Abstract
Background and the purpose of the study
Silymarin, a standardized extract of the milk thistle (Silybum marianum), is believed to exert some of its hepatoprotective effects though inhibition of free radicals and inflammation. In this study the effect of some pro- and anti-inflammatory cytokines and also antioxidant genes polymorphisms on the hepatoprotective effects of silymarin in the occupationally exposed individuals to hydrogen sulfide (H2S) in the sour natural gas refinery was investigated.
Methods
We genotyped seven polymorphisms in six genes reported by others as modifiers of oxidative stress (NQO1, mEPXH1, GSTT1 and GSTM1) and inflammation (TNF-α and TGF-β1) for an association in effect of decreasing in liver function tests (LFTs). The LFTs of 77 sour gas refinery workers were measured before and after administration of silymarin (140 mg, three times per day for 1 month).
Results
A significant reduction of blood AST, ALT and ALP was observed after 30 days of consumption (p < 0.001). The decreasing effect of silymarin on ALT in the subjects with high producer genotype (A allele carriers) was less than low producers. There were no significant associations between TGF-β1 and the studied genes of oxidative stress pathway and the effectiveness of silymarin.
Conclusion
This is the first report about the effectiveness of silymarin in the subjects exposed chronically to H2S. Meanwhile, the modulatory effect of TNF-α on the effectiveness of silymarin might be used for individualize therapy.
Keywords: Silymarin, Hepatoprotective, Hydrogen sulfide, Occupational toxicology, Polymorphism
Introduction
The workers of natural gas refineries are exposed to potentially toxic substances like hydrogen sulfide (H2S) and benzene. Recently we have shown that the oxidative stress biomarkers increased in the workers of sweet and, in higher levels, sour gas refineries [1]. Meanwhile the levels of liver function tests (LFTs) were high in the H2S exposed subjects [1]. Although pathogenesis of liver toxicity induced by H2S has not been elucidated so far, there is experimental evidence that induction of oxidative stress plays a key role [2,3]. Several observations suggest that increased reactive oxygen species (ROS) and resulting oxidative stress is a critically important determinant of liver injury [4,5]. Oxidative stress is involved in the release of pro-inflammatory mediators (cytokines and chemokines) and injury to the liver cells [4,5]. Silymarin, a mixture of flavonolignans isolated from Silybum marianum (milk thistle), has been used to treat liver diseases for hundreds of years [6,7]. The hepatoprotective effect of silymarin is mainly due to its strong antioxidant activity and scavenging the free radicals [8]. It also effectively inhibits the inflammation [9,10], which can also damage the liver [6,11]. Silybin is an effective antioxidant, conserving GSH in liver cells while stabilizing the liver cell membranes against oxidative attack [4,12].
There are inter-individual variations in detoxifying enzymes and cytokines that could be translated into differences in susceptibility for xenobiotic toxicity and treatment outcomes [13-17]. So in this study, we decided to investigate the association of polymorphisms in the enzymes involved in general oxidative stress defence including NAD(P)H:quinone oxidoreductase-1 (NQO1), Glutathione S-transferases (GST) M1 and T1, and microsomal epoxide hydrolase (EPHX1) enzymes and the two most important cytokines involved in the pathogenesis of liver disease with the basis of inflammation, namely tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) cytokines and the treatment outcome of silymarin in the subjects chronically exposed to H2S.
Materials and methods
Materials
Silymarin containing tablets (Livergol) was purchased from Goldaru, Isfahan, Iran. PCR master mix was obtained from CinnaClone (Iran). Ethylenediamine tetraacetic acid (EDTA), Tris, agarose, Sodium Dodecyl Sulfate (SDS), isopropanol and ethanol were purchased from Merck (Germany). Proteinase K and DNA ladder were obtained from Fermentas (Germany).
