Abstract
The ε-caprolactam is the monomer of the synthetic non-degradable nylon-6 and often found as nonreactive component of nylon-6 manufacturing waste effluent. Environmental consequences of its toxicity to natural habitats and humans pose a global public concern. Soil samples were collected from three designated solid waste dumpsites, namely, Abule-Egba, Olusosun and Isheri-Igando in Lagos State, Nigeria. Sixteen bacteria isolated from these samples were found to utilize the ε-caprolactam as a sole source of carbon and nitrogen at concentration of ≤20 g l−1. The isolates were characterized using their 16S rRNA gene sequence and showed similarity with Pseudomonas sp., Proteus sp., Providencia sp., Corynebacterium sp., Lysinibacillus sp., Leucobacter sp., Alcaligenes sp. and Bordetella sp. Their optimal growth conditions were found to be at temperature range of 30 to 35 °C and pH range of 7.0–7.5. High Performance liquid chromatography analysis of the ε-caprolactam from supernatant of growth medium revealed that these isolates have potential to remove 31.6–95.7 % of ε-caprolactam. To the best of our knowledge, this study is first to report the ability of Proteus sp. and Bordetella sp. for ε-caprolactam utilization.
Keywords: ε-Caprolactam, Biodegradation, Bacteria, 16S rRNA, HPLC
Introduction
ε-caprolactam is an organic compound consisting of carbon, Nitrogen, oxygen and hydrogen which is an exclusive raw material for nylon-6, produced by hydrolytic polymerization of ε-caprolactam [16]. It is found as unreacted monomer in waste water effluents of nylon-6 producing factories, constituting significant pollution load with toxic effect on plants, animals and humans [7]. Also, its solid oligomers and used nylon-6 fibre constitute solid waste materials that end up in dumpsites, eventually to uncontrolled open environment. Reports have shown the ability of some bacteria to remove ε-caprolactam from waste water treatment sludge. Biochemical and genetic aspects of ε-caprolactam biodegradation have been described by Prijambada et al. [14] where Pseudomonas and Flavobacterium were found to degrade ε-caprolactam isolated from soil and effluent water released by nylon-6 producing factories. Baxi and Shah [3] isolated Alcaligenes faecalis, Arthrobacter citrus, Bacillus sphaericus and Rhodococcus rhodochrous from different soils of a nylon-6 manufacturing site and these isolates were able to degrade ε-caprolactam in solution. In a recent study [16], ε-caprolactam denitrifying bacteria were isolated including Hyphomicrobium species, Methylosinus pucelena and Magnetospirillum species from the waste water treatment system of a chemical manufacturing company.
Scarcity of information on potential caprolactam degraders from solid waste dumpsites, which are the ultimate repository of the sludge from the treatment plants as well as the recalcitrant solid waste of the caprolactam products, stimulated this research. The current study focused on isolation and the characterization of ε-caprolactam degrading bacteria from different solid waste dumpsites in Nigeria with the view of investigating their potential on nylon-6 fibre degradation.
Materials and Methods
Isolation of ε-Caprolactam Utilising Bacteria
Soil samples were collected from the three major solid waste dumpsites in Lagos State, South Western Nigeria, namely Olusosun, Abule-Egba and Isheri-Igando dump. 1 g of soil sample was mixed in 10 ml phosphate buffer saline (PBS; 8 g NaCl, 0.2 g KCl, 1.44 g NaH2PO4, 0.24 g KH2PO4), serially diluted and plated on nutrient agar. Pure cultures of bacterial isolates were obtained and screened for their ability to utilize ε-caprolactam on solid basal medium containing 0.2 g KH2PO4, 0.6 g K2HPO4, 0.3 g NaCl, 0.2 g MgSO4 7H2O, 0.1 g CaCl2 2H2O, 0.1 g FeCl3 with 10 g ε-caprolactam per litre supplemented with 15 g agar and varied pH and temparatures [4]. All chemicals and reagents were obtained from Zayo–Sigma Chemicals Limited, Nigeria. For each isolate, four test tubes containing 5 ml phosphate buffer of pH 6.0, 6.4, 7.0, 7.4, 8.0 and 8.5 were prepared with suppliment of 1 % ε-caprolactam. Each tube was then inoculated with 0.5 ml overnight-grown culture and incubated separately at 27, 30, 35 and 40 °C respectively. Growth of the microorganisms was measured spectrophotometrically at 600 nm at the zero hour blank and at 24 h incubation. Isolates that were able to grow on this agar medium in 24 h were selected to possess the ability to utilise the substrate as sources of both carbon and nitrogen.
