPV1: Transient receptor potential cation channel subfamily V member 1.
Competing interests
The authors declare that they have no financial or non-financial competing interests.
Authors’ contributions
AN designed and performed the experiments, analysed the data and wrote the manuscript. OJB and LBM coordinated the project, helped to interpret the data and edited the manuscript. LSD and ML carried out the motor behavioural studies. AMH, ATM, ED and TSJ revised the article. All authors have discussed, commented and approved the final manuscript.
Supplementary Material
De novo pathway of BH4 biosynthesis and its functions. BH4 synthesis proceeds from GTP via three steps catalysed by GTPCH1, PTS and SR. BH4 is an essential cofactor for the aromatic amino acid hydroxylases (PAH, TH and TPH) as well as for all isoforms of NOS. The regeneration pathway involves two steps catalysed by PCD and DHPR. The red line indicates the enzyme targeted in the hph-1 mouse model. Abbreviations: GTPCH1, GTP cyclohydrolase 1; PTS, 6-pyruvoyltetrahydrobiopterin synthase; SR, sepiapterin reductase; PAH, phenylalanine hydroxylase; TH, tyrosine hydroxylase; TPH, trypthophan hydroxylase; NOS, nitric oxide synthase; AADC, aromatic L-amino acid decarboxylase; PCD, pterin-4-acarbinolamine dehydratase; DHPR, dihydropteridine reductase.
Intact paw thickness in hph mice following CFA injection. The dorsal-plantar paw thickness was measured using a digital caliper. A significant increase in paw thickness was found in both genotypes as compared with baseline values (n = 5). WT: ***p < 0.001 and hph: †††p < 0.001. No significant difference was found between genotypes (p > 0.05). Two-way RM-ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean + SEM.
No heat hypersensitivity was observed following capsaicin injection in both WT and hph mice. Heat sensitivity was examined before as well as 15, 30, 45 and 60 minutes after intraplantar injection of capsaicin. Capsaicin did not induce heat hypersensitivity in both WT and hph mice (p > 0.05, n = 4). Two-way RM-ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean ± SEM.
Contributor Information
Arafat Nasser, Email: Arafat.Nasser@regionh.dk.
Ole J Bjerrum, Email: ojb@sund.ku.dk.
Anne-Marie Heegaard, Email: amhe@sund.ku.dk.
Anette T Møller, Email: torvin@ki.au.dk.
Majbritt Larsen, Email: majbrittlarsen@gmail.com.
Louise S Dalbøge, Email: louise@dalboege.net.
Erik Dupont, Email: d007242@dadlnet.dk.
Troels S Jensen, Email: tsjensen@ki.au.dk.
Lisbeth B Møller, Email: Lisbeth.Birk.Moeller@regionh.dk.
Acknowledgments
We would like to thank medical laboratory technologist Pia Hougaard for performing the genotyping of mice. The work was supported in part by (i) A.P. Møller Fund, (ii) Brødrene Hartmann Fund, (iii) Beckett Fund and (iiii) Danielsens Fund to AN, and by grants to LBM from Lundbeck Fonden and Danish Medical Research Council. The hph-1 mouse model was a generous gift from Dr. Keith M. Channon from Welcome Trust Center for Human Genetics, Oxford.
