Abstract
The present work studied the differences in accumulation, transformation and volatilization of different heavy metals ions on molecular and macromorphological features of Aspergillus niger wild type strains. Four different strains of A. niger (An) were used. Three strains (An-P, An-N, An-S) were isolated from acid and ultra acid mining regions with higher concentration of As and Sb. The fourth strain (An-G) was used as the comparative one. Environmental burden strongly affected biochemical, macro and micromorphological characteristics of studied strains. The RAMP profiles showed 90 % similarity among the studied strains. The strain An-S showed its own characteristic RAMP profile, different to the others ones. Analyzed strains can be clustered into two groups on the basis of the changes in gene expression and morphological parameters. Differences were found in both acid β-1,3-glucanases and peroxidases. Main quantitative and qualitative differences by A-PAGE and SDS-PAGE were registered for proteins with Mr ~ 50; 34; 28–27 and 11 kDa. Presence of living mutants of A. niger strains in old environmental burden indicate on the adaptation and mutation processes of soil microorganisms from the point of long-term effect.
Keywords: Aspergillus niger, RAMP PCR, Proteins profile, Ecological burden
Introduction
The Aspergillus niger Tiegh is a saprotrophic mitosporic wide-spread fungus commonly found in different components of the environment as soils, pictures, walls, indoor air etc. [1]. This fungus belongs to acidophilic and acid-tolerant fungi with internal pH regulation. It is capable of maintaining a relatively neutral pH by pumping protons out of the cell and by establishing a low proton membrane permeability [2]. Wild type strains are very often used in studies of biosorption and bioaccumulation of different heavy metals and toxic elements [3–7].
Differences in the accumulation and rate of chemical elements sorption are connected with their functional groups as well as with the affinity of the fungus cell wall to these chemical elements. This affinity affected amount of receptors in the cell wall and membrane channels for input and output of hydrated heavy ions from the environment [8]. Affinity and sorption of hydrated heavy ions to the cell wall changed not only membrane potential but regulated amount and composition of extracellular proteins [9].
Heavy metals which are accumulated in soils, water and sewage sludge may, at sufficiently, in high concentrations affect the growth, sporulation and metabolism of fungi [6, 10, 11]. Wide spectrum of heavy metal ions effecting soil microorganisms led us to study their effects on selected strains of A. niger from various localities with different level of old ecological burden. The present study compares the differences in biochemical, molecular and morphological features of four A. niger wild strains.
Materials and Methods
Isolation of Fungi
Four A. niger (An) wild type strains were used in this study. Three strains were isolated from three different former mining areas of Slovakia. The strain An-P was isolated from the stream sediment of the Blatina River in the Pezinok mining region, the strain An-N was isolated from the brown coal dust in the Nováky mining region and the strain An-S was recovered from Dystric Cambisol without vegetation at the mining locality Šobov. The strain An-G was the control comparative strain and it was isolated from Eutric Fluvisol cover with plant community structure of Salici-Populetum from floodplain forest without the presence of heavy metals at the locality Gabčíkovo.
All used strains were isolated using dilution plate method (10 g of fine soil was diluted on 10−4 CFU—colony forming units) from a mixed culture on Sabouraud Agar (Sabouraud Maltose Agar—SAB, Himedia, Mumbai, India).
Macro and Micromorphological Features
Macromorphological features, diameter of colonies and time of sporulation, were observed visually on the 3rd, 5th and 8th day of cultivation on media containing SAB agar (Fig. 1). Every experiment and the measurements were repeated independently three times. Micromorphological features were observed under the light microscope Jenalumar Carl Zeiss Jena (Germany) on the 7th day of cultivation fixed in a drop of lactic acid enriched with cotton blue stain (0.01 %). Figures were made with Olympus digital camera (Japan).
Fig. 1.