Identification of Bacterial Isolates
Identification of bacterial isolates was performed using 16S rRNA gene sequencing approach. Genomic DNA was isolated as described previously [2]. The PCR assay was performed using Applied Biosystems, model 9800 with 50 ng of DNA extract in a total volume of 25 μl. The PCR master mix contained 2.5 μl of 10X PCR reaction buffer (with 1.5 M MgCl2), 2.5 μl of 2 mM dNTPs, 1.25 μl of 10 pm μl−1 of each oligonucleotide primer 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1391R (5′-GACGGGCGGTGTGTRCA-3′), 0.2 μl of 3 U/μl Taq DNA polymerase and 15.76 μl of glass-distilled PCR water [13]. Initially denaturation accomplished at 94 °C for 3 min. Thirty-two cycles of amplification consisted of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1.5 min. A final extension phase at 72 °C for 10 min was performed. The PCR product was purified by PEG-NaCl method [13]. The thermocycling for the sequencing reactions began with an initial denaturation at 94 °C for 2 min, followed by 25 cycles of PCR consisting of denaturation at 94 °C for 10 s, annealing at 50 °C for 10 s, and extension at 60 °C for 4 min using primers 704 F (5′-GTAGCGGTGAAATGCGTAGA-3′) and 907 R (5′-CCGTCAATTCMTTTGAGTTT-3′) [5]. The samples were purified using standard protocols described by Applied Biosystems®, Foster City, USA. To this, 10 μl of Hi-Di formamide was added and vortexed briefly. The DNA was denatured by incubating at 95 °C for 3 min, kept on ice for 5–10 min, and was sequenced in a 3,730 DNA analyzer (Applied Biosystems) following the manufacturer’s instructions. Obtained sequences were analysed using Sequence Scanner (Applied Biosystems) software. The rRNA sequence contigs were analysed using EzTaxon [8] and NCBI-BLAST [1] to find the closest match of the contiguous sequence.
Phylogenetic Analyses
Obtained 16S rRNA gene sequences were aligned using BioEdit® [6] and edited in DAMBE® [17]. The evolutionary history was inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa are clustered together in the bootstrap test (1,000 replicates) is recorded next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree using Molecular Evolutionary Genetics Analysis (MEGA, V. 5) software [15].
ε-Caprolactam Utilization Experiment
Degradation experiment for ε-caprolactam was conducted in the basal medium fortified with ε-caprolactam. The minimum inhibatory concentration was determined on the same medium at varied concentrations of 10, 15, 20, 25, and 30 g l−1 of ε-caprolactam. Bacterial isolates that grew on the solid basal medium at concentration of 10 g l−1 were selected and enriched in peptone broth for 24 h. 1 ml of each cultured isolate was then inoculated into 100 ml of the 1 % ε-caprolactam basal medium in 150 ml conical flasks and incubated at 35 °C on the G24 Environmental incubator shaker (New Brunswick Scientific Co., Inc., Edison, NJ, USA) at 180 rpm. The experiments were monitored at time interval of 24, 72 and 120 h. The cells were harvested by centifugation at 10,000 rpm at 4 °C for 10 min and cell free supernatants were used for the High Performance Liquid Chromatography (HPLC) analysis to estimate unutilised ε-caprolactam in the medium. A mixture of ε-caprolactam and 6-aminohaxanoic acid was used as a standard (50:50 v/v). The mobile phase consisted of methanol: water using concentration of 60:40 (v/v). The analyses were performed on Cecil-Adept System 4 (Analytical); UV–Visible detector; CE 4900 Power stream software at 200 nm and the flow rate of the mobile phase was 1 ml min−1 in TSK-GEL ODS-80TM 4.6 mm × 7.5 cm column [17].