Study subjects
In this cross-sectional study, 77 employees of sour gas refinery were recruited. According to local authorities, the atmospheric concentration of H2S in the place was 10–15 ppm [1]. The eligibility criteria for the cases were as follow: working at least 5 years in the refinery and no history of liver disease. Demographic data, general health conditions, lifestyle, years of employment and smoking habits were registered in a questionnaire. Written informed consent was obtained from the subjects after describing the aim of the study. The study was conducted according to the declaration of Helsinki and subsequent revisions and approved by the Ethical Committee of Kerman University of Medical Sciences (No: 91/91/k, KMU). The liver function tests (LFTs) including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were determined before and after administration of silymarin at the dosage of 150 mg three times per day (Livergol, Goldaru, Esfahan, Iran).
DNA extraction and GST genotyping
DNA was extracted from 5 ml peripheral blood using Miller method [18]. The subjects were genotyped for the deletion in GSTM1 and GSTT1 by a multiplex polymerase chain reaction (PCR). The PCR conditions and primers were as described previously [19]. TNF-α -308G/A and TGF-β1 codon 10 and codon 25 genotyping methods were based on the PCR-ARMS, and were similar to those previously described [20]. The amplified fragments were 184-bp, 241-bp and 233-bp respectively for TNF-α, TGF-β1 codon 10 and 25. EPXH1 exon 4 (His139Arg), NQO1 (C609T) and TNF-α (G-308A, for confirmation of PCR-ARMS for TNF-α -308G/A) genotyping methods were based on PCR-RFLP and as described before [21-23]. The products of each PCR reaction and digestion were resolved by electrophoresis on 3% agarose gels stained with ethidium bromide. Reliability and validity of the PCR and RFLP methods were assessed through re-conducting the genotype assays using at least a 10% sample of our DNA samples. The polymorphisms of heterozygous, homozygous and wild-type TNF-α which obtained by PCR-ARMS were re-checked with PCR-RFLP method. In addition, for TGF-β1 the method was also assessed through re-conducting the genotype assays using most of DNA samples with mutations (both heterozygous and homozygous). The results for all re-assessments were 100% concordant.
Statistical analysis
For the comparison of continuous variables, we firstly checked the assumption that they were normally distributed. If the distribution was normal, the results were expressed as means and standard error (SE) and a classical t-test or one-way ANOVA was used accordingly. According to the genes polymorphisms, genotypes were collapsed into two categories: homozygous (common genotypes) versus heterozygous + variant homozygous and they were coded as follow: GSTM1*0 (code 0) and GSTM1-positive (code 1), GSTT1*0 (code 0) and GSTT1-positive (code 1), NQO1 CC allele (code 0) and CT/TT alleles (code 1), EPXH exon 4 AA allele (code 0) and AG/GG alleles (code 1), TGF-β1 codon 10 TT allele (code 0, high producer) and TC/CC alleles (code 1, low producer), and TGF-β1 codon 25 GG allele (code 0, high producer) and GC/CC alleles (code 1, low producer). Combination of TGF-β1 codons 10 and 25 was as follow: TT/GG (code 0, high producer) and all other combinations (code 1, low producer). Simple and multiple linear regression was used to screen the independent variables including genetic polymorphism to best predict the value of the LFTs and LFTs difference. Age and BMI variables believed to potentially confound the association with LFTs’ level were included in the multivariate model. The significance (P) of genetic polymorphisms factors from multivariate analysis was considered as adjusted p-value. Differences between LFTs before and after administration of silymarin were analyzed using paired-sample t-test. For all the tests, a p-value less than 0.05 was considered to be significant. All analyses were conducted using SPSS statistical software (version 11.5).