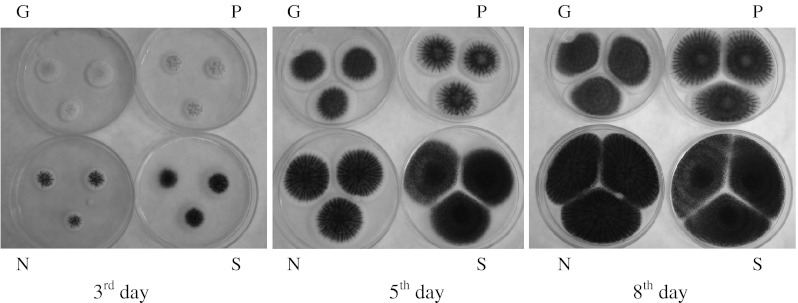
Micromorphological features of A. niger strains on the 3rd, 5th and 8th day of cultivation on SAB. G Gabčíkovo, P Pezinok, N Nováky, S Šobov
DNA Extraction
The four Aspergillus niger wild type strains were inoculated in Sabouraud broth liquid medium (Himedia, Mumbai, India) at 28 °C. After 8 days cultivation, they were separated from the media by filtration through the sterile filter paper. DNA was isolated by the DNA-easy Blood & Tissue Kit (Qiagen, Hilden, Germany), according to enclosed protocol for animal and vegetable tissue (DNAeasy Handbook, July 2006).
Internal Transcribed Spacer (ITS) Fungal Identification and Characterization
The A. niger strains were identified by the amplification of the ITS fragment using the primers ITS1 (5′-tccgtaggtgaacctgcgg-3′) and ITS4 (5′-tcctccgcttattgatatgc-3′) [12] and subsequent sequencing. PCR mixture contained 20 pmol of each primer, 200 μmol/l dNTP, 1 U HotStarTaq plus DNA polymerase, 1 × PCR buffer and 6 μl of template DNA in the total reaction volume of 25 μl. The PCR products were purified using ExoSAP-IT (Affymetrix, Cleveland, Ohio, USA) and sequenced for both strands by a commercial facility (Macrogen, Seoul, South Korea). The sequences of Aspergillus strains were compared directly with database in GenBank by FASTA search program (http://www.ebi.ac.uk/fasta33/). The RAMP (Random Amplified Microsatellite Polymorphism) PCR method was used to characterize the four A. niger wild type strains [13].
Protein isolation and separation
Proteins were isolated into 0.1 M Na-phosphate buffer pH 7 [14] from 350 μg A. niger mycelia in the stationary growth phase. Mycelium was homogenized in the liquid nitrogen and frozen powder was solubled in the extraction buffer. After 24 h isolation at 10 °C, samples were frozen at −35 °C. Quantitative content of soluble proteins was determined [15]. Separation of native acid proteins was done on 12.5 % discontinual polyacrylamide gel [16]. β-1,3-glucanases were identified directly on the slab gels [17]. Peroxidases were identified according to [14, 22]. SDS (sodium dodecyl sulphate)-PAGE was done according to Laemmli [18]. Fermentas wide molecular ladder (Fermentas, Life Science, EU.) was used as a molecular mass marker. Gels were silver stained [19].
Results and Discussion
The strain An-G represent the control strain isolated from alkaline to weakly alkaline floodplain forest soil without heavy metals. Very acid to acid stream sediment of the Blatina River with natural amounts of As (363 mg/kg) and Sb (93 mg/kg) was the source of the 2nd A. niger strain (An-P). From the brown coal dust containing As (400 mg/kg) in the Nováky mining region was isolated the 3rd A. niger strain (An-N). This substrate was ultra acid. The 4th A. niger strain (An-S) was isolated from the old mine locality Šobov from Dystric Cambisol contaminated and eroded. This locality has ultra acid pH and is impacted by an acid sulphate weathering with high exchangeable contents of Fe3+ [20].
During the cultivation of studied A. niger wild type strains conspicuous differences were noticed in growth rate. The growth rate was the slowest for the strains An-G and An-P. Diameter of colony was 1.6–1.4 cm on the 3rd day of cultivation, 3.0–2.8 cm on the 5th day and 4.8–4.9 cm on the 8th day of cultivation consequently. Mycelium of both strains was brown to dark brown with very slow rate of sporulation. Fast growing colonies and noticable sporulation on the 3rd day of cultivation after inoculation was noticed for the strains An-N and An-S. The diameter of colony was 1.6–1.8 cm on the 3rd day of cultivation and 3.4–3.7 cm on the 5th day and 5.6–6.2 cm on the 8th day of cultivation. Intensive sporulation was noticed at the 3rd day of cultivation. Both strains were isolated from the ultra acid substrates produced black mycelia (Table 1, Fig. 1).
Table 1.