Result and Discussion
Bacterial Identification and Phylogenetic Analysis
Sixty four different bacterial isolates were obtained from the soil samples based on difference in colony morphology. Identity of the isolates possessing the ability to utilize ε-caprolactam as a source of carbon and nitrogen were confirmed using 16S rRNA gene sequence approach (Table 1). Result of phylogenetic study of the bacteria showed that the isolates belong to three major taxonomic groups including Proteobacteria, Actinobacteria and Firmicutes (Fig. 2). All these bacterial groups have been reported in studies involving solid waste dumpsites [9–12] except isolate identified as Proteus sp. (strain NTS2). Figure 1 shows a phylogenetic tree of the isolates constructed with Molecular Evolution Genetics Analysis (MEGA) version 5. Phylogenetic analysis of the isolates using the neighbour-joining method grouped the isolates in to four different clusters. In this study, the diversity of the ε-caprolactam degrading bacteria isolates was presented in three phylogenetic divisions, the Proteobacteria (γ and β), Actinobacteria and Firmicutes constituting 43.75 % γ-Proteobacteria, 25 % β-Proteobacteria, 25 % Actinobacteria and 65 % Firmicutes. However, this study revealed that the Proteobacteria and the Actinobacteria are the dominant ε-caprolactam degrading groups in the studied dumpsites.
Table 1.
Characterisation of ε-caprolactam utilising bacterial isolates used in this study
| Isolate | Colony morphology | Gram character | Optimum temperature ( °C) | Optimum pH | 16S rRNA sequence length | Accession number (GenBank) | Accession number of closest match | Closest match using EzTaxon online database | Present similarity |
|---|---|---|---|---|---|---|---|---|---|
| 2ABA2 | CR, PY, Rd | −ve | 35 | 7.5 | 1228 | HE858273 | FN391024 | Paenalcaligenes hominis CCUG 53761A(T) | 100 |
| 2ABA4 | IR, R, CCR | −ve | 35 | 7.5 | 1168 | HE858274 | AM902716 | Bordetella petrii DSM 12804(T) | 100 |
| ABC3 | IR, W, Rd | −ve | 35 | 7.5 | 1165 | HE858275 | Z76651 | Pseudomonas aeruginosa LMG 1242(T) | 99.91 |
| BO2 | CR, W, Rd | +ve | 35 | 7.5 | 1188 | HE858276 | AJ781047 | Leucobacter aridicollis CIP 108388(T) | 99.66 |
| BO3 | CR, W, Rd | +ve | 35 | 7.5 | 1215 | HE858277 | AJ781047 | Leucobacter aridicollis CIP 108388(T) | 99.58 |
| FBC1 | CR, BR, Rd | −ve | 35 | 7.5 | 1210 | HE858278 | AM040495 | Providencia vermicola OP 1(T) | 98.92 |
| FBC2 | CR, W, Rd | +ve | 35 | 7.5 | 1219 | HE858279 | ADNS01000011 | Corynebacterium ammoniagenes DSM 20306(T) | 100 |
| FBC3 | CR, W, Rd | +ve | 35 | 7.5 | 1162 | HE858280 | ADNS01000011 | Corynebacterium ammoniagenes DSM 20306(T) | 100 |
| ISC1 | CR, W, Rd | +ve | 35 | 7.5 | 1207 | HE858281 | CP000817 | Lysinibacillus sphaericus C3-41 | 99.42 |