Results
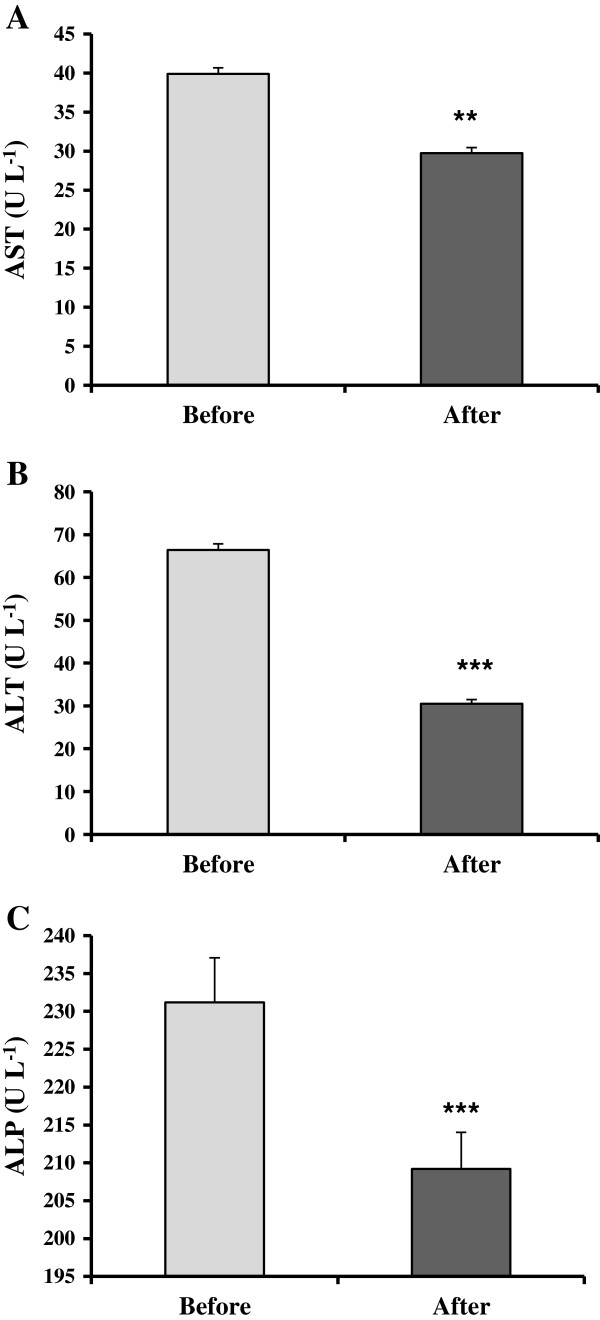
Significant decreases in AST (P < 0.01), ALT (P < 0.001) and ALP (P < 0.001) were observed by use of silymarin (Figure 1). The mean ± SE of AST before and after using were 39.8 ± 0.78 and 29.7 ± 0.07 U L-1 (Figure 1A). A significant (P < 0.001) decrease in ALT was observed after administration of silymarin (66.4 ± 1.4 U L-1 before versus 30.5 ± 0.9 U L-1 after (Figure 1B). After use of silymarin, the ALP level decreased significantly (P < 0.001). The mean ± SE values before and after were 231.2 ± 5.9 and 209.2 ± 4.8 U L-1 (Figure 1C).
Figure 1.
Effect of the consumption of silymarin on serum LFTs. AST (A), ALT (B) and ALP (C) levels in the chronically H2S exposed subjects (n = 77) were measured before and after administration of silymarin. Results are expressed as mean ± SE. ** p < 0.001 and *** p < 0.001 compared with before treatment by Pair sample t-test.
Simple and multivariate linear regression were performed to analyze the association of GSTM1, GSTT1, NQO1 and EPXH1 genes polymorphisms with changes in LFTs after administration of silymarin (Table 1). There was no association between the abovementioned genes’ polymorphisms and decreasing in LFTs after administration of silymarin.
Table 1.