References
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Geisslinger G, Tegeder I. Genetic modulation of the pharmacological treatment of pain. Pharmacol Ther. 2009;124(2):168–184. doi: 10.1016/j.pharmthera.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, Carmona C, Uhart M, Wand G, Carteret A, Lim YK, Frost J, Campbell JN. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141(1–2):114–118. doi: 10.1016/j.pain.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lötsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12(11):1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lötsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008;12(8):1069–1077. doi: 10.1016/j.ejpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Dionne RA. Lack of influence of GTP cyclohydrolase gene (GCH1) variations on pain sensitivity in humans. Mol Pain. 2007;3:6. doi: 10.1186/1744-8069-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee WI, Lee YS, Kim DH, Chang JW, Kim SW, Lee H. Effective relief of neuropathic pain by adeno-associated virus-mediated expression of a small hairpin RNA against GTP cyclohydrolase 1. Mol Pain. 2009;5:67. doi: 10.1186/1744-8069-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Myrczek T, Rûckert S, Weigert A, Häussler A, Ferreirós N, Brüne B, Lötsch J, Tegeder I. Inhibition of GTP cyclohydrolase reduces cancer pain in mice and enhances analgesic effects of morphine. J Mol Med (Berl) 2012. Epub ahead of print. [DOI] [PubMed]
- Romstad A, Dupont E, Krag-Olsen B, Østergaard K, Guldberg P, Güttler F. Dopa-responsive dystonia and Tourette syndrome in a large Danish family. Arch Neurol. 2003;60(4):618–622. doi: 10.1001/archneur.60.4.618. [DOI] [PubMed] [Google Scholar]
- Bode VC, McDonald JD, Guenet JL, Simon D. hph-1: A mouse mutant with hereditaryhyperphenylalaninemia induced by ethylnitrosourea mutagenesis. Genetics. 1988;118(2):299–305. doi: 10.1093/genetics/118.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JP, Nicoli T, Alp NJ, Fullerton J, Flint J, Channon KM. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol Genet Metab. 2004;82(3):251–254. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Tatham AL, Crabtree MJ, Warrick N, Cai S, Alp NJ, Channon KM. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J Biol Chem. 2009;284(20):13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland K, Gunasekara RS, Munk-Martin TL, Arnold LA, Engle T. The hph-1 mouse: a model for dominantly inherited GTP-cyclohydrolase deficiency. Ann Neurol. 2003;54(Suppl 6):S46–S48. doi: 10.1002/ana.10695. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Cotton RG, Jennings I, Ledley FD, Woo SL, Bode VC. Biochemical defect of the hph-1 mouse mutant is a deficiency in GTP-cylcohydrolase activity. J Neurochem. 1988;50(2):655–657. doi: 10.1111/j.1471-4159.1988.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacological inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain. 2005;119(1–3):113–123. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee S, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(1–2):67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27(42):11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535(1):155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- Dallel R, Raboisson P, Clavelou P, Saade M, Woda A. Evidence for a peripheral origin of the tonic nociceptive response to subcutaneous formalin. Pain. 1995;61(1):11–16. doi: 10.1016/0304-3959(94)00212-W. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Early nociceptive events influence the temporal profile, but not the magnitude, of the tonic response to subcutaneous formalin: effects with remifentanil. J Pharmacol Exp Ther. 1997;280(2):876–883. [PubMed] [Google Scholar]
- Brand MP, Heales SJ, Land JM, Clark JB. Tetrahydrobiopterin deficiency and brain nitric oxide synthase in the hph1 mouse. J Inherit Metab Dis. 1995;18(1):33–39. doi: 10.1007/BF00711370. [DOI] [PubMed] [Google Scholar]
- Gütlich M, Ziegler I, Witter K, Hemmens B, Hültner L, McDonald JD, Werner T, Rödl W, Bacher A. Molecular characterization of HPH-1: a mouse mutant deficient in GTP cyclohydrolase I activity. Biochem Biophys Res Commun. 1994;203(3):1675–1681. doi: 10.1006/bbrc.1994.2379. [DOI] [PubMed] [Google Scholar]
- Shimoji M, Hirayama K, Hyland K, Kapatos G. GTP cyclohydrolase I gene expression in the brains of male and female hph-1 mice. J Neurochem. 1999;72(2):757–764. doi: 10.1046/j.1471-4159.1999.0720757.x. [DOI] [PubMed] [Google Scholar]