| ISC6 | IR, W, Rd | −ve | 35 | 7.5 | 1196 | HE858282 | Z76651 | Pseudomonas aeruginosa LMG 1242(T) | 99.91 |
| ISC7 | IR, W, Rd | −ve | 35 | 7.5 | 1207 | HE858283 | Z76651 | Pseudomonas aeruginosa LMG 1242(T) | 99.91 |
| NTS1 | IR, W, Rd | −ve | 30 | 7.0 | 1207 | HE858284 | Z76651 | Pseudomonas aeruginosa LMG 1242(T) | 99.83 |
| NTS2 | IR, W, Rd | −ve | 35 | 7.5 | 1192 | HE858285 | ACLE01000013 | Proteus mirabilis ATCC 29906(T) | 100 |
| NTS6 | IR, W, Rd | −ve | 35 | 7.5 | 1192 | HE858286 | Z76651 | Pseudomonas aeruginosa LMG 1242(T) | 99.91 |
| ORB5 | CR, W, Rd | −ve | 35 | 7.5 | 1215 | HE858287 | D88008 | Alcaligenes faecalis IAM 12369(T) | 97.25 |
| ORC1 | CR, W, Rd | −ve | 35 | 7.5 | 1198 | HE858288 | D88008 | Alcaligenes faecalis IAM 12369(T) | 97.63 |
CR Circular, IR Irregular, PY Pale Yellow W White, R Red, CCR Coccoid rods, Rd Rods
The 16S rRNA based identification was done using EzTaxon database at its online ‘identify’ tool
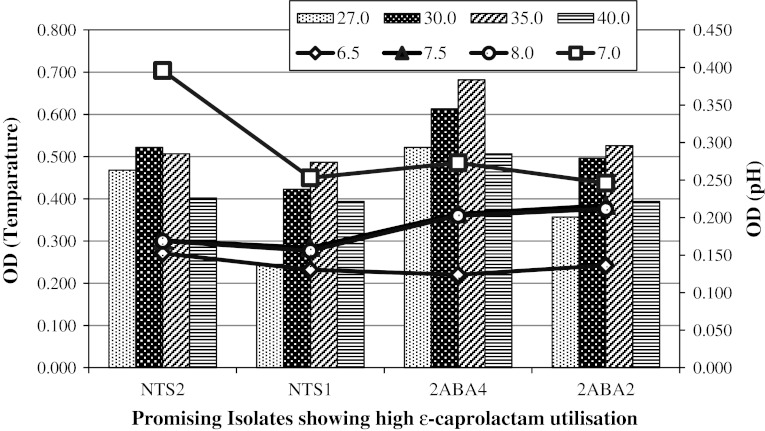
Fig. 2.
Temperature and pH growth parameters of four isolates viz. Proteus sp. strain NTS2, Pseudomonas sp. strain NTS1, Bordetella sp. strain 2ABA4 and Alcaligenes sp. strain 2ABA2 which showed promising ε-caprolactam utilisation activity. Bar graph for temperature and line graph for pH shows spectrophotometric optical density (OD) readings recorded at 600 nm
Fig. 1.
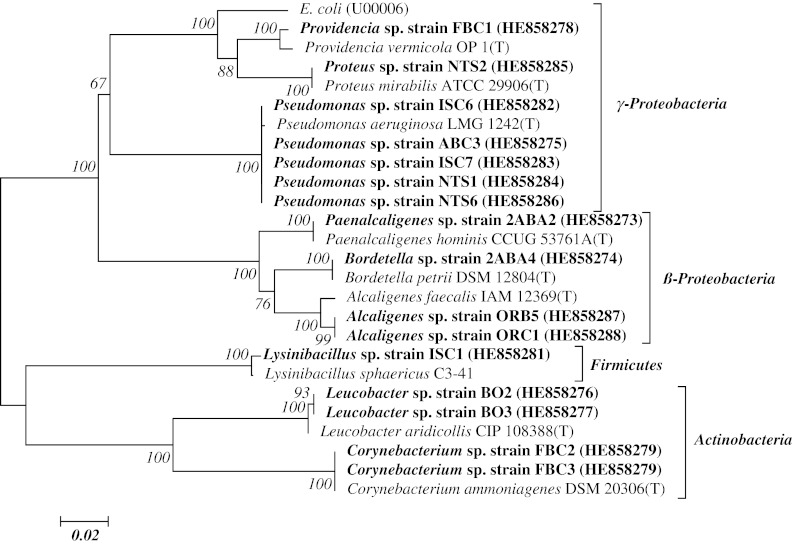
Neighbour- joining inferred tree based on 16S rRNA gene sequences showing the evolutionary relationship of bacterial isolates found utilising ε-caprolactam from solid waste dumpsites within previously characterised species. E. coli was used as ‘outgroup’. Type strains are indicated by T. Bar length represent the number of the base substitution per site. Bootstrap values expressed as percentage of 1,000 re-samplings of the neighbour-joining dataset are showed at nodes
Isolation and ε-Caprolactam Utilization Screening of Bacteria
The minimum inhibitory concentration of the caprolactam for all the sixteen bacterial isolates showed ε-caprolactam degrading ability was found to be 20 g l−1. Wang and Lee (2007) reported Paracocus versutus MDC-3 growing at the concentration of 1.6 g l−1 isolated from wastewater. Unlike previous report, isolates identified as Bordetella sp. strain 2ABA4, Alcaligenes sp. strain 2ABA2, Pseudomonas sp. strain ISC7 and Proteus sp. strain NTS2 from this study showed an exceptional growth potential on media containing ε-caprolactam at the concentration of 25 g l−1. Efficient ε-caprolactam degrading potential and high concentration tolerance capacity for caprolactam makes our isolates as ideal candidate for clean-up of caprolactam contaminated environment.