Selected characteristics of wild type A. niger strains
| Strain | Locality/source | pH H2O/KCl | Sequence similarity | CMF ISB České Budějovice |
|---|---|---|---|---|
| An-G | Gabčíkovo/Eutric Fluvisol | 7.7/7.4 | 100 % AM270051 | 1670 |
| An-P | Pezinok/stream sediment As 363 mg/kg, Sb 93 mg/kg | 5.3/4.8 | 99.8 % AM270051 | 1671 |
| An-N | Nováky/coal dust As 400 mg/kg | 3.3/2.9 | 99.8 % AM270051 | 1669 |
| An-S | Šobov/dystric cambisol (contaminated, eroded) Fe3+ 346 mg/kg | 3.0/2.7 | 99.6 % AM270051 | 1674 |
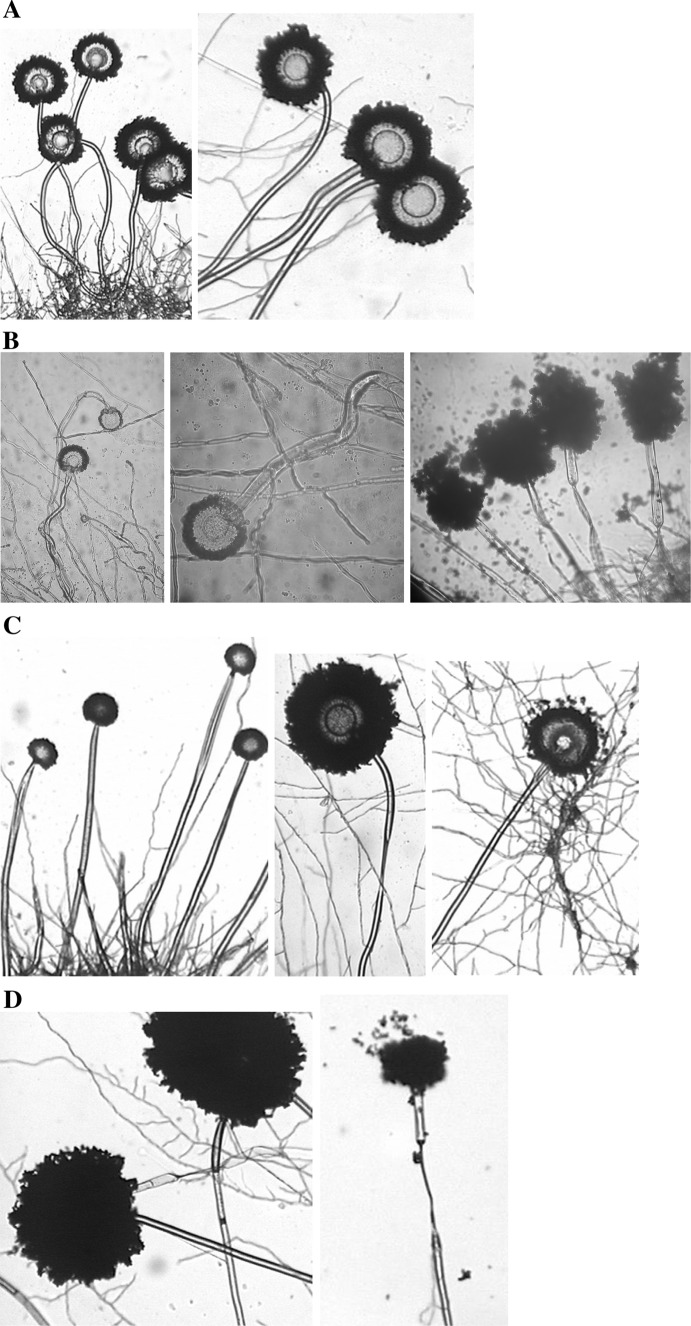
Environmental burden strongly affected habitus of A. niger wild type strains. Despite of the differences in the growth rate, micromorphological anomalies as constriction, splitting and swelling of hyphaes, bad developed heads with phialides and conidia were observed. Multiplay constriction and swelling in hyphae were detected in A. niger wild type strains isolated from the locality Pezinok (Fig. 2b). Very small or irregularly differentiated heads were detected at the strain An-N. Ultra acid soil caused swelling of hyphaes and abnormal sporulation of strain An-S. Isolated mutant strains had irregular heads with many deep layers of the cells which contained minimal amount of melanin (Fig. 2c–d). Strain An-G isolated from the control locality is without any visible changes (Fig. 2a). High concentrations of Zn and Cd affected the growth and sporulation of A. terreus strain [10, 11] where abnormal conidial head and hyphal swellings were observed. Very thin mycelium of Trichoderma viride with minimal sporulation was described under the influence of Cd and Hg [21].
