Abstract
The fungal endophyte Chaetomium globosum No.04 was isolated from the medicinal plant Ginkgo biloba. The crude extract of the fungus fermentation were active in the agar-diffusion tests against the phytopathogenic fungi Rhizopus stolonifer and Coniothyrium diplodiella. Further bioassay-guided chemical investigation led to the isolation and purification of six alkaloids and three non-targeted compounds from 50 L fermentation of this endophytic fungus and their structures were elucidated as chaetoglobosin A, C, D, E, G, R (1-6), ergosterol, allantoin and uracil, by means of spectroscopic analysis. Compounds 1-6 showed significant growth inhibitory activity against R. stolonifer and C. diplodiella at a concentration of 20 μg/disc. We present here, for the first time, the potent antifungal activity of chaetoglobosins from endophytic fungi against two important phytopathogenic fungi R. stolonifer and C. diplodiella.
Keywords: Chaetomium globosum, Antifungal activity, Chaetoglobosins
Introduction
Rhizopus stolonifer and Coniothyrium diplodiella are phytopathogenic fungi that caused plant diseases such as peach rot disease and grape white rot. Over several decades, various methods had been used to prevent, control, oreradicate plant diseases, and development of synthetic fungicides were primarily investigated [1, 2]. However, the global trend appears to be shifting towards reduced use of fungicides on produce and hence, there is a strong public and scientific desire to seek safer and eco-friendly alternatives to treat plant diseases in agriculture for reducing the decay loss [3]. Biological control provides an alternative to the use of synthetic fungicides with the advantages of greater public acceptance and reduced environ environmental impact [4].
Natural products have been a promising source of new lead compounds, and chemical agents in the agrochemical and pharmaceutical industries. Ever since the antibiotic drug penicillin was identified from Penicillium fungi in 1929, chemists have been focused interest on the discovery of novel bioactives from microbial metabolites. despite engaged in a synthetic products, bioactive natural products retain an profound effect on modern medicine and agriculture.
Endophytes can be found in virtually all terrestrial plants and play important role for the growth of hosts [5]. Recently, endophytes have been recognized as rich sources of structurally unique and biologically active secondary metabolites [6–9].
During the course of our ongoing search for biologically active secondary metabolites from endophytic fungus [10–13], we investigated antifungal metabolites produced by cultures of the endophytic fungus Chaetomium globosum No.04, harbored inside the healthy barks of medicinal plant Ginkgo biloba. against phytopathogenic fungi R. stolonifer and C. diplodiella (Speq.) Sacc. Bioassay-guided fractionation of the crude extract of cultures of this fungus led to the isolation of six active cytochalasan type alkaloids along with three non-target metabolites. In the present paper, we report, for the first time, the potent antifungal activity of chaetoglobosins against these two agricultural important phytopathogenic fungi R. stolonifer and C. diplodiella, which can cause serious plant diseases.
Materials and Methods
Microorganisms and Culture Conditions
The fungal strain C. globosum No.04 was separated from the fresh barks of the host Ginkgo biloba, a medicinal plant growing in Linyi, Shandong province, China. It was authenticated by Prof. Hongyu Pan based on morphological studies and has been deposited at College of Plant Science, Jilin University. R. stolonifer and C. diplodiella were isolated from infectious peach and grape, respectively.
Fermentation
After growing on potato dextrose agar (PDA) medium at 25 °C for 4 days, the fresh mycelium of strain C. globosum No.04 was inoculated in liquid medium containing: Oat flour 80 g, maltose 10 g, yeast extracts 2 g dissolved in 1,000 mL dH2O. The pH was adjusted to 6.0 before autoclaving. Fermentation was carried out in 2 L flasks each containing 1 L medium on a rotary shaker at 180 r/min, 25 °C for 8 days, and the cultures were used for the extraction and isolation.