Optimal growth conditions
Optimum pH for the growth of the isolates was 7.5; however, four isolates those showed good ε-caprolactam utilisation activity were found growing at pH 7.0 as shown in Fig. 2. The optimal growth temperature range for these isolates found falling within the mesophilic temperature range (30–35 °C). Optimum temperature for the growth of the isolates in ε-caprolactam medium is given in Fig. 2. Our result indicates that 35 °C is the best temperature for all the isolates except for Proteus sp. strain NTS2 which shows its highest growth at 30 °C. Optimal growth at circumneutral pH and in mesophilic temperature range facilitates maintenance and mass production for bioremediation purpose under normal laboratory conditions in lesser cost and labour.
Biodegradation Experiments
Figure 3 shows percentage reduction in ε-caprolactam concentration by each isolate was calculated using High Performance Liquid Chromatography (HPLC). We reported highest ε-caprolactam reduction (95.7 %) by Proteus sp. strain NTS2 followed by Bordetella sp. strain 2ABA4 (89.1 %), Pseudomonas sp. strain NTS1 (86 %) and Alcaligenes sp. strain 2ABA2 (74.2 %). The least reduction of 31.6 % was recorded in Corynebacterium sp. strain FBC3. Although, most of the bacteria isolated in this study may have been found to be associated with environmental samples, there is no earlier report of such degradation ability showed by Proteus sp. strain NTS2. The expanded metabolic range as observed in these bacterial genera may be due to physiological adaptation as a result of alteration in enzyme substrate specificity or mutation in plasmid DNA harbouring genes coding ε-caprolactam utilising enzymes, which is focus of upcoming study on these isolates. The percentage of the ε-caprolactam utilized by the isolates from this study ranges between 99 and 31 % in the least performing bacteria isolates at 10 g l−1 concentration within 5 days. However, the bacteria strains isolated in this study displayed a better ε-caprolactam utilization potential compared to those of earlier reports and will be expected to play a better role in remediation of ε-caprolactam polluted water samples.
Fig. 3.
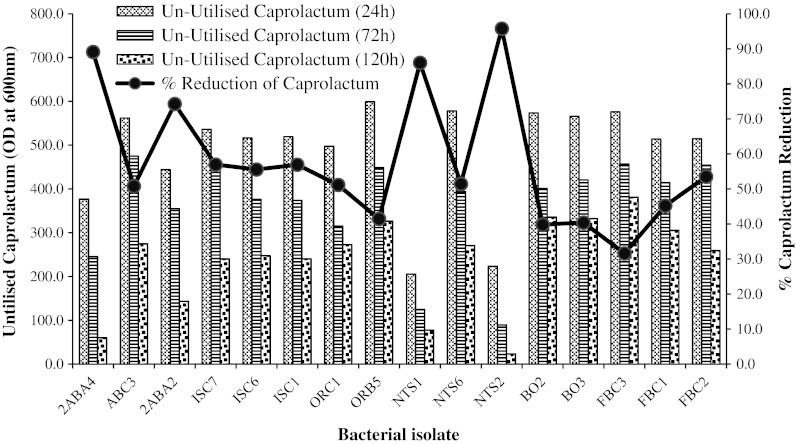
Percentage utilised caprolactam concentration by sixteen bacterial isolates after 120 h. Bar graph shows HPLC readings (OD at 600 nm) taken at the time intervals of 24, 48 and 120 h of un-utilised ε-caprolactam in the medium
Conclusion
Phylogenetic studies of 16 ε-caprolactam degrading bacteria from solid waste indicated that these isolates belong to 3-different phyla. Screening for degradation potential using HPLC indicates that our isolates have good potential for degradation of ε-caprolactam. Furthermore, we reported Proteus and Bordetella for the first time as ε-caprolactam degraders. The findings of our study indicate that these organisms can be a good tool for decontamination of ε-caprolactam contaminated site and will be valuable for study of pathway and genetics of ε-caprolactam degradation.