Association of oxidative stress related genes polymorphisms with changes in LFTs’ levels by consumption of silymarin
|
LFT |
Gene |
Unadjusted |
|
Adjusted |
|
|---|---|---|---|---|---|
| B (CI) | p-value | B (CI) | p-value | ||
|
AST |
NQO1 |
−0.73 (−3.38, 4.84) |
0.72 |
−0.05 (−4.10, 3.92) |
0.98 |
|
GSTM1 |
−0.58 (−4. 02, 3.03) |
0.75 |
−0.64 (−4.25, 2.97) |
0.72 |
|
|
GSTT1 |
1.27 (−3.10, 5.64) |
0.56 |
1.92 (−2.55, 6.38) |
0.40 |
|
|
EPXH4 |
1.34 (−3.10, 5.77) |
0.54 |
1.72 (−2.73, 6.17) |
0.44 |
|
|
ALT |
NQO1 |
0.87 (−4.20, 5.92) |
0.73 |
0.79 (−4.40, 5.98) |
0.76 |
|
GSTM1 |
−4.24 (−8. 62, 0.13) |
0.05 |
4.21 (−8.67, 0.25) |
0.06 |
|
|
GSTT1 |
1.30 (−4.23, 6.83) |
0.64 |
1.22 (−4.50, 6.92) |
0.65 |
|
|
EPXH4 |
1.04 (−3.02, 4.67) |
0.54 |
0.89 (−4.46, 6.24) |
0.74 |
|
|
ALP |
NQO1 |
−12.83 (−39.89, 14.22) |
0.35 |
−2.64 (−20.22, 14.95) |
0.76 |
|
GSTM1 |
1.21 (−23.79, 26.21) |
0.92 |
2.27 (−13.67, 18.22) |
0.77 |
|
|
GSTT1 |
21.28 (−8.93, 51.50) |
0.16 |
10.21 (−9.46, 29.87) |
0.30 |
|
| EPXH4 | −14.20 (−47.62, 19.22) | 0.40 | 1.73 (−17.78, 21.24) | 0.86 |
Multivariate regression: Parameters adjusted for age and BMI;
B: Partial regression coefficient.
Simple and multivariate linear regression were performed to analyze the association of TNF-α, TGF-β1 codon 10, TGF-β1 codon 25 and combination of these two codons polymorphisms with changes in LFTs after administration of silymarin (Table 2). TNF-α G-308A polymorphism was significantly associated with deceasing in the AST (p = 0.04) and ALT (p = 0.003). These results show that decreasing in the AST and ALT levels are lower in the subjects with high producer TNF-α genotype. These associations remained significant after adjustment with age and BMI. There was also negative significant association between TGF-β1 codon 10 polymorphisms and decreasing in ALP that this association was abolished when age and BMI added to the model.
Table 2.
Association of inflammation related cytokines polymorphisms with changes in LFTs’ levels by consumption of silymarin
|
LFT |
Gene |
Unadjusted |
|
Adjusted |
|
|---|---|---|---|---|---|
| B (CI) | p-value | B (CI) | p-value | ||
|
AST |
TNF-α |
−4.27 (−8.57, 0.03) |
0.05 |
−4.58 (−8.97, -0.21) |
0.04* |
| TGF-β1 codon 10 |
−0.77 (−5. 17, 3.61) |
0.72 |
−0.07 (−4.56, 4.41) |
0.97 |
|
| TGF-β1 codon 25 |
2.78 (−3.36, 8.93) |
0.36 |
2.79 (−3.40, 8.98) |
0.37 |
|
| TGF-β1 codons 10/25 |
0.99 (−3.04, 5.03) |
0.62 |
1.37 (−2.73, 5.46) |
0.50 |
|
|
ALT |
TNF-α |
−8.30 (−13.73, -2.86) |
0.003* |
−8.93 (−14.62, -3.23) |
0.003* |
| TGF-β1 codon 10 |
−3.62 (−8.92, 1.67) |
0.17 |
−3.43 (−9.00, 2.14) |
0.22 |
|
| TGF-β1 codon 25 |
5.87 (−1.29, 13.03) |
0.10 |
5.94 (−1.36, 13.24) |
0.10 |
|
| TGF-β1 codons 10/25 |
−0.33 (−5.28, 4.63) |
0.89 |
0.03 (−5.11, 5.24) |
0.99 |
|
|
ALP |
TNF-α |
−6.17 (−41.08, 28.75) |
0.72 |
−0.85 (−21.93, 20.21) |
0.93 |
| TGF-β1 codon 10 |
−31.45 (−61.31, -1.59) |
0.03 |
−9.05 (−27.43, 8.32) |
0.33 |
|
| TGF-β1 codon 25 |
16.67 (−25.38, 58.73) |
0.43 |
−12.00 (−37.17, 13.17) |
0.34 |
|
| TGF-β1 codons 10/25 | −16.16 (−44.41, 12.07) | 0.25 | −8.65 (−24.63, 7.33) | 0.28 |
Multivariate regression: Parameters adjusted for age and BMI. *Significant effect. B: Partial regression coefficient.