General
Optical rotation was measured on a Perkin-Elmer 141 polarimeter. The melting point was determined on an XRC-1 micro-melting point apparatus and is uncorrected. IR spectra were obtained with a Nexus 870 FT-IR with KBr pellets. 1H NMR spectra: Varian Inova 400 (399.95 MHz), 500 (499.8 MHz), 600 (600 MHz). 13C NMR spectra: Varian Inova 500 (125.7 MHz), 600 (150.9 MHz). Chemical shifts were measured relative to tetramethylsilane as an internal standard, δ were expressed in ppm. Mass spectra: EI-MS at 70 eV with Varian MAT 731, Varian 311A, AMD-402, high resolution with perflurokerosine as standard. DCI-MS: Finnigan MAT 95 A, 200 eV, Reactant gas NH3. Column chromatography was carried out on silica gel (200–300 mesh, Merck, German) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden). All solvents used in this study were analytical grade. Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 10 % H2SO4 in ethanol.
Extraction and Isolation
The culture broth (50 L) of C. globosum No.04 was filtered to give the mycelium and water phase, The mycelium was dried at 45 °C, smashed and followed by ultrasonic extraction with ethyl acetate and acetone three times successively to generated crude extract M (15.6 g). The culture filtrate was extracted by ethyl acetate three times and dried under vacuum to get crude extract F (20.5 g). Extracts M and F were combined due to both of them showed antifungal activity by agar-diffusion method. The total crude extract was chromatographed on a silica gel, eluted with a gradient system of CH2Cl2/MeOH (CH2Cl2, 50:1, 20:1, 10:1, MeOH) to give five fractions designated fractions A–E. Fraction B (8.5 g) was chromatographed over Sephadex LH-20 (CH2Cl2 : MeOH = 6:4) and Sephadex LH-20 (MeOH) successively, one subfraction B-3-2 was repeatedly chromatographed over silica column (CH2Cl2:MeOH) and reversed-phase (ODS) column to afford the compound 1 (262.2 mg) and 2 (17.5 mg). Subfraction B-2-2 was repeatedly subjected to silica column (CH2Cl2: MeOH) to afford compound 5 (8.7 mg). Fraction C (7.2 g) was further separated by Sephadex LH-20 (CH2Cl2 :MeOH = 6:4) and Sephadex LH-20 (MeOH) successively, and one subfraction C-4-2 was repeatedly chromatographed over silica column (CH2Cl2:MeOH) and reversed-phase (ODS) column to harvest compound 3 (6.8 mg) and 4 (6.0 mg). Subfraction C-5-2 was first fractionated on silica gel (CH2Cl2:MeOH), and then purified by reversed-phase (ODS) column to produce compound 6 (8.1 mg).
Antifungal Bioassay
For evaluation of the antagonistic potential of C. globosum No.04 against phytopathogenic fungi, we conducted the confrontation culture method [14, 16]. The C. globosum and phytopathogenic fungi were co-cultured on the 9-cm petri dish filled with PDA medium plate at 28 °C for 2–4 days to check the confrontation effect between them. The distance between the two inoculation sites (strain No.04 and fungal plug) on each plate was 45 mm.
The antifungal effect of the extract, fraction and active compounds was tested by Bauer-Kirby method (agar-diffusion method) [15] with 6-mm diameter filter paper discs. The paper disc was impregnated with 4 μL of dimethyl sulfoxide (DMSO) solution of 5 mg/mL compounds 1-6 (20 μg/disc) and the discs for negative control was treated with 4μL of DMSO. Hygromycin B (25 mg/mL) was used as positive drug, (the paper discs were treated with same amounts of DMSO and dried). After the 6-mm diameter filter papers were completely dry on both sides, they were placed on the side margins of the assay PDA plates, tested phytopathogenic fungi were inoculated in the center of plates, The distance between the two inoculation sites (each filter paper and fungal plug) on each plate was about 35 mm. The inhibition zones around each disc of R. stolonifer and C. diplodiella were measured when the after 24 and 72 h respectively. Each treatment contains triplicate petri dishes and the experiment was repeated three times.