Fig. 2.
Micromorphological features of A. niger wild strains (720×). a strain A. niger from control environment. b, c, d deformations of the A. niger after long-term effect of As, Sb, extreme acid and ultra acid pH.
The PCR identification through the amplification of ITS fragment and its consequent sequencing confirmed that our wild type strains belonged to the species A. niger (Table 1). The RAMP PCR analysis was able to characterize the four A. niger strains. RAMP profiles comparison displayed a similarity of 90 % among the strains An-N, An-G and An-P; the strain An-S showed its own characteristic RAMP profile, dissimilar to the others isolates (Fig. 3). This result has a certain degree of homology with the analysis of protein patterns which located the strains An-G and An-P into same cluster (Table 2).
Fig. 3.

The RAMP PCR analyse of four A. niger wild type strains
Table 2.
Differences in the protein patterns of A. niger wild type strains
| Locality/Strain | Proteins up regulated Mr (kDa) | Proteins down regulated Mr (kDa) |
|---|---|---|
| Pezinok/An-P | 70; 54–53; 41; 34; 16 | 20 |
| Nováky/An-N | 50; 34; 28–27; 24; 22–21; 16; 15–12; 10 | 20 |
| Šobov/An-S | 34; 28–27; 22–21; 16 | 20; 13; 11.5–11 |
Comparison of the protein spectras was done to the pattern of control strain A.n-G (isolated from the locality Gabčíkovo)
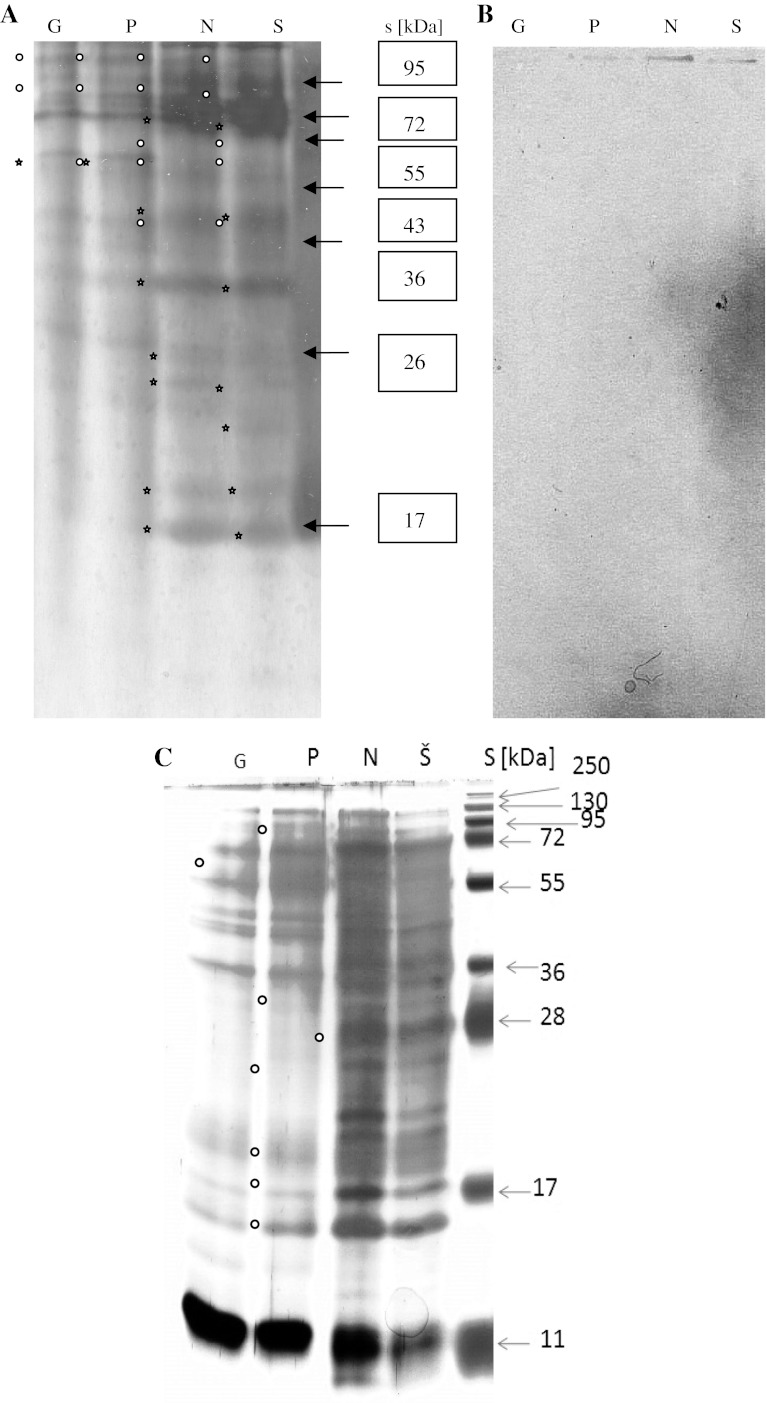
Patterns of both acid and denaturated proteins (Fig. 4a–c) showed that analyzed A. niger strains can be clusterized in two groups on the base of changes in the gene expression and morphological parameters (Figs. 2, 4). The first groups is formed by the strains isolated from the locality Pezinok and Gabčíkovo and the second group by the strains isolated from the localities Nováky and Šobov (Table 1). Differences were found as well as between acid β-1,3-glucanases (Glu; Mr ~ 45–20 kDa) and peroxidases (PRX; Mr ~ 70–40 kDa, Fig. 4a–b). Main quantitative and qualitative differences by A-PAGE (polyacrylamide gel electrophoresis of acid native proteins) and SDS-PAGE were registered for proteins with Mr ~ 50; 34; 28–27 and 11 kDa (Fig. 4c). Table 3 shows their up and down regulated amount of proteins represented in the patterns. Main changes were observed by the strains of A. niger isolated from locality Nováky and Šobov. Results in the protein patterns (Table 3; Fig. 