Discussion
In light of our recent study demonstrating increase in the LFT levels in the workers exposed chronically to H2S, we became interested in determining whether a well-known hepatoprotective natural product like silymarin could decrease the LFTs in the subjects and if the genetics of the subjects could determine responding to treatment. Administration of silymarin (140 mg tablets, thrice per day for 1 month) significantly reduced the LFTs in the subjects. The hepatoprotective mechanisms of silymarin on H2S-induced elevation of LFTs have not been addressed so far. Recently we have also shown that stress oxidative biomarkers were higher in the workers in exposure to H2S [1]. Lines of evidence demonstrate that stress oxidative is one of the main mechanisms for the toxic effect of xenobiotics on the liver [4,15,24]. Antioxidant activity and scavenging the free radicals have been reported as the main mechanism for the hepatoprotective effect of silymarin [6].
Our data showed significant association (B = −8.30, CI: -13.73,-2.86) between the polymorphism of TNF-α G-308A and the changes in ALT and AST after administration of silymarin (Table 2). In other words, decrease in ALT and AST in the high TNF-α producers treated with silymarin is significantly lower than low producers. TNF-α is a pro-inflammatory cytokine involved in the pathogenesis of variety of liver injuries [25]. TNF-α, in concert with other cytokines, mediates the recruitment of neutrophils to the liver that induce inflammation and cell death [26]. Production of TNF-α is under genetic control which determined by a number of single nucleotide polymorphisms in this gene. The G-308A polymorphism in the promoter of the gene is the most studied one that has been linked to higher TNF-α production [27,28] as homozygosity for TNF1 (TNF1/TNF1) is associated with low production (TNF-αLo), whereas the TNF1/TNF2 and TNF2/TNF2 genotypes are high producers (TNF-αHi). The effect of TNF-α on the production of ROS was also shown elsewhere [25]. TNF-induced ROS production activates JNK (c-jun N-terminal kinase) by oxidizing and inactivating several members of the MAP kinase phosphates (MKPs). Taking together, one might relate the less hepatoprotective effect of an antioxidant compound like silymarin in high producer of TNF-α to higher production of ROS in these subjects.
Our data also showed no association between the polymorphisms of TGF-β and some of detoxifying enzymes and the changes in LFTs after administration of silymarin. Univariate regression analysis showed a significant association between TGF-β1 gene polymorphism at codon 10 and ALP levels that this association was abolished after adjusting the regression with confounders like age and BMI. This is in consistent with the previous reports about the role of BMI on the LFTs [29].
Conclusion
Administration of silymarin (140 mg tablets, thrice per day for 1 month) significantly reduced the LFTs in the subjects exposed chronically to H2S. Meanwhile, increasing the dose of silymarin in the high TNF-α producers to obtain therapeutic effects might be needed.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM participated in the design of the study, performed the statistical analysis, participated in the sequence alignment and drafted the manuscript. AS carried out sampling and carried out genotyping of some genes. AE carried out genotyping of some genes and filed the data. VM carried out genotyping of some genes. ESh carried out DNA extraction and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Ali Mandegary, Email: alimandegary@kmu.ac.ir.
Arastoo Saeedi, Email: saeediarastoo@yahoo.com.
Aziz Eftekhari, Email: azizeftekhari@yahoo.com.
Vahideh Montazeri, Email: v_montazeri1360@gmail.com.
Elham Sharif, Email: elhamsharif86@yahoo.com.
Acknowledgements
This works is supported by Deputy of Research, Kerman University of Medical Sciences. The authors would like to thank Ms. Maryam Sezavar and Dr. Samaneh Soleymani for their helps. This article also has been derived from the thesis of MSc students (AS and AE) in Kerman University of medical Sciences, Faculty of Pharmacy, Kerman, Iran.