Evaluation of the Antifungal Activity of Chaetoglobosin A
The antifungal activities of chaetoglobosin A against R. stolonifer and C. diplodiella were determined on solid PDA mediums. The freshly prepared and sterilized PDA medium (45 °C) was mixed with chaetoglobosin A (5 mg/mL) for the final concentrations of 0.015, 0.030, 0.0625, 0.125, 0.250, 0.500, 1.000, 2.000 μg/mL and then evenly distributed to several Petri dishes. PDA Petri dishes which contain little DMSO but without chaetoglobosin A was used as negtive control. Then three mycelia discs from 3-day-old fungal cultures were cultured in an equal space in the Petri dishes and incubated at 26 °C. The mycelia diameters of phytopathogenic fungi were recorded after 24–72 h culturing and the inhibitive percentage was calculated by the following formula: 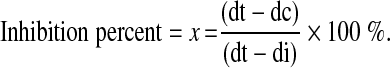 Where dc is the mean colony diameter (or radius) of negtive control sets; dt is the mean colony diameter (or radius) of treatment sets; di is the initial colony diameter (or radius) of fungal PDA discs. For each treatment, three replicates were performed. The minimal inhibitory concentrations (MICs) were recorded by reading the lowest concentrations that inhibited visible growth. Effective concentrations for inhibition of 50 and 90 % of fungi radial growth (EC50 and EC90) were calculated by the program DPS.
Where dc is the mean colony diameter (or radius) of negtive control sets; dt is the mean colony diameter (or radius) of treatment sets; di is the initial colony diameter (or radius) of fungal PDA discs. For each treatment, three replicates were performed. The minimal inhibitory concentrations (MICs) were recorded by reading the lowest concentrations that inhibited visible growth. Effective concentrations for inhibition of 50 and 90 % of fungi radial growth (EC50 and EC90) were calculated by the program DPS.
Data Analysis
Data were statistically processed and subjected to analysis of variance (ANOVA) using the program DPS and means significantly different were separated by the least significant difference (LDS) with p < 0.05.
Results and Discussion
Isolation and Identification of Antifungal Metabolites
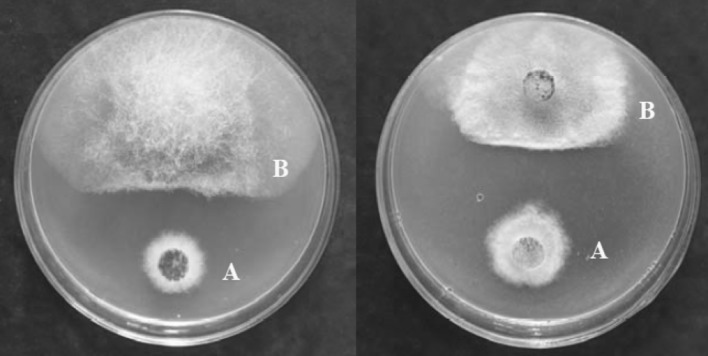
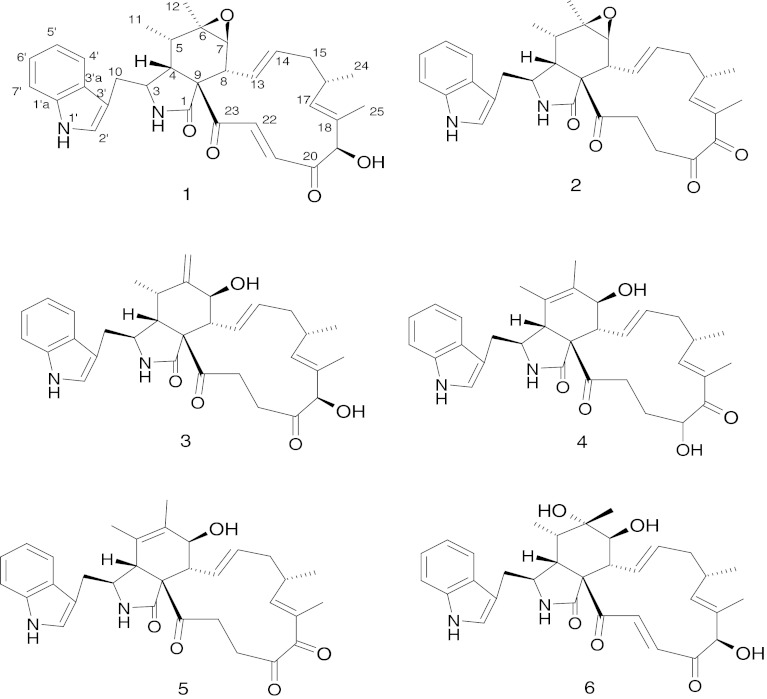
Primary screening for the antagonism of the endophytic fungus by confrontation assay [14–16] was performed against phytopathogenic fungi R. stolonifer and C. diplodiella (Speq.) Sacc. (Fig. 1). Activity-directed fractionation of the crude extract of cultures of this fungus led to the isolation of six active cytochalasan type alkaloids along with three non-target metabolites. Their structures were determined as chaetoglobosin A (1), C (2), D (3), E (4), G (5), R (6) (Fig. 2), ergosterol, allantoin and uracil on the basis of ESI-MS, 1H and 13C NMR together with 2D-NMR spectroscopic analysis, as well as comparison of chemical-physic properties with those of previously reported data in the literatures [17–22]. In the present paper, we report, for the first time, the potent antifungal activity of chaetoglobosins against these two agricultural important phytopathogenic fungi R. stolonifer and C. diplodiella, which can cause serious plant diseases, such as peach rhizopu rot and grape white rot, respectively.