4a–c) indicate on the possible role of the different concentration of the heavy metal ions in the soil of original localities. What was reflected on the morphological features (Fig. 2a–d). Qualitative and quantitative differences between Glu indicate on the changes in the enzymes activity connected with different intensity of biosynthesis of saccharides and oligosaccharides [22]. Biosynthesis pathways of saccharides and oligosaccharides are connected with activity of additional enzymes as β-glucosidases, manases, galactosidases and galactomanases (Table 3). Pattern of acid β-1,3-glucanase isozymes of the studied A. niger strains are shown in Fig. 4b. Their content was changed in the dependence of the heavy ions concentration and acidity of the soil burden. Glu150 (Fig. 4b) was found by all strains. Differences in their quantitative content answered degree of pollution. Other isozymes of Glu are probably caused of different level of gene suppression/inhibition for oligosaccharides. The morphological changes in the structure of the mycelia cell wall indicated on the different absorption and sorption ability which can affected the rate and intensity of active ion transport via channels in and out direction. This probability is reflected on SDS-PAGE protein patterns for the last two A. niger strains. Differences in the PRX patterns (Fig. 4a) indicate on the changes in the rate and quality of oxido-reduction pathways during stationary growth phase. One for the melanin binding proteins had Mr ~ 11 kDa. Our results are in agreement with the results obtained by Tsang et al. [9].
Fig. 4.
Protein patterns of wild strains of A. niger. a Acid protein patterns of A. niger. Asterisk indicated up regulated acid proteins synthesized in the stationary phase of the growth. Dots indicated proteins with peroxidase activity. G Gabčíkovo; P Pezinok; N Nováky; Š Šobov; s molecular mass standards ladder. Load per lane was 1.3 μg of soluble proteins; Gel was stained with AgNO3 [19]. b. A. niger—acid proteins with β-1,3-glucanase activity [17]. Glu activity is strongly growing from left to right. Load per lane was 5 μg of soluble proteins. c 1-D-SDS-PAGE protein patterns of selected A. niger strains. Dots designated proteins/polypeptides with strong up/down regulation of gene expression. Load per lane was 0.8 ± 0.05 μg of proteins
Table 3.