Acknowledgments
Authors are grateful to Dr. Om Prakash Sharma, Microbial Culture Collection (MCC), National Centre for Cell Science (NCCS), Pune for critical reading of the manuscript.
References
- 1.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel FM, Brent R, Kingston RE, Moore DM, Smith JA, Struhl K. Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 3.Baxi NN, Shah AK. Biological treatment of the components of solid oligomeric waste from nylon-6 production plant. World J Microbiol Biotechnol. 2000;16(8–9):835–840. doi: 10.1023/A:1008971216941. [DOI] [Google Scholar]
- 4.Baxi NN, Shah AK. ε-caprolactam degradation by Alcaligenes faecalis for remediation of wastewater of nylon-6 production plant. Biotechnol Lett. 2002;24(14):1177–1180. doi: 10.1023/A:1016187103682. [DOI] [Google Scholar]
- 5.Ben-Dov E, Shapiro OH, Siboni N, Kushmaro A. Advantage of using inosine at the 3′ termini of 16S rRNA gene universal primers for the study of microbial diversity. Appl Environ Microbiol. 2006;72:6902–6906. doi: 10.1128/AEM.00849-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 7.Johnson V, Patel SJ, Shah D, Patel KA, Mehta MH. Caprolactam waste liquor degradation by various yeasts. World J Microbiol Biotechnol. 1994;10:524–526. doi: 10.1007/BF00367658. [DOI] [PubMed] [Google Scholar]
- 8.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee CM, Wang CC. Denitrification with e-caprolactam by acclimated mixed culture and by pure culture of bacteria isolated from polyacrylonitrile fibre manufactured wastewater treatment system. Water Sci Technol. 2004;49(5):341–354. [PubMed] [Google Scholar]
- 10.Nabegu AB. An analysis of municipal solid waste in Kano metropolis. Niger J Hum Ecol. 2010;31(2):111–119. [Google Scholar]
- 11.Obire O, Nwaubeta O, Adue SBN. Microbial community of a waste-dump site. J Appl Sci Environ Manag. 2002;6(1):78–83. [Google Scholar]
- 12.Oviasogie FE, Ajuzie CU, Ighodaro UG. Bacterial analysis of soil from waste dumpsite. Arch Appl Sci Res. 2010;2(5):161–167. [Google Scholar]
- 13.Pidiyar VJ, Kaznowski A, Badri Narayan N, Patole MS, Shouche YS. Aeromonas culicicola sp. nov. from the midgut of Culex quinquefasciatus. Int J Syst Evol Microbiol. 2002;52:1723–1728. doi: 10.1099/ijs.0.02019-0. [DOI] [PubMed] [Google Scholar]
- 14.Prijambada ID, Negoro S, Yomo T, Urabe I. Emergence of nylon oligomer degradation enzymes in Pseudomonas aeruginosa PAO through experimental evolution. Appl Environ Microbiol. 1995;61(5):2020–2022. doi: 10.1128/aem.61.5.2020-2022.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2732. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CC, Lee CM. Isolation of the ε-caprolactam denitrifying bacteria from a wastewater treatment system manufactured with acrylonitrile-butadiene-styrene resin. J Hazard Mater. 2007;145(1–2):136–141. doi: 10.1016/j.jhazmat.2006.10.092. [DOI] [PubMed] [Google Scholar]
- 17.Xia X, Xie Z. DAMBE: Data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]