Fig. 1.
Influence of Chaetomium globosum No.04 (A) on Rhizopus stolonifer (B, left) and Coniothyrium diplodiella (B, right) by confrontation culture method
Fig. 2.
Structures of compounds 1-6
Chaetoglobosins belong to the general group of cytochalasans with the phenyl group in the molecules being replaced by an indolyl group [22], and to date around 40 chaetoglobosins and analogues have been characterized from cultures of the fungus genus Chaetomium, Iplodia, Calonectria, Cylindrocladium, Discosia, Penicillium and Phomopsis [23, 24], which includes both marine- and terrestrial-derived species. However, Chaetomium species was revealed to be a excellent rich source of chaetoglobosins [25].
In Vitro Antifungal Activity of Isolated Compounds
In vitro antifungal effects of compounds 1-6 were evaluated by agar-diffusion method [15]. Compounds 1-6 show moderate antifungal effects against R. stolonifer with observed acute inhibition percent range from 31.37–51.53 %, and display remarkable inhibitory effects against C. diplodiella with average inhibition percent in the range 54.73–73.07 % (Fig. 3), at a concentration of 20 μg/disc. The average inhibition zone radius of positive control hygromycin B (100 μg/disc) against R. stolonifer and C. diplodiella was 4.5 and 32.5 mm, respectively. There was no clear sterile zone was observed around negative control.
Fig. 3.
Percentage inhibition of phytopathogenic fungi (Rhizopus stolonifer and Coniothyrium diplodiella) at compounds 1-6. Each point is the mean of three independent experiments. Error bars denote SE
Reportedly, chaetoglobosins, such as chaetoglobosin A (1), were known as cytochalasan alkaloids exhibits strong cytotoxicity on various of human cancer cell lines [17, 23]. Chaetoglobosin A (1), C (2), D (3), E (4) and G (5) displayed acute toxic effects against Hela cells with IC50 values in the range 3.2–20 μM [21]. Compounds 1, 3 and 6 possess significant toxicity against P388 murine leukemia cell line [22]. Compounds 2, 3 and 5 showed cytotoxicities against BC1 cell lines, KKU-100 cells and KKU-OCA17 cells with IC50 values in the range 2.54–21.29 μM [26]. Compounds 1 and 2 showed significant growth inhibitory activity against the brine shrimp (Artemia salina) [10], also exhibited highly toxic effects to mice and rats cells and chick embryos [28, 29]. 1 and 2 also showed phytotoxicity against alfalfa seedlings, antibacterial activity against Helicobacter pylori and Staphylococcus aureus [22, 27], as well as marked inhibitory effects on the fungus Mucor miehei in an agar-diffusion assay [10].