Possible biochemical function of some acid proteins synthesized in the selected A. niger wild type strains during the stationary stage of the growth
| Enzyme | Mr (kDa) | Study strains | References | |||
|---|---|---|---|---|---|---|
| An-G | An-P | An-N | An-S | |||
| Endoglucanase I | 25; 43 | −; + | −; + | −; + | +; − | [9, 26] |
| Endoglucanase II | 25 | − | − | + | + | |
| β-Glucosidase A | 96 | + | + | + | + | |
| β-Glucosidase B | 120 | − | − | + | + | |
| β-1,3-glucanase | 150; 49 | +; − | +; − | +; − | +; + | Present work |
| Endoxylanase | 33; 20.8 | −; + | −; + | +; + | +; + | [26] |
| β-D-manase | 56; 45-40 | +; + | +; + | −; + | −; + | |
| β-D-manoxidase | 80; 51 | +; + | +; + | +; − | +; − | |
| Endopolygalactouranase | 40; 43 | +; − | +; + | −; + | −; + | |
| Pectinlyase | 83; 60 | +; − | +; − | +; + | +; + | |
| Aranbinofuranoxidase A | 43 | − | − | + | + | |
| Aranbinofuranoxidase B | 95; 82 | +; + | +; + | +; + | +; + | |
| Endoarabinase | 78; 45 | +; - | +; + | +; + | +; + | |
| β-Galactosidase | 60 | − | − | + | + | |
| Endo-β-1,6-galactomanase | 60 | − | − | + | + | |
| Feruryl-acetyl-methyl-esterase II | 75.8 | + | + | + | + | |
| Acetylanesterase | 40 | + | + | + | + | |
| Acetyl-galacto-glucomannan esterase | 42 | + | + | + | + | |
| Ramanan-galactouronanesterase | 49 | + | + | − | − | |
| Pectine-methyl-esterase | 43 | − | − | + | + | |
| Peroxidase | 150; 90; 60; 50 | +; +; −; + | −; +; +; − | +; +; −; + | +; +; −; + | Present work |
| Melanine-binding proteins | 11.5; 11 | −; − | −; − | +; + | +; + | |
− missing, + present
Bold are marked the differences, respect the previous published works, evidenced in this study
Differences in the activity and amount of other enzymes connected with low and very low pH were found by different authors [9, 23–25]. Long-term effect of heavy metal ions, low pH and changes in the gene expression were represented on the morphological characteristics of hyphae and heads carrying spores (Fig. 2a–d). Presence of living mutant A. niger strains in old burden indicated on the adaptation processes of soil microorganisms from the point of long-term effects.
In conclusions, our results showed that long-term effect of extreme acid (pH 4) and ultra acid (pH 2.2) environment conditions combined with presence of heavy metal ions as As3+, Hg2+, Cd2+ in the soil of old environmental burden caused mutation in the soil micromycetes A. niger. Both factors influenced gene expression, the metabolism and consequently their morphological appearance.
Acknowledgments
This research was supported by Slovak Grant Agency VEGA 1/0156/11.
References
- 1.Domsch KH, Gams W, Anderson TH (2007) Compendium of soil fungi. 2nd edn. taxonomically revised by Walter Gams. IHW-Verlag, Eching, pp 1–672
- 2.Gross S, Robbins EI. Acidophilic and acid-tolerant fungi and yeasts. Hydrobiologia. 2000;433:91–109. doi: 10.1023/A:1004014603333. [DOI] [Google Scholar]
- 3.Joshi PK, Swarup A, Maheshwari S, Kumar R, Singh N. Bioremediation of heavy metals in liquid media through fungi isolated from contaminated sources. Indian J Microbiol. 2011;51(4):482–487. doi: 10.1007/s12088-011-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal TK, Bhattacharyya S, Basumajumdar A. Cellular distribution of bioaccumulated toxic heavy metals in Aspergillus niger and Rhizopus arrhizus. Int J Pharm BioSci. 2010;V1(2):1–6. [Google Scholar]
- 5.Amini M, Younesi H, Bahramifar N. Biosorption of nickel(II) from aqueous solution by Aspergillus niger: response surface methodology and isotherm study. Chemosphere. 2009;75:1483–1491. doi: 10.1016/j.chemosphere.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi P, Srivastava S. Development and characterization of nickel accumulating mutants of Aspergillus nidulans. Indian J Microbiol. 2007;47:241–250. doi: 10.1007/s12088-007-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Žemberyová M, Sherman A, Šimonovičová A, Hagarová I. Bio-accumulation of As(III) and As(V) species from water samples by two strains of Aspergillus niger using hydride generation atomic absorption spectrometry. Int J Environ Anal Chem. 2009;89:569–581. doi: 10.1080/03067310802716107. [DOI] [Google Scholar]