References
- Mandegary A, Sezavar M, Saeedi A, Amirheidari B, Naghibi B. Oxidative stress induced in the workers of natural gas refineries, no role for GSTM1 and GSTT1 polymorphisms. Hum Exp Toxicol. 2012;31:1271–1279. doi: 10.1177/0960327112450898. [DOI] [PubMed] [Google Scholar]
- Eghbal MA, Pennefather PS, O’Brien PJ. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology. 2004;203:69–76. doi: 10.1016/j.tox.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455–459. doi: 10.1158/1541-7786.MCR-06-0439. [DOI] [PubMed] [Google Scholar]
- Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY, Shyu JC, Hsieh YS, Tsai CC, Liu YC. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J Viral Hepat. 2008;15:508–514. doi: 10.1111/j.1365-2893.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- De La Puerta R, Martinez E, Bravo L, Ahumada MC. Effect of silymarin on different acute inflammation models and on leukocyte migration. J Pharm Pharmacol. 1996;48:968–970. doi: 10.1111/j.2042-7158.1996.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects (Review) Int J Oncol. 2005;26:169–176. [PubMed] [Google Scholar]
- Luster MI, Simeonova PP, Gallucci RM, Bruccoleri A, Blazka ME, Yucesoy B. Role of inflammation in chemical-induced hepatotoxicity. Toxicol Lett. 2001;120:317–321. doi: 10.1016/S0378-4274(01)00284-3. [DOI] [PubMed] [Google Scholar]
- Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Fabris C, Soardo G, Falleti E, Toniutto P, Vitulli D, Federico E, Del Forno M, Mattiuzzo M, Gonano F, Pirisi M. Relationship among hepatic inflammatory changes, circulating levels of cytokines, and response to IFN-alpha in chronic hepatitis C. J Interferon Cytokine Res. 1998;18:705–709. doi: 10.1089/jir.1998.18.705. [DOI] [PubMed] [Google Scholar]
- Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- Abbas Z, Moatter T, Hussainy A, Jafri W. Effect of cytokine gene polymorphism on histological activity index, viral load and response to treatment in patients with chronic hepatitis C genotype 3. World J Gastroenterol. 2005;11:6656–6661. doi: 10.3748/wjg.v11.i42.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P, Gomez J, Mozo L, Gutierrez C, Suarez A. Cytokine polymorphisms influence treatment outcomes in SLE patients treated with antimalarial drugs. Arthritis Res Ther. 2006;8:R42. doi: 10.1186/ar1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandegary A, Rostami S, Alimoghaddam K, Ghavamzadeh A, Ghahremani MH. Gluthatione-s-transferase T1-null Genotype predisposes adults to acute promyelocytic leukemia; a case–control study. Asian Pacific J Cancer Prev. 2011;12:1279–1282. [PubMed] [Google Scholar]
- Perrey C, Turner SJ, Pravica V, Howell WM, Hutchinson IV. ARMS-PCR methodologies to determine IL-10, TNF-alpha, TNF-beta and TGF-beta 1 gene polymorphisms. Transpl Immunol. 1999;7:127–128. doi: 10.1016/S0966-3274(99)80030-6. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Yu CJ, Chen CJ, Yang PC. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur Respir J. 2004;23:818–824. doi: 10.1183/09031936.04.00104904. [DOI] [PubMed] [Google Scholar]
- Naoe T, Takeyama K, Yokozawa T, Kiyoi H, Seto M, Uike N, Ino T, Utsunomiya A, Maruta A, Jin-nai I, Kamada N, Kubota Y, Nakamura H, Shimazaki C, Horiike S, Kodera Y, Saito H, Ueda R, Wiemels J, Ohno R. Analysis of genetic polymorphism in NQO1, GST-M1, GST-T1, and CYP3A4 in 469 Japanese patients with therapy-related leukemia/ myelodysplastic syndrome and de novo acute myeloid leukemia. Clin Cancer Res. 2000;6:4091–4095. [PubMed] [Google Scholar]
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol. 2009;1:72–78. doi: 10.4254/wjh.v1.i1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- Marcos M, Gomez-Munuera M, Pastor I, Gonzalez-Sarmiento R, Laso FJ. Tumor necrosis factor polymorphisms and alcoholic liver disease: a HuGE review and meta-analysis. Am J Epidemiol. 2009;170:948–956. doi: 10.1093/aje/kwp236. [DOI] [PubMed] [Google Scholar]
- Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/S0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Choi JW. Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann Clin Lab Sci. 2003;33:257–264. [PubMed] [Google Scholar]