Antifungal Activity of Chaetoglobosin A on Phytopathogenic Fungi
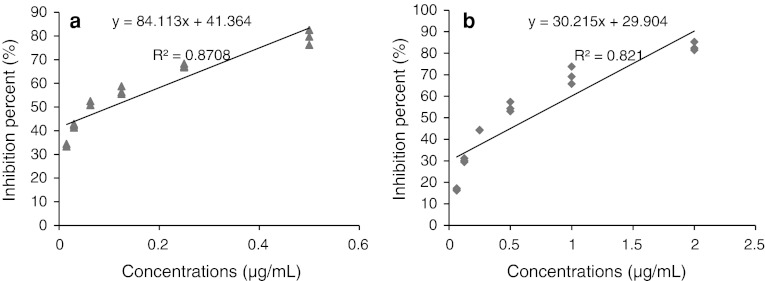
Antifungal activities of different concentrations of chaetoglobosin A on phytopathogenic fungi were evaluated. As shown in Table 1, the mycelia growths were inhibited in the presence of chaetoglobosin A, and the inhibitory efficiency were enhanced with the increment of the concentration of chaetoglobosin A. The mycelia growths of 24 h cultivation of R. stolonifer and 72 h cultivation of C. diplodiella were nearly completely inhibited as chaetoglobosin A concentration 40 and 20 μg/mL, respectively. Linear regressions of colonies inhibition percent of R. stolonifer and C. diplodiella by different concentrations of chaetoglobosin A shown in (Fig. 4). As the MIC, EC50 and EC90 of chaetoglobosin A aginst R. stolonifer and C. diplodiella shown in (Table 1), chaetoglobosin A had acute antifungal activity against phytopathogenic fungi, which indicated that the studied propolis had the potential to be a natural preservative that can be applied to control plant diseases.
Table 1.
Bioactivity of chaetoglobosin A against phytopathogenic fungi
| Phytopathogenic fungi | MIC (μg/mL) | EC50 (μg/mL) | EC90 (μg/mL) |
|---|---|---|---|
| Coniothyrium diplodiella | <0.0150 | 0.1073 ± 0.0164a | 0.5800 ± 0.0232a |
| Rhizopus stolonifer | <0.0625 | 0.6663 ± 0.0465b | 1.9862 ± 0.0580b |
Each point is the mean of three independent experiments. Means (±SE) in the same column followed by different letters (a and b) within the same column indicated differ significantly (p < 0.05) according to the Fisher’s protected LDS test
Fig. 4.
a Linear regressions of colonies inhibition percent of Coniothyrium diplodiella at different concentrations of chaetoglobosin A (0.015, 0.030, 0.0625, 0.125, 0.250, 0.500 μg/mL). b Linear regressions of colonies inhibition percent of Rhizopus stolonifer at different concentrations of chaetoglobosin A (0.0625, 0.125, 0.250, 0.500, 1.000, 2.000 μg/mL)
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (31271991),The ministry of education doctoral program funds(20120061110082), the Ministry of Agriculture Public Benefit Industry of the People’s Republic of China (20113016), and the project of National Key Technology R&D Program in the 12th Five year Plan of China (2012BAD19B04).
Footnotes
Guizhen Zhang and Yanhua Zhang contributed equally to this work and are considered co-first authors.