- 8.Soni SK, Magdum A, Khire JM. Purification and characterization of two distinct acidic phytases with broad pH stability from Aspergillus niger NCIM 563. World J Microbiol Biotechnol. 2010;26:2009–2018. doi: 10.1007/s11274-010-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang A, Butler G, Powlowski J, Panisko EA, Baker SE. Analytical and computational approaches to define the Aspergillus niger secretome. Fungal Genet Biol. 2009;46:S153–S160. doi: 10.1016/j.fgb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Ezzouhri L, Castro E, Moya M, Espinola F, Lairini K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr J Microbiol Res. 2009;3:035–048. [Google Scholar]
- 11.Azab MS, Peterson PJ, Young TWK. Scanning electron microscopy of Aspergillus terreus growth in the presence of zinc and cadmium compounds. Trans Br Mycol Soc. 1986;86:469–474. doi: 10.1016/S0007-1536(86)80191-7. [DOI] [Google Scholar]
- 12.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. New York: Academic Press; 1990. pp. 315–321. [Google Scholar]
- 13.Pangallo D, Chovanová K, Šimonovičová A, Ferianc P. Investigation of microbial community isolated from indoor artworks and air environment: identification, biodegradative abilities, and DNA typing. Can J Microbiol. 2009;55:277–287. doi: 10.1139/w08-136. [DOI] [PubMed] [Google Scholar]
- 14.Hlinková E, Ondřej M. Changes in the protein spectra of transgenic tobacco plants carrying different Agrobacterium tumefaciens C58 T-DNA genes. Biol Plant. 1994;36:29–36. doi: 10.1007/BF02921264. [DOI] [Google Scholar]
- 15.Bradford MR. A rapid and sensitive method for the quantification of protein utilising the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Smith JA (1998) Analysis of proteins. In: Ausubel FM, Bent R, Smith JA, Struhe K (eds) Current Protocols in Molecular Biology. Wiley, New York. 10.0.3-10.3.11. 1988
- 17.Pan SQ, Ye XS, Kuc J. Direct detection of beta-1,3-glucanase isozymes on polyacrylamide electrophoresis and isoelectrofocusing gels. Anal Biochem. 1989;182:136–140. doi: 10.1016/0003-2697(89)90730-6. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Beňová A, Hlinková E, Šimonovičová A. Changes in protein pattern of microscopic fungi of Aspergillus niger Tiegh. and Aspergillus clavatus Desm. by stress conditions. Phytopedon (Bratislava) 2005;4:19–27. [Google Scholar]
- 20.Výbohová M, Šimonovičová M, Dlapa P, Madaras M. Microbial activity in soils under the influence of pyrite weathering. Geol Carpath. 1999;50:389–394. [Google Scholar]
- 21.Šimonovičová A, Ševc J, Iró S. Trichoderma viride Pers. ex Gray as biosorbent of heavy metals (Pb, Hg and Cd) Ekológia (Bratislava) 2002;21:298–306. [Google Scholar]
- 22.Kákoniová D, Hlinková E, Lišková D, Kollárová K. Oligosaccharides induce changes in protein patterns of regenerating spruce protoplast. Cent Eur J Biol. 2010;5(3):353–363. doi: 10.2478/s11535-010-0018-0. [DOI] [Google Scholar]
- 23.Kim T, Mullaney EJ, Porres JM, Roneker KR, Crowe S, Rice S, Ko T, Ullah AHJ, Daly CB, Welch R, Lei XG. Shifting the pH profile of Aspergillus niger PhyA phytase to match the stomach pH enhances its effectiveness as an animal feed additive. Appl Environ Microbiol. 2006;72:4397–4403. doi: 10.1128/AEM.02612-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JA, Murphy RA, Power RFG. Purification and physico-chemical characterization of genetically modified phytases expressed in Aspergillus awamori. Bioresour Technol. 2006;97:1703–1708. doi: 10.1016/j.biortech.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Bučková M, Godočíková J, Šimonovičová A, Polek B. Production of catalases by Aspergillus niger isolates as a response to pollutant stress by heavy metals. Curr Microbiol. 2005;50:175–179. doi: 10.1007/s00284-004-4458-5. [DOI] [PubMed] [Google Scholar]
- 26.de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev. 2001;65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]