Abstract
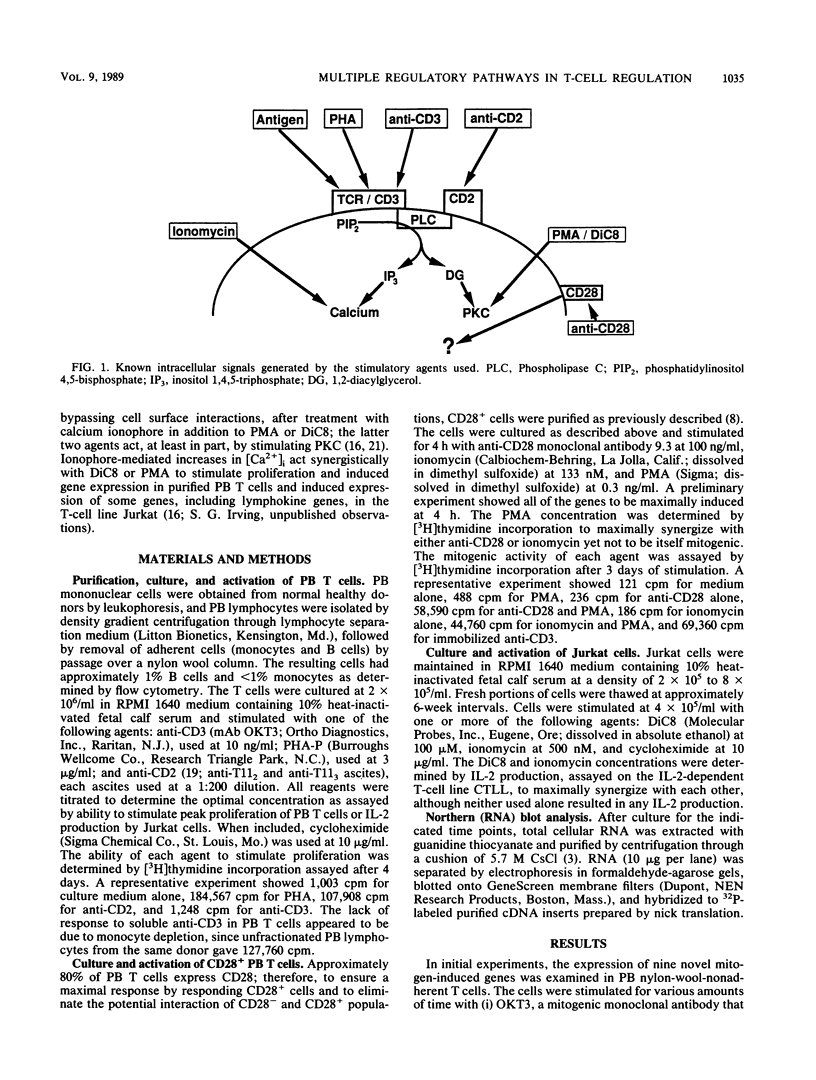
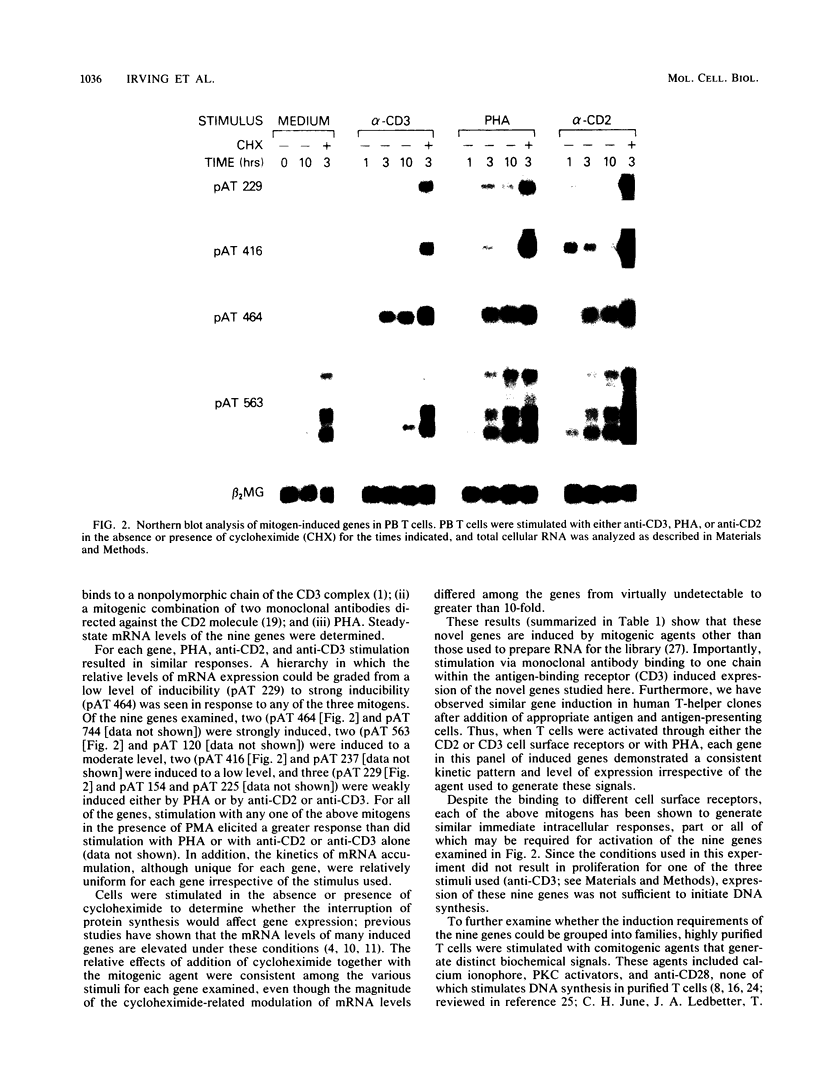
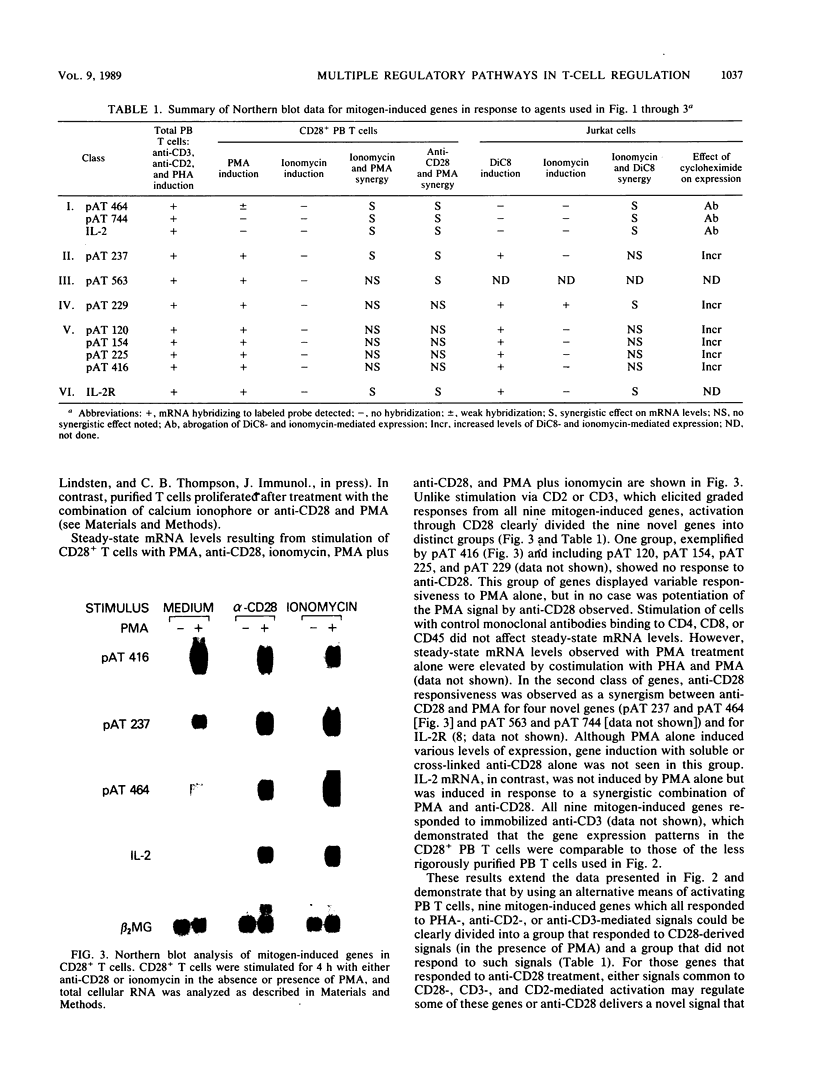
The delivery of a mitogenic signal to T cells via any one of several cell surface molecules elicits a variety of intracellular responses, some or all of which regulate subsequent gene expression events. The expression of nine novel mitogen-induced genes in response to various T-cell-activating agents was examined to evaluate the diversity of pathways which regulate such genes. The relative contribution of distinct secondary signals, individually or together, to mitogen-stimulated gene induction and the capability of individual genes to respond to the sometimes divergent signals generated from different cell surface structures is addressed. The activation of T cells with mitogenic monoclonal antibodies directed against the CD2 or CD3 cell surface molecules, or with phytohemagglutinin, induced all nine genes. Thus, stimulation by fully mitogenic agents regardless of cell surface-binding specificity correlated with the expression of all of the genes studied. However, heterogeneous patterns of gene expression, encompassing five regulatory classes, were revealed by the use of phorbol 12-myristate 13-acetate, calcium ionophore, and anti-CD28 monoclonal antibody, agents which mediated only a subset of intracellular events and thus an incomplete mitogenic signal. Interleukin-2 and two novel lymphokines represented one regulatory class that appeared to require unique transcriptional