References
- 1.Ippolito C, Luciana A, Laura DM, Vincenzo DF, Emilia M, Gian LR. In vitro control of post-harvest fruit rot fungi by some plant essential oil components. Int J Mol Sci. 2012;13:2290–2300. doi: 10.3390/ijms13022290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YS, Kim J, Lee SG, Oh E, Shin SC, Park IK. Effects of plant essential oils and components from Oriental sweetgum (Liquidambar orientalis) on growth and morphogenesis of three phytopathogenic fungi. Pestic Biochem Physiol. 2009;93:138–143. doi: 10.1016/j.pestbp.2009.02.002. [DOI] [Google Scholar]
- 3.Mari X, Rochelle-Newall EJ, Torreton JP, Pringault O, Jouon A, Migon C. Water residence time: a regulatory factor of the DOM to POM thansfer efficiency. Limnol Oceanogr. 2007;52(2):808–819. doi: 10.4319/lo.2007.52.2.0808. [DOI] [Google Scholar]
- 4.Reino JL, Guerrero RF, Hernandez-Galan R, Collado IG. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem Rev. 2008;7:89–123. doi: 10.1007/s11101-006-9032-2. [DOI] [Google Scholar]
- 5.Saikkonen K, Wali P, Helander M, Faeth SH. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004;9:275–280. doi: 10.1016/j.tplants.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Strobel GA. Rainforest endophytes and bioactive products. Crit Rev Biotechnol. 2002;22:315–333. doi: 10.1080/07388550290789531. [DOI] [PubMed] [Google Scholar]
- 7.Strobel GA. Endophytes as sources of bioactive products. Microbes Infect. 2004;5:535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 8.Krohn K, Kock I, Elsässer B, Flörke U, Schulz B, Draeger S, Antus S, Kurtán T. Bioactive natural products from the endophytic fungus ascochyta sp. from meliotus dentatus–configurational assignment by solid-state CD and TDDFT calculations. Eur J Org Chem. 2007;16:1123–1129. doi: 10.1002/ejoc.200600907. [DOI] [Google Scholar]
- 9.Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 10.Qin JC, Zhang YM, Gao JM, Bai MS, Yang SX, Laatsch H. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg Med Chem Lett. 2009;19:1572–1574. doi: 10.1016/j.bmcl.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Qin JC, Gao JM, Zhang YM, Yang SX, Bai MS, Ma YT, Laatsch H. Polyhydroxylated steroids from an endophytic fungus, Chaetomium globosum ZY-22 isolated from Ginkgo biloba. Steroids. 2009;74:786–790. doi: 10.1016/j.steroids.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Qin JC, Gao JM, Zhang YM, Hu L, Ma YT. Cytotoxic Metabolites produced by Alternaria No.28, an endophytic fungus isolated from Ginkgo biloba. Nat Prod Commun. 2009;11:1473–1476. [PubMed] [Google Scholar]
- 13.Zhang AL, He YL, Gao JM, Xu X, Li SQ, Bai MS, Qin JC. Metabolites from an endophytic fungus Sphaceloma sp. LN-15, isolated from the leaves of Melia azedarach. Lipids. 2009;44:745–751. doi: 10.1007/s11745-009-3317-3. [DOI] [PubMed] [Google Scholar]
- 14.Gopalakrishnan S, Humayun P, Kiran BK, Kannan LGK, Vidya MS, Deepthi K, Rupela O. B Evaluation of bacteria isolated from rice rhizosphere for biological control of charcoal rot of sorghum caused by Macrophomina phaseolina (Tassi) Goid. World J Microb Biot. 2011;27:1313–1321. doi: 10.1007/s11274-010-0579-0. [DOI] [PubMed] [Google Scholar]
- 15.Yang SZ, Peng LT, Su XG, Chen F, Cheng YJ, Fan G, Pan SY. Bioassay-guided isolation and identification of antifungal components from propolis againat penicillium italicum. Food Chem. 2011;127:210–215. doi: 10.1016/j.foodchem.2010.12.011. [DOI] [Google Scholar]
- 16.Zhu N, Zhao PJ, Shen YM. Selective isolation and ansamycin-targeted screenings of commensal actinomycetes from the‘‘Maytansinoids-Producing’’ arboreal Trewia nudiflora. Curr Microbiol. 2009;58:87–94. doi: 10.1007/s00284-008-9284-8. [DOI] [PubMed] [Google Scholar]
- 17.Sekita S, Yoshihira K, Natori S, Kuwano H. Structures of chaetoglobosin A and B, cytotoxic metabolites of chaetomium globosum. Tetrahedron Lett. 1973;14:2109–2112. doi: 10.1016/S0040-4039(01)86820-9. [DOI] [Google Scholar]
- 18.Silverton JV, Akiyama T, Kabuto C, Sekita S, Yoshihira K, Natori S. X-ray analysis of chaetoglobosin A, an indol-3-yl-[13]cytochalasan from Chaetomium globosum. Tetrahedron Lett. 1976;17:1349–1350. doi: 10.1016/S0040-4039(00)78061-0. [DOI] [Google Scholar]
- 19.Sekita S, Yoshihira K, Natori S, Kuwano H. Structures of chaetoglobosins C, D, E, and F, cytotoxic indol-3-yl-[13]cytochalasans from Chaetomium globosum. Tetrahedron Lett. 1976;17:1351–1354. doi: 10.1016/S0040-4039(00)78062-2. [DOI] [Google Scholar]
- 20.Sekita S, Yoshihira K, Natori S. Chaetoglobosins, cytotoxic 10-(indol-3-yl)-[13]cytochalasans from Chaetomium spp. IV. Carbon-13 nuclear magnetic resonance spectra and their application to a biosynthetic study. Chem Pharm Bull. 1983;31:490–498. doi: 10.1248/cpb.31.490. [DOI] [Google Scholar]
- 21.Sekita S, Yoshihira K, Natori S, Kuwano H. Chaetoglobosins, cytotoxic 10-(indol-3-yl)-[13]cytochalasans from Chaetomium spp. III. Structures of chaetoglobosins C, E, F, G, and J. Chem Pharm Bull. 1982;30:1629–1638. doi: 10.1248/cpb.30.1629. [DOI] [PubMed] [Google Scholar]
- 22.Jiao WX, Feng YJ, John WB, Anthony LJ, Murray HG. Chaetoglobosins Q, R, and T, Three further new metabolites from Chaetomium globosum. J Nat Prod. 2004;67:1722–1725. doi: 10.1021/np030460g. [DOI] [PubMed] [Google Scholar]
- 23.Scherlach K, Boettger D, Remme N, Hertweck C. The chemistry and biology of cytochalasans. Nat Prod Rep. 2010;27:869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 24.Ding G, Song YC, Chen R, Xu C, Ge HM, Wang XT, Tan RX. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J Nat Prod. 2006;69:302–304. doi: 10.1021/np050515+. [DOI] [PubMed] [Google Scholar]
- 25.Cui C, Li X, Li C, Proksch P, Wang B. Cytoglobosins A-G, cytochalasans from a marine-derived endophytic fungus, Chaetomium globosum QEN-14. J Nat Prod. 2010;73:729–733. doi: 10.1021/np900569t. [DOI] [PubMed] [Google Scholar]
- 26.Thohinung S, Kanokmedhakul S, Kanokmedhakul K, Kukongviriyapan V, Tusskorn O, Soytong K. Cytotoxic 10-(indol-3-yl)-[13]cytochalasans from the fungus Chaetomium elatum ChE01. Arch Pharm Res. 2010;33:1135–1141. doi: 10.1007/s12272-010-0801-5. [DOI] [PubMed] [Google Scholar]
- 27.Ichihara A, Katayama K, Teshima H, Oikawa H, Sakamura S. Chaetoglobosin O and other phytotoxic metabolites from Cylindrocladium floridanum, a causal fungus of alfalfa [Medicago sativa] black rot disease. Biosci Biotech Bioch. 1996;60:360–361. doi: 10.1271/bbb.60.360. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsubo K, Saito M, Sekita S, Yoshihira K, Natori S. Acute toxic effects of chaetoglobosin A, a new cytochalasan compound produced by Chaetomium globosum, on mice and rats. Jpn J Exp Med. 1978;48:105–110. [PubMed] [Google Scholar]
- 29.Veselý D, Veselá D, Jelínek R. Penicillium aurantiogriseum Dierckx produces chaetoglobosin A toxic to embryonic chickens. Mycopathologia. 1995;132:31–33. doi: 10.1007/BF01138601. [DOI] [Google Scholar]