- Lam AA, Hyland K, Heales SJ. Tetrahydrobiopterin availability, nitric oxide metabolism and glutathione status in the hph-1 mouse; implications for the pathogenesis and treatment of tetrahydrobiopterin deficiency states. J Inherit Metab Dis. 2007;30(2):256–262. doi: 10.1007/s10545-006-0502-x. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Klepstad P, Doehring A, Dale O. A GTP cyclohydrolase 1 genetic variant delays cancer pain. Pain. 2010;149(1):103–106. doi: 10.1016/j.pain.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Inturrisi CE. Feeling pain? Who’s your daddy…. Nat Med. 2006;12(11):1243–1244. doi: 10.1038/nm1106-1243. [DOI] [PubMed] [Google Scholar]
- Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain. 2007;11(7):810–818. doi: 10.1016/j.ejpain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. 2010;6:13. doi: 10.1186/1744-8069-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gühring H, Tegeder I, Lötsch J, Pahl A, Werner U, Reeh PW, Rehse K, Brune K, Geisslinger G. Role of nitric oxide in zymosan induced paw inflammation and thermal hyperalgesia. Inflamm Res. 2001;50(2):83–88. doi: 10.1007/s000110050728. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4(10):e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2(4):287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- Barker JE, Strangward HM, Brand MP, Hurst RD, Land JM, Clark JB, Heales SJ. Increased inducible nitric oxide synthase protein but limited nitric oxide formation occurs in astrocytes of the hph-1 (tetrahydrobiopterin deficient) mouse. Brain Res. 1998;804(1):1–6. doi: 10.1016/S0006-8993(98)00603-9. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE. Transgenic studies of pain. Pain. 1998;77(2):107–128. doi: 10.1016/S0304-3959(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Thöny B, Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-Pyruvoyl-tetrahydrobiopterin synthase, Sepiapterin reductase, Carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum Mutat. 2006;27(9):870–878. doi: 10.1002/humu.20366. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Fekkes D, Voskuilen-Kooijman A. Quantitation of total biopterin and tetrahydrobiopterin in plasma. Clin Biochem. 2007;40(5–6):411–413. doi: 10.1016/j.clinbiochem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sato K, Sumi-Ichinose C, Kaji R, Ikemoto K, Nomura T, Nagatsu I, Ichinose H, Ito M, Sako W, Nagahiro S, Graybiel AM, Goto S. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proc Natl Acad Sci USA. 2008;105(34):12551–12556. doi: 10.1073/pnas.0806065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithientybutenyl- and dithientylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Callahan BL, Gil AS, Levesque A, Mogil JS. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain. 2008;9(2):174–184. doi: 10.1016/j.jpain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14(1):69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31(12):1279–1285. doi: 10.1016/0028-3908(92)90057-V. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67(1):179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
De novo pathway of BH4 biosynthesis and its functions. BH4 synthesis proceeds from GTP via three steps catalysed by GTPCH1, PTS and SR. BH4 is an essential cofactor for the aromatic amino acid hydroxylases (PAH, TH and TPH) as well as for all isoforms of NOS. The regeneration pathway involves two steps catalysed by PCD and DHPR. The red line indicates the enzyme targeted in the hph-1 mouse model. Abbreviations: GTPCH1, GTP cyclohydrolase 1; PTS, 6-pyruvoyltetrahydrobiopterin synthase; SR, sepiapterin reductase; PAH, phenylalanine hydroxylase; TH, tyrosine hydroxylase; TPH, trypthophan hydroxylase; NOS, nitric oxide synthase; AADC, aromatic L-amino acid decarboxylase; PCD, pterin-4-acarbinolamine dehydratase; DHPR, dihydropteridine reductase.
Intact paw thickness in hph mice following CFA injection. The dorsal-plantar paw thickness was measured using a digital caliper. A significant increase in paw thickness was found in both genotypes as compared with baseline values (n = 5). WT: ***p < 0.001 and hph: †††p < 0.001. No significant difference was found between genotypes (p > 0.05). Two-way RM-ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean + SEM.
No heat hypersensitivity was observed following capsaicin injection in both WT and hph mice. Heat sensitivity was examined before as well as 15, 30, 45 and 60 minutes after intraplantar injection of capsaicin. Capsaicin did not induce heat hypersensitivity in both WT and hph mice (p > 0.05, n = 4). Two-way RM-ANOVA with pair-wise comparisons using the Fisher’s LSD test. Data are presented as mean ± SEM.