activation signals relative to the other mitogen-induced genes. As demonstrated in the accompanying paper (P. F. Zipfel, S. G. Irving, K. Kelly, and U. Siebenlist, Mol. Cell. Biol. 9:1041-1048, 1989), the immediate transcriptional response of T cells to mitogenic stimulation is quite complex, involving numerous genes beyond those which have been previously described. Furthermore, the discrimination of several regulatory phenotypes among these nine genes suggests that a multiplicity of signaling pathways extends from the cell surface to the level of transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst J., Alexander S., Elder J., Terhorst C. The T3 complex on human T lymphocytes involves four structurally distinct glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5135–5141. [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985 Feb 10;260(3):1562–1566. [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W. Association of protein kinase C activation with IL 2 receptor expression. J Immunol. 1986 Feb 15;136(4):1266–1273. [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Martin P. J., Spooner C. E., Hansen J. A., Meier K. E. Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol. 1986 Jun 1;136(11):3945–3952. [PubMed] [Google Scholar]
- Ledbetter J. A., Parsons M., Martin P. J., Hansen J. A., Rabinovitch P. S., June C. H. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol. 1986 Nov 15;137(10):3299–3305. [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger B., Weiss A., Imboden J., Laing T., Stobo J. D. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987 Oct 15;139(8):2755–2760. [PubMed] [Google Scholar]
- May W. S., Lapetina E. G., Cuatrecasas P. Intracellular activation of protein kinase C and regulation of the surface transferrin receptor by diacylglycerol is a spontaneously reversible process that is associated with rapid formation of phosphatidic acid. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1281–1284. doi: 10.1073/pnas.83.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moretta A., Poggi A., Olive D., Bottino C., Fortis C., Pantaleo G., Moretta L. Selection and characterization of T-cell variants lacking molecules involved in T-cell activation (T3 T-cell receptor, T44, and T11): analysis of the functional relationship among different pathways of activation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1654–1658. doi: 10.1073/pnas.84.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Kozumbo W. J., Moretta L., Moretta A. Transmembrane signalling via the T11-dependent pathway of human T cell activation. Evidence for the involvement of 1,2-diacylglycerol and inositol phosphates. Eur J Immunol. 1987 Jan;17(1):55–60. doi: 10.1002/eji.1830170110. [DOI] [PubMed] [Google Scholar]
- Saito T., Germain R. N. The generation and selection of the T cell repertoire: insights from studies of the molecular basis of T cell recognition. Immunol Rev. 1988 Jan;101:81–113. doi: 10.1111/j.1600-065x